Abstract
Background
Oesophageal carcinoma is one of the most common cancers worldwide. Its incidence and mortality rates show a wide geographical variation at a world and regional level. Geographic mapping of age-standardized, cause-specific death rates at a municipal level could be a helpful and powerful tool for providing clues leading to a better understanding of its aetiology.
Methods
This study sought to describe the geographic distribution of oesophageal cancer mortality for Spain's 8077 towns, using the autoregressive spatial model proposed by Besag, York and Mollié. Maps were plotted, depicting standardised mortality ratios, smoothed relative risk (RR) estimates, and the spatial pattern of the posterior probability of RR being greater than 1.
Results
Important differences associated with area of residence were observed in risk of dying from oesophageal cancer in Spain during the study period (1989–1998). Among men, excess risk appeared across the north of the country, along a band spanning the length of the Cantabrian coastline, Navarre, the north of Castile & León and the north-west of La Rioja. Excess risk was likewise observed in the provinces of Cadiz and part of Seville in Andalusia, the islands of Tenerife and Gran Canaria, and some towns in the Barcelona and Gerona areas. Among women, there was a noteworthy absence of risk along the mid-section of the Cantabrian seaboard, and increases in mortality, not observed for men, in the west of Extremadura and south-east of Andalusia.
Conclusion
These major gender- and area-related geographical differences in risk would seem to reflect differences in the prevalence of some well-established and modifiable risk factors, including smoking, alcohol consumption, obesity and diet. In addition, excess risks were in evidence for both sexes in some areas, possibly suggesting the implication of certain local environmental or socio-cultural factors. From a public health standpoint, small-area studies could be very useful for identifying locations where epidemiological research and intervention measures ought to receive priority, given the potential for reducing risk in certain places.
Similar content being viewed by others
Background
Oesophageal carcinoma is one of the most common cancers worldwide, ranking eighth in terms of incidence and sixth in terms of mortality in 2002 [1]. Its incidence and mortality rates show a wide geographical variation at an international level, with remarkable differences between high- and low-risk areas [1–3]. In Western countries it is a tumour that, on the whole, is relatively infrequent in men and very infrequent in women. However, it is an important problem in economically less developed countries, where the sex ratio is also much closer to unity [1].
Geographic variability is also present at a regional and local level. In Europe, the highest incidence rates among men appear in France and the United Kingdom, where oesophageal cancer is 10 times more frequent than in Greece and Macedonia [2]. Among women, the highest rates are registered by the United Kingdom and Ireland, while the lowest rates are recorded for Mediterranean countries such as Greece and Spain. The most recent incidence data furnished by Spanish tumour registries indicate that European-population-adjusted rates range from 11.51 per 100,000 population in the Canary Islands to 4.45 in Cuenca for men, and from 1.37 in the Canary Islands to 0.55 in Albacete for women [4]. Mortality rates are close to those for incidence, due to the low survival for this tumour (13% and 23% at 5 years in men and women respectively) [5].
The main histopathologic types of oesophageal cancer are squamous cell carcinoma (SCC) and adenocarcinoma (AC). The dominant histological type worldwide is SCC, tobacco and alcohol being the main agents involved in its aetiology [6–8]. For oesophageal AC, the principal risk factors described are gastro-oesophageal reflux and obesity [9–13]. Recent decades have witnessed a surprising increase in the incidence of oesophageal adenocarcinomas in the United States and Europe [14–16], and, though this trend might be partially explained by improvement in the accuracy of histological diagnosis, some authors insist that we are facing a real increase in disease burden [17, 18]. SCC, in contrast, registered a relatively stable or declining trend in Western countries over the same time period [14, 19]. While the SCC mortality trend could be linked to the decrease in tobacco use in the West, obesity, gastro-oesophageal reflux disease and, possibly, reductions in Helicobacter pylori prevalence have all been suggested as contributors to the upward trends in oesophageal AC rates [20].
Despite the fact that an increased risk of oesophageal cancer has also been described in the offspring of affected parents [21], the strong temporal and geographical differences in incidence and mortality at a world and regional level suggest the importance of environmental factors in its aetiology [22]. In Spain, a previous study highlighted the existence of important geographical differences in mortality due to this tumour [23]. Geographic mapping of age-standardized, cause-specific death rates using a greater level of disaggregation could be a helpful and powerful tool for providing clues leading to a better understanding of its aetiology. Hence, this study set out to analyse the spatial distribution of oesophageal cancer mortality at a municipal level in Spain, with the aim of highlighting interprovincial or district patterns.
Methods
Individual mortality data were used to ascertain the number of oesophageal cancer deaths (International Classification of Diseases, 9th revision [ICD-9], code 150) for the period 1989–1998. These data, which include information on town or city of residence at death, were supplied by the National Statistics Institute for the production of a municipal cancer mortality atlas, after its institutional review board approved the study. The municipal populations, broken down by age group (18 groups) and sex, were drawn from the 1991 census and 1996 municipal roll. These years correspond to the midway points of the two quinquennia that comprise the study period (1989–1993 and 1994–1998). The person-years for each five-year period were estimated by multiplying these populations by 5.
Standardised mortality ratios (SMR) were calculated as the ratio between observed and expected deaths. For the calculation of expected cases, the overall Spanish mortality rates for the above two 5-year periods were applied to each town's person-years by age group, sex and quinquennium.
To draw up these maps, smoothed municipal relative risks (RRs) were calculated using the autoregressive conditional model initially proposed by Clayton and Kaldor [24] and developed by Besag, York and Mollié [25]. Such models are based on fitting spatial Poisson models with two random-effects terms that take the following into account: a) the effects which vary in a structured manner in space (municipal contiguity); and, b) a component that models the effects which vary among municipalities in an unstructured manner (municipal heterogeneity). The model takes the following form
O i ~ Po(E i λ i )
log(λ i ) = α + h i + b i
where: λ i is the relative risk in area i; O i is the number of deaths in area i; E i are the expected number of cases; α is the intercept; h i is the municipal heterogeneity term; and b i is the spatial term.
The models were fitted using bayesian Markov chain Monte Carlo simulation methods with improper priors [26, 27]. In Bayesian modelling, prior distributions have to be specified for all unknown parameters even when there is no external information available. Priors, and distributions in general, are improper when they do not integrate to 1. In most cases, improper priors can be used in Bayesian analysis without major problems given that a proper posterior distribution can be obtained [27]. Posterior distributions of RR were obtained using WinBugs [28]. The criterion of contiguity used was municipal adjacency.
It was possible to compile and ascertain the posterior distribution of relative risk on the basis of a single Bayesian spatial model covering all of Spain's 8077 municipal areas. Convergence of the simulations was verified using the BOA (Bayesian Output Analysis) R programme library [29]. Given the great number of parameters of the models, the convergence analysis was performed on a randomly selected sample of 10 towns and cities, taking strata defined by municipal size. Convergence of the estimators was achieved before 100,000 iterations. In the present study, a "burn-in" (iterations discarded to ensure convergence) of 300,000 iterations was performed, and the posterior distribution was derived using 5,000 iterations. The CPU time on a Pentium 2 GHz was 18 hours.
A Geographic Information System (GIS) was used to create municipal maps of SMRs, smoothed RR estimates and the posterior probability that RR>1.
Results
From 1989 to 1998, a total of 17618 oesophageal cancer deaths were registered in Spain (15274 in men and 2344 in women). Table 1 displays basic descriptive statistics for the population and disease data on both sexes.
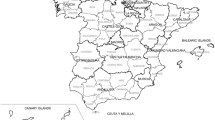
By way of reference, Figure 1 shows the situation of Spain's autonomous regions and provinces. Figure 2 depicts the distribution pattern of: a) SMR; and b) smoothed RR (both sexes). The SMR map mainly shows towns with and without cases. Despite the presence of a greater number of towns with high risks in some areas of the province of Cadiz, Seville or the provinces along the Cantabrian seaboard (Bay of Biscay), in this map no clear pattern is discernable in the distribution of risk. The smoothed risk map, on the other hand, enables homogeneous areas to be more clearly delimited, showing three areas with excess risk, namely: one in the south of mainland Spain, in the provinces of Cadiz and Seville; one in the north-west of the country, which basically encompasses the coastal provinces of Galicia, Asturias and northern León; and another to the north, covering Cantabria, the Basque Country, the north of Navarre and Burgos, stretching down as far as La Rioja, part of Palencia, Valladolid and even Segovia. Outside mainland Spain, a clear excess risk was likewise in evidence in the Canary Islands. Lastly, some towns in areas around Barcelona and Gerona also registered an excess risk, albeit more moderate.
Figures 3 and 4 depict smoothed relative risk by sex and the likelihood of the estimated RR being greater than 1. As will be seen, the pattern plotted for men determined the combined pattern, since the number of deaths among men was 6-fold that of women. However, the contrast between the maps for men and women enabled differences in the distribution of risk to be observed. Among males, the two areas in the north of Spain that had registered excess risk for both sexes, merged and expanded to include the Cantabrian coastline, from Galicia to Navarre, and the north of the provinces of León, Palencia and Burgos, whereas in women the increased risk in these regions was far more geographically defined, with statistically significant excess risks appearing mainly in the Galician provinces of La Coruña (Corunna), Pontevedra and Lugo. In the south the opposite effect took place, however, with the excess risk observed for men in Andalusia in the provinces of Cadiz and part of Seville, spreading to cover a wider area in women, which included the western area of the province of Malaga, the coast of Malaga and Granada, and the west of Extremadura. A noteworthy feature in men and women alike was the excess mortality in the Canary Islands, which proved statistically significant in towns on the two largest islands, Tenerife and Gran Canaria. Finally, statistically significant RR were also in evidence in the City of Barcelona for both sexes, and in towns along the Barcelona coast for men.
Tables 2 and 3 show a list of towns and cities with 10 or more deaths among men and 5 or more deaths among women, an excess risk of over 1.5, and a posterior probability of over 0.90 that RR would be higher than 1. These arbitrary selection criteria were used in order to select a reasonable number of municipalities with a high and statistically significant risk excess. In line with the findings observed in the maps, towns and cities with risks of over 1.5 were mainly situated: in the case of men, in Corunna, Pontevedra, Guipúzcoa, Vizcaya, Asturias and the Canary Islands; and in the case of women, in Corunna, Lugo, Vizcaya, Cadiz, Malaga and the Canary Islands.
Discussion
This study recorded marked differences in the distribution of oesophageal cancer mortality at a small-area level in Spain and reveals the similarities and differences in the patterns for men and women. Among men, mortality was far greater in the north of the country, though an area with excess was also detected in the south, in the provinces of Cadiz and Seville, as well as in the Canary Islands. Among women, the excess risk in the north of the country was more closely delimited, and there was a noteworthy degree of excess mortality in Galicia and the south-west of the country in particular. These results confirm the existence of some geographical risk patterns partially highlighted in previous studies [23], and suggest the existence of important differences -both geographical and sex-related- in the prevalence of some well-established and modifiable risk factors, including smoking, alcohol consumption, overweight and diet, and to some extent, differences in the distribution of the two main histological types. Furthermore, the fact that in some areas excess risks appear in both sexes suggests the implication of certain local environmental or socio-cultural factors.
Disease maps typically show standardised mortality or morbidity ratios for geographical units [30]. However, the use of SMR for the study of spatial disease patterns in small areas introduces an extra source of variability in the form of random variability, since sparsely populated areas with few or zero cases can generate extreme SMR rates [30]. The Bayesian approach, in contrast, helps identify the underlying geographical pattern. Great part of the variability that appears in the maps before "smoothing" is removed by smoothing, with the result that smoothed RR maps pinpoint high-risk areas. This approach is not free of limitations, however, and there are authors who feel that Bayesian disease-mapping models are essentially conservative [31].
In Spain, mortality represents the only comprehensive and homogeneous source of information on cancer nationwide. Oesophagus is slightly overrepresented in Spanish mortality data, basically due to the inclusion of adjacent gastric cancers [32]. If misclassification were associated with geographical area, then spatial distribution of oesophageal cancer mortality could be affected by this problem. However, death-certificate quality studies undertaken in various regions of Spain do not suggest the existence of important geographical differences in misclassification [32].
Unfortunately, available mortality data do not enable the distribution of the main histological types in Spain to be studied. AC and SCC, though sharing some risk factors, display different spatial distributions and temporal trends worldwide, denoting that they are basically different diseases. Data from existing Spanish tumour registries indicate that incidence of SCC is three to ten times higher than ACC. SCC/ACC ratio do not present a clear geographical pattern, though incidence data are not available for most of the regions with high mortality rates in our country [4].
The implication of tobacco and alcohol in the aetiology of SCC of the oesophagus has been highlighted by a number of studies [6, 22, 33]. Risk of adenocarcinoma is also increased by tobacco (though the association is weaker than in the case of SCC), but there appears to be little connection with alcohol consumption [6, 34]. Furthermore, combined consumption of alcohol and tobacco has a synergic effect that increases risk exponentially, not only for oesophageal cancer, but also for other malignant tumours, such as those of the larynx, oral cavity and pharynx. The fact that in Spain the distribution of male mortality due to oesophageal cancer displays similarities with that of mortality due to the above tumours [23], with risks excesses in south-western Andalusia, in the north of Spain (along the central part of the Cantabrian coastline and the Atlantic coastline of Galicia), as well as in the Canary Islands, points to the importance of these two variables when it comes to explaining the variability encountered in this study among men, given that men drink and smoke more than women [35]. In this respect, risk excesses of dying from bladder and lung cancer have also been registered in south-western Andalusia in men, reflecting the role of tobacco smoking in this pattern [36]. However, the different distribution patterns of these habits [35] are not sufficient to explain the enormous differences found between higher- and lower-risk regions. Studies in high-risk populations have described the implication of certain smoking habit characteristics, such as intensity, duration, or type of tobacco, in the geographical differences in this tumour [37, 38], as well as differences in the type of alcoholic beverages consumed locally. In the Canary Islands, moreover, existing tumour registries show the incidence of oesophageal cancer to be the highest in Spain for men and women alike [4], so that it would be of great interest to study which factors might be determining such excess risk in this area.
Gastroesophageal reflux and obesity have been described as the strongest risk factors for oesophageal adenocarcinoma [9–12]. Adenocarcinomas account for around 20% of total oesophageal cancers in some Spanish tumour registries, such as Granada, Mallorca and Murcia, whereas they represent only a 10% of cases in Navarre, Girona and Asturias [4]. Lower exposure to alcohol and tobacco in women could be associated with the greater relative weight of oesophageal adenocarcinoma in Spain. In this respect, the geographic pattern observed for women could be partly associated with differences in the prevalence of obese women, since Extremaduran and Andalusian women register the greatest percentage of obesity in Spain [33]. Galicia and the Canary Islands also have a higher prevalence of obese women than the average level. In the United Kingdom, which has the highest rates of female oesophageal cancer in the EU, high body mass index in early adulthood and low consumption of fruit were associated with increased risk of oesophageal AC in this sex [39], whilst no relationship with alcohol and only a moderate association with smoking were found for SCC [40]. In Scotland, where the highest cumulative rates of oesophageal adenocarcinomas in the world have been reported [3], high rates are observed in both sexes. High intake of saturated fats and high rates of obesity and cigarette smoking have been proposed as factors that could be implicated in these findings [41].
Insofar as diet is concerned, there is consistent evidence to show that consumption of fruit and vegetables reduces the risk of oesophageal cancer (both SCC and AC) [42–47] while higher intake of dietary fat and nutrients found primarily in foods of animal origin and pickled vegetables have been associated with increased risk [44, 46, 48]. Some evidence of a protective effect of olive oil [45] has been observed, as well as a positive association with butter consumption [45, 49]. Furthermore, diet implies exposure to other carcinogens ingested with foods and drinking water, such as N-nitroso compounds [47, 50, 51] or diarrhetic shellfish-poisoning toxins [52]. In France, it has been suggested that differences in risk of oesophageal cancer might be due to exposure to okadaic acid, a tumour promoter that is the main toxin present in French coastal waters. Regular shellfish consumers could be exposed to okadaic acid, also detected in mussels in Galicia, a site of important industrial mussel production [53, 54]. It would be interesting to investigate if these marine biotoxins might be linked to the excess risk of oesophageal cancer that appears in this and other geographical areas in our country in subsequent studies.
Similarly connected with dietary habits, consumption of very hot beverages has also been associated with a raised risk of oesophageal cancer in various populations, though its role is not clear [55]. In Europe, the high incidence of squamous cell carcinoma of the oesophagus in the Department of Calvados, the highest risk area in France, was attributed to the local habit of consuming hot alcoholic beverages [49]. With regard to other hot beverages, a recent study has suggested that tea consumption might be the cause of the excess risk of SCC registered by women in the United Kingdom [40]. In South America, the drinking of mate, a tea-like infusion, has also been associated with an increased risk of oesophageal SCC [56–58]. In Spain, the role of differences in the consumption of hot beverages in different geographical areas has not been studied.
Social class has also been linked to the occurrence of oesophageal cancer, with a higher incidence being observed in groups having a low socio-economic level [34, 59]. In Western countries, low socio-economic level reflects greater exposure to tobacco, alcohol and a worse diet, as well as a greater likelihood of residing in more polluted areas or having little access to the health system. Brown et al [60] estimated that for SCC of the oesophagus the combination of low income, moderate/heavy alcohol consumption, tobacco use, and low daily consumption of raw fruit and vegetables accounted for almost all SCC of the oesophagus in whites (98%) and blacks (99%). They concluded that lifestyle modifications, especially a lower intake of alcoholic beverages, would markedly decrease the incidence of this cancer in both races. This "clustering" of risk factors could be linked to the excess risk detected in Cadiz -where the highest risk of death from cancer in Spain was already described over 15 years ago- ranking it first for the following cancers: lung, oral cavity and pharynx, oesophagus, larynx, and prostate for males; and oesophagus, other uterus, and bone for females [61]. More recently, Benach et al [62] reported that the areas with the highest increased risk of death in both sexes in Spain are clustered in the south-western region of the country, in the provinces of Huelva, Seville and Cadiz. These authors did not elucidate the causes of this clustering of increased mortality but suggested that environmental pollution, occupational exposures and social factors could be involved.
With regard to possible environmental factors which might, at least in part, be determining the pattern detected, the south-west of Andalusia is documented as being exposed to a number of pollutants, including heavy metals and radioactivity in water [63]. In addition, both Galicia and Extremadura are areas with high exposure to naturally produced radioactivity, by reason of the high granite content of their soils [64]. Yet, in Galicia excess risk appears in both sexes, whilst in Extremadura it appears solely in women.
Finally, different infectious agents have likewise been implicated in the aetiology of oesophageal cancer. Whereas infection with Helicobacter pylori, an established carcinogen for gastric cancer, has been associated with reduced risk of oesophageal AC [20], it has also been proposed as a risk factor for oesophageal SCC [65]. Concerning other infectious agents, some studies have suggested that oesophageal human papillomavirus (HPV) infection may be implicated in the development of oesophageal SCC, particularly in high risk areas, in association with known risk factors [66–68] though other studies have found no evidence of a positive association between HPV infection and either form of oesophageal cancer [69]. An association between herpes simplex and Epstein-Barr viruses and oesophageal carcinoma has also been reported in a high-incidence population in China [70]. Though the responsibility of infectious agents in the aetiology of this tumour is yet to be established, it would be an interesting issue to explore in high risk areas in Spain. At present, information of the prevalence of these agents is absent for most Spanish regions.
Conclusion
The excess risks observed in Galicia, Cadiz and the Canary Islands for both sexes, suggest that the related risk factors could be different to those implicated in the excesses detected in Asturias, Cantabria, the Basque Country, Navarre or the north of Castile & León, which only affect males, and those in Extremadura or the coastal areas of Malaga and Granada, where the excess risks only affect women. Among men, the important differences point, above all, to variations in alcohol and tobacco consumption, including the fact that differing tobacco and alcohol use patterns might be responsible for part of the differences. Among women, differences in the prevalence of obesity, in tandem with different smoking, alcohol use and dietary patterns, might explain, at least in part, the variations in risk linked to geographical area. The possible implication of environmental factors, e.g., drinking water, and infectious agents cannot be ignored. This type of study can serve: to detect areas in which greater stress should be laid on prevention and early detection; and to stimulate further epidemiological study of specific situations.
Abbreviations
- AC:
-
adenocarcinoma
- HPV:
-
human papillomavirus
- ICD-9:
-
international classification of diseases, 9 th revision
- RR:
-
relative risk
- SCC:
-
squamous cell carcinoma
- SMR:
-
standardised mortality ratios.
References
Parkin DM, Bray F, Ferlay J, Pisani P: Global cancer statistics, 2002. CA Cancer J Clin. 2005, 55: 74-108.
Ferlay J, Bray F, Pisani P, Parkin DM: GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. 2004, Lyon, IARCPress
Corley DA, Buffler PA: Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001, 30: 1415-1425. 10.1093/ije/30.6.1415.
Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB: IARC scientific publication. Cancer Incidence in Five Continents Vol. VIII. 2002, Lyon, International Agency for Research on Cancer
Sant M, Aareleid T, Berrino F, Bielska LM, Carli PM, Faivre J, Grosclaude P, Hedelin G, Matsuda T, Moller H, Moller T, Verdecchia A, Capocaccia R, Gatta G, Micheli A, Santaquilani M, Roazzi P, Lisi D: EUROCARE-3: survival of cancer patients diagnosed 1990-94--results and commentary. Ann Oncol. 2003, 14 Suppl 5: v61-118. 10.1093/annonc/mdg754.
Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, West AB, Blaser M, Blot WJ, Gail MH, Fraumeni JF: Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003, 95: 1404-1413.
IARC: Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004, 83: 1-1438.
Corrao G, Bagnardi V, Zambon A, La Vecchia C: A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004, 38: 613-619. 10.1016/j.ypmed.2003.11.027.
Brown LM, Swanson CA, Gridley G, Swanson GM, Schoenberg JB, Greenberg RS, Silverman DT, Pottern LM, Hayes RB, Schwartz AG: Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst. 1995, 87: 104-109. 10.1093/jnci/87.2.104.
Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, Stanford JL, Dubrow R, Schoenberg JB, Mayne ST, Farrow DC, Ahsan H, West AB, Rotterdam H, Niwa S, Fraumeni JF: Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998, 90: 150-155. 10.1093/jnci/90.2.150.
Lagergren J, Bergstrom R, Nyren O: Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999, 130: 883-890.
Lagergren J, Bergstrom R, Lindgren A, Nyren O: Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999, 340: 825-831. 10.1056/NEJM199903183401101.
Crew KD, Neugut AI: Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004, 31: 450-464. 10.1053/j.seminoncol.2004.04.021.
Devesa SS, Blot WJ, Fraumeni JF: Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998, 83: 2049-2053. 10.1002/(SICI)1097-0142(19981115)83:10<2049::AID-CNCR1>3.0.CO;2-2.
Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA: Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000, 29: 645-654. 10.1093/ije/29.4.645.
Bollschweiler E, Wolfgarten E, Gutschow C, Holscher AH: Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001, 92: 549-555. 10.1002/1097-0142(20010801)92:3<549::AID-CNCR1354>3.0.CO;2-L.
Pohl H, Welch HG: The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005, 97: 142-146.
Lindblad M, Ye W, Lindgren A, Lagergren J: Disparities in the classification of esophageal and cardia adenocarcinomas and their influence on reported incidence rates. Ann Surg. 2006, 243: 479-485. 10.1097/01.sla.0000205825.34452.43.
Vizcaino AP, Moreno V, Lambert R, Parkin DM: Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer. 2002, 99: 860-868. 10.1002/ijc.10427.
Brown LM, Devesa SS: Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002, 11: 235-256. 10.1016/S1055-3207(02)00002-9.
Hemminki K, Rawal R, Chen B, Bermejo JL: Genetic epidemiology of cancer: from families to heritable genes. Int J Cancer. 2004, 111: 944-950. 10.1002/ijc.20355.
Muñoz N, Day NE: Esophageal cancer. Cancer Epidemiology and Prevention. Edited by: Schottenfeld D and Fraumeni J. 1996, New York, Oxford University Press, 681-706.
López-Abente G, Pollán M, Escolar A, Errezola M, Abraira V: Atlas de mortalidad por cáncer y otras causas en España, 1978-1992. 2001, Madrid, Instituto de Salud Carlos III
Clayton D, Kaldor J: Empirical Bayes estimates of age-standardized relative risks for use in disease mapping. Biometrics. 1987, 43: 671-681. 10.2307/2532003.
Besag J, York J, Mollie A: Bayesian Image-Restoration, with Applications in Spatial Statistics. Annals of the Institute of Statistical Mathematics. 1991, 43: 1-20. 10.1007/BF00116466.
Gilks W, Richardson S, Spiegelhalter D: Markov Chain Montecarlo in practise. 1996, London, Chapman Hall
Best N, Richardson S, Thomson A: A comparison of Bayesian spatial models for disease mapping. Stat Methods Med Res. 2005, 14: 35-59. 10.1191/0962280205sm388oa.
Spiegelhalter D, Thomas A, Best N: WinBUGS user manual. Version 1.4.1. 2003, Cambridge, MRC
Smith BJ: Bayesian Output Analysis Program (BOA), Version 1.1.5 for R. 2006, [http://www.public-health.uiowa.edu/boa]
Elliott P, Wartenberg D: Spatial epidemiology: current approaches and future challenges. Environ Health Perspect. 2004, 112: 998-1006.
Richardson S, Thomson A, Best N, Elliott P: Interpreting posterior relative risk estimates in disease-mapping studies. Environ Health Perspect. 2004, 112: 1016-1025.
Perez-Gomez B, Aragonés N, Pollán M, Suarez B, Lope V, Llácer A, Lopez-Abente G: Accuracy of cancer death certificates in Spain: A summary of available information. Gacet Sanit. 2007,
Nyrén O, Adami HO: Esophageal Cancer. Textbook of Cancer Epidemiology. Edited by: Adami HO, Hunter D and Trichopoulos D. 2002, New York, Oxford University Press, 137-161.
Gammon MD, Schoenberg JB, Ahsan H, Risch HA, Vaughan TL, Chow WH, Rotterdam H, West AB, Dubrow R, Stanford JL, Mayne ST, Farrow DC, Niwa S, Blot WJ, Fraumeni JF: Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997, 89: 1277-1284. 10.1093/jnci/89.17.1277.
INE: Encuesta Nacional de Salud 2003. Instituto Nacional de Estadística. Ministerio de Sanidad, 2003. http://www.ine.es/inebase/menu3_soc.htm#3,
Lopez-Abente G, Aragones N, Ramis R, Hernandez-Barrera V, Perez-Gomez B, Escolar-Pujolar A, Pollan M: Municipal distribution of bladder cancer mortality in Spain: possible role of mining and industry. BMC Public Health. 2006, 6: 17-10.1186/1471-2458-6-17.
Gallus S, Altieri A, Bosetti C, Franceschi S, Levi F, Negri E, Dal Maso L, Conti E, Zambon P, La Vecchia C: Cigarette tar yield and risk of upper digestive tract cancers: case-control studies from Italy and Switzerland. Ann Oncol. 2003, 14: 209-213. 10.1093/annonc/mdg074.
Launoy G, Milan C, Faivre J, Pienkowski P, Gignoux M: Tobacco type and risk of squamous cell cancer of the oesophagus in males: a French multicentre case-control study. Int J Epidemiol. 2000, 29: 36-42. 10.1093/ije/29.1.36.
Cheng KK, Sharp L, McKinney PA, Logan RF, Chilvers CE, Cook-Mozaffari P, Ahmed A, Day NE: A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer. 2000, 83: 127-132. 10.1054/bjoc.2000.1121.
Sharp L, Chilvers CE, Cheng KK, McKinney PA, Logan RF, Cook-Mozaffari P, Ahmed A, Day NE: Risk factors for squamous cell carcinoma of the oesophagus in women: a case-control study. Br J Cancer. 2001, 85: 1667-1670. 10.1054/bjoc.2001.2147.
Eksteen JA, Latchford A, Thomas SJ, Jankowski JA: Commentary: Regional variations in oesophageal and gastric cardia cancers--implications and practice. Int J Epidemiol. 2001, 30: 1425-1427. 10.1093/ije/30.6.1425.
Launoy G, Milan C, Day NE, Pienkowski MP, Gignoux M, Faivre J: Diet and squamous-cell cancer of the oesophagus: a French multicentre case-control study. Int J Cancer. 1998, 76: 7-12. 10.1002/(SICI)1097-0215(19980330)76:1<7::AID-IJC2>3.0.CO;2-4.
Chen H, Ward MH, Graubard BI, Heineman EF, Markin RM, Potischman NA, Russell RM, Weisenburger DD, Tucker KL: Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002, 75: 137-144.
Brown LM, Swanson CA, Gridley G, Swanson GM, Silverman DT, Greenberg RS, Hayes RB, Schoenberg JB, Pottern LM, Schwartz AG, Liff JM, Hoover R, Fraumeni JF: Dietary factors and the risk of squamous cell esophageal cancer among black and white men in the United States. Cancer Causes Control. 1998, 9: 467-474. 10.1023/A:1008861806923.
Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, Trichopoulos D, Lagiou P, Bardini R, Franceschi S: Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000, 87: 289-294. 10.1002/1097-0215(20000715)87:2<289::AID-IJC22>3.0.CO;2-9.
Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H, West AB, Rotterdam H, Blot WJ, Fraumeni JF: Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001, 10: 1055-1062.
Yokokawa Y, Ohta S, Hou J, Zhang XL, Li SS, Ping YM, Nakajima T: Ecological study on the risks of esophageal cancer in Ci-Xian, China: the importance of nutritional status and the use of well water. Int J Cancer. 1999, 83: 620-624. 10.1002/(SICI)1097-0215(19991126)83:5<620::AID-IJC9>3.0.CO;2-W.
IARC: Pickled vegetables. IARC Monogr Eval Carcinog Risks Hum. 1993, 56: 83-113.
Launoy G, Milan C, Day NE, Faivre J, Pienkowski P, Gignoux M: Oesophageal cancer in France: potential importance of hot alcoholic drinks. Int J Cancer. 1997, 71: 917-923. 10.1002/(SICI)1097-0215(19970611)71:6<917::AID-IJC1>3.0.CO;2-0.
Terry PD, Lagergren J, Wolk A, Steineck G, Nyren O: Dietary intake of heterocyclic amines and cancers of the esophagus and gastric cardia. Cancer Epidemiol Biomarkers Prev. 2003, 12: 940-944.
Zhang XL, Bing Z, Xing Z, Chen ZF, Zhang JZ, Liang SY, Men FS, Zheng SL, Li XP, Bai XL: Research and control of well water pollution in high esophageal cancer areas. World J Gastroenterol. 2003, 9: 1187-1190.
Cordier S, Monfort C, Miossec L, Richardson S, Belin C: Ecological analysis of digestive cancer mortality related to contamination by diarrhetic shellfish poisoning toxins along the coasts of France. Environ Res. 2000, 84: 145-150. 10.1006/enrs.2000.4103.
Comesana Losada M, Leao JM, Gago-Martinez A, Rodriguez Vazquez JA, Quilliam MA: Further studies on the analysis of DSP toxin profiles in galician mussels. J Agric Food Chem. 1999, 47: 618-621. 10.1021/jf971043a.
Gago-Martinez A, Rodriguez-Vazquez JA, Thibault P, Quilliam MA: Simultaneous occurrence of diarrhetic and paralytic shellfish poisoning toxins in Spanish mussels in 1993. Nat Toxins. 1996, 4: 72-79.
Terry P, Lagergren J, Wolk A, Nyren O: Drinking hot beverages is not associated with risk of oesophageal cancers in a Western population. Br J Cancer. 2001, 84: 120-121. 10.1054/bjoc.2000.1561.
Castellsague X, Munoz N, De Stefani E, Victora CG, Castelletto R, Rolon PA: Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer. 2000, 88: 658-664. 10.1002/1097-0215(20001115)88:4<658::AID-IJC22>3.0.CO;2-T.
De Stefani E, Munoz N, Esteve J, Vasallo A, Victora CG, Teuchmann S: Mate drinking, alcohol, tobacco, diet, and esophageal cancer in Uruguay. Cancer Res. 1990, 50: 426-431.
Sewram V, De Stefani E, Brennan P, Boffetta P: Mate consumption and the risk of squamous cell esophageal cancer in uruguay. Cancer Epidemiol Biomarkers Prev. 2003, 12: 508-513.
Polednak AP: Geographic pattern of cancers related to tobacco and alcohol in Connecticut: implications for cancer control. Cancer Detect Prev. 2004, 28: 302-308. 10.1016/j.cdp.2004.03.005.
Brown LM, Hoover R, Silverman D, Baris D, Hayes R, Swanson GM, Schoenberg J, Greenberg R, Liff J, Schwartz A, Dosemeci M, Pottern L, Fraumeni JF: Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol. 2001, 153: 114-122. 10.1093/aje/153.2.114.
Errezola M, Lopez-Abente G, Escolar A: Geographical patterns of cancer mortality in Spain. Recent Results Cancer Res. 1989, 114: 154-162.
Benach J, Yasui Y, Martinez JM, Borrell C, Pasarin MI, Daponte A: The geography of the highest mortality areas in Spain: a striking cluster in the southwestern region of the country. Occup Environ Med. 2004, 61: 280-281. 10.1136/oem.2002.001933.
Alcaraz Pelegrina JM, Martinez-Aguirre A: Natural radioactivity in groundwaters around a fertilizer factory complex in South of Spain. Appl Radiat Isot. 2001, 55: 419-423. 10.1016/S0969-8043(00)00380-8.
Quindos Poncela LS, Fernandez PL, Gomez AJ, Sainz C, Fernandez JA, Suarez ME, Martin Matarranz JL, Cascon MC: Natural gamma radiation map (MARNA) and indoor radon levels in Spain. Environ Int. 2004, 29: 1091-1096. 10.1016/S0160-4120(03)00102-8.
Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, Nyren O: Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst. 2004, 96: 388-396.
Syrjanen KJ: HPV infections and oesophageal cancer. J Clin Pathol. 2002, 55: 721-728. 10.1136/jcp.55.10.721.
Benamouzig R, Pigot F, Quiroga G, Validire P, Chaussade S, Catalan F, Couturier D: Human papillomavirus infection in esophageal squamous-cell carcinoma in western countries. Int J Cancer. 1992, 50: 549-552.
Farhadi M, Tahmasebi Z, Merat S, Kamangar F, Nasrollahzadeh D, Malekzadeh R: Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World J Gastroenterol. 2005, 11: 1200-1203.
Lagergren J, Wang Z, Bergstrom R, Dillner J, Nyren O: Human papillomavirus infection and esophageal cancer: a nationwide seroepidemiologic case-control study in Sweden. J Natl Cancer Inst. 1999, 91: 156-162. 10.1093/jnci/91.2.156.
Wu MY, Wu XY, Zhuang CX: Detection of HSV and EBV in esophageal carcinomas from a high-incidence area in Shantou China. Dis Esophagus. 2005, 18: 46-50. 10.1111/j.1442-2050.2005.00423.x.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/7/3/prepub
Acknowledgements
This study was funded by Grant No. EPY-1176/02 from the Carlos III Institute of Health (ISCIII) and RCESP FIS-C03/09 (Spanish Network for Co-operative Research in Epidemiology and Public Health). The authors would like to thank Michael Benedict for his help with the English, Maria José Sánchez del Corral for her assistance with the bibliographic material, and Eva Ardanaz for her useful comments and help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
GLA, MP, NA, and BPG were all involved in designing the study. GLA and RR performed the statistical analysis. NA wrote the first draft of the manuscript to which all authors subsequently contributed. All authors made contribution to statistical analyses and interpretation of results, and revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Aragonés, N., Ramis, R., Pollán, M. et al. Oesophageal cancer mortality in Spain: a spatial analysis. BMC Cancer 7, 3 (2007). https://doi.org/10.1186/1471-2407-7-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-7-3