Abstract
Background
Breast cancer (BC) detection in women with a genetic susceptibility or strong family history is considered mandatory compared with BC screening in the general population. However, screening modalities depend on the level of risk. Here we present an update of our screening programs based on risk classification.
Methods
We defined different risk categories and surveillance strategies to identify early BC in 1325 healthy women recruited by the Modena Study Group for familial breast and ovarian cancer. Four BC risk categories included BRCA1/2 carriers, increased, intermediate, and slightly increased risk. Women who developed BC from January 1, 1994, through December 31, 2005 (N = 44) were compared with the number of expected cases matched for age and period. BRCA1/2 carriers were identified by mutational analysis. Other risk groups were defined by different levels of family history for breast or ovarian cancer (OC). The standardized incidence ratio (SIR) was used to evaluate the observed and expected ratio among groups. All statistical tests were two-sided.
Results
After a median follow-up of 55 months, there was a statistically significant difference between observed and expected incidence [SIR = 4.9; 95% confidence interval (CI) = 1.6 to 7.6; p < 0.001]. The incidence observed among BRCA carriers (SIR = 20.3; 95% CI = 3.1 to 83.9; P < 0.001), women at increased (SIR = 4.5; 95% CI = 1.5 to 8.3; P < 0.001) or intermediate risk (SIR = 7.0, 95% CI = 2.0 to 17.1; P = 0.0018) was higher than expected, while the difference between observed and expected among women at slightly increased risk was not statistically significant (SIR = 2.4, 95% CI = 0.9 to 8.3; P = .74).
Conclusion
The rate of cancers detected in women at high risk according to BRCA status or strong family history, as defined according to our operational criteria, was significantly higher than expected in an age-matched general population. However, we failed to identify a greater incidence of BC in the slightly increased risk group. These results support the effectiveness of the proposed program to identify and monitor individuals at high risk, whereas prospective trials are needed for women belonging to families with sporadic BC or OC.
Similar content being viewed by others
Background
Following the discovery the mutant BRCA1 and BRCA2 genes which predispose carriers for BC and OC [1, 2], many high-risk women request genetic testing and counselling on strategies to reduce their risk of death from BC. There are several options available for the management and care of women at risk for developing BC. Primary prevention can be achieved by prophylactic mastectomy and/or other risk-reducing strategies, e.g. chemoprevention with Tamoxifen, or oophorectomy. Prophylactic mastectomy is usually not proposed as the first choice for the management of women at high risk for developing BC, although it has been demonstrated to be efficient in reducing the incidence of BC either in women belonging to families with familial [3] or hereditary BC [4, 5]. Otherwise, secondary prevention through intensified surveillance to identify the earliest possible diagnosis of familial BC at a prognostically favourable stage is considered a feasible and acceptable strategy. If intensified surveillance is chosen, we have to keep in mind that there is difference between women with a positive genetic test who face a lifetime risk of 46–87% [6, 7] and women with a calculated lifetime risk of ≥ 18%, according to specific models, e.g. Claus [8, 9]or Gail [10]. Depending on BRCA status or family history, management of women at increased risk for developing breast cancer has to be considered as an individual surveillance program. The most widely recommended strategy for BRCA carriers entails frequent, intense surveillance that begins at age 25 to 35 years. How to screen these patients is also debatable. There is no consensus on the optimum modality and screening interval of women identified to be at moderate or high risk of breast cancer. [11, 12]. We know that the "lead time" will be short, in BRCA related BC, due to this forms rapid growth rate, and the screening performed at shorter intervals [13]. Furthermore, although mammography remains the gold standard tecnique, the radiation risk, due to the fact that BRCA genes are implicated in DNA repair of double stranded breaks typically caused by ionizing radiation [14, 15], and the diminished sensitivity in dense breasts, lead to evaluate the efficacy and safety of other imaging screening modalities for breast cancer, such as ultrasound and MRI. In particular, ultrasound has an established role in complementing diagnostic mammography in young patients with dense breasts [16–18]. Considering MRI, two retrospective [19, 20] and five prospective studies [21–25] have suggested that this imaging technique is useful in screening high risk women.
Several guidelines for hereditary breast cancer (HBC) and familial BC have been published. Particularly, for familial breast cancer, three groups of risk have been defined (high, moderate and low), according to the number of affected relatives, the age at onset and the cancers associated. All women belonging to moderate/high risk should be offered annual mammography, but MRI and ultrasound should not be used in routine surveillance [26, 27]. With the objective of improving the impact of different BC screening strategies in women in different risk categories, we developed operational criteria for the selection of family groups at risk of developing BC. Four categories were defined and four different BC screening strategies were established, in terms of age to begin screening, screening intervals, and imaging techniques. Here we describe the results of our surveillance and document the effectiveness of the proposed program in selecting individuals at risk.
Methods
Study population
Beginning in 1994, 1628 family histories were collected by the Modena Study Group for Familial Breast and Ovarian Cancer, in accordance with an oncologist-based model of cancer genetic counselling for HBC and hereditary OC (HOC) [28]. The family histories were obtained through detailed questionnaires and interviews. Family pedigrees were traced as far backward and laterally as possible, including a minimum of four generations and extended to paternal lines. The BC risk estimate was assessed according to the Gail model [10], Claus tables [8, 9] and a slightly modified BRCAPro model, adapted to the Italian population as suggested by Marroni et al. [29]. Furthermore, risk estimation was also determined according to the following criteria: 1) at least three relatives diagnosed with BC or OC in two different generations; 2) at least one of the three relatives must be a first-degree relative of one of the other two; in the case of male interposition, a relationship of different degree is allowed; 3) at least one BC must be diagnosed before the age of 40 years or be bilateral; 4) at least one BC diagnosed at age ≤ 35, regardless of family history; 5) at least one BC and one OC in the same woman, regardless of family history; 6) at least one male BC, regardless of family history; 7) one sporadic BC or OC. Applying these criteria, subjects were classified at high, intermediate, or slightly increased risk as described in Table 1. We defined the lifetime risk cut-off, calculated by the Gail model, at 30–50% in the high risk group, 18–29% in the intermediate risk group, and 6–18% in the slightly increased risk group. Finally, a group defined by a genetic predisposition due to the presence of the mutant BRCA genes was established to have a lifetime 50–85% risk of developing BC.
After disclosure of the BC risk to the consultants, they were instructed to convey suggestions concerning surveillance to relatives who were at an increased risk with respect to the general population.
To date, 1325 asymptomatic women accepted to be enrolled in our surveillance program, of which 1072 were first degree, and 253 were second degree relatives; 48 women belonged to carrier group (37 were BRCA1 mutation carriers, and 11 BRCA2 mutation carriers), 674 belonged to the high risk group, 257 to the intermediate risk group, and 346 to the slightly increased risk group (Table 2).
Additionally, 299 healthy women belonging to collected pedigrees did not adhere to our surveillance program for the following reasons: a) consultants failed to communicate the information to other relatives due to a poor relationship between family member; b) although they were informed of belonging to an at-risk family, they decided not to undergo surveillance due to low interest or because they lived too far from the clinic.
Genetic testing
Genetic testing to identify deleterious BRCA1 and BRCA2 mutations included direct automated sequencing on the entire coding sequence. From 1995 to 2005, 385 index cases affected by BC or OC, in the high risk categories, were tested for BRCA1/2 mutations of which, 82 carrier patients (21.3%) were identified. Of these 82 carriers, 79 asymptomatic relatives were found to carry a BRCA mutation.
Surveillance
An open prospective nonrandomized study was designed and approved by the Ethical Committee of Modena. Carriers of BRCA1 or BRCA2 mutations discovered through genetic testing or subjects at risk according to our previously described criteria, who were at least 18 years of age were eligible. Women with symptoms that were suggestive of BC or women who had a personal history of BC were excluded.
From January 1994 to September 2000, surveillance consisted of mammography (oblique and craniocaudal views and, if necessary, compression views and magnifications), ultrasonography and clinical breast examination (CBE) for BC prevention, and transvaginal ultrasound plus Ca.125 serum levels for early diagnosis of OC were proposed at different intervals based on the assessed risk. In October 2000, surveillance for carriers of BRCA1 or BRCA2 mutations was modified by introducing a dynamic breast MRI with gadolinium-containing contrast medium (Table 3). Whenever possible, the 4 independent exams for each screening event were planned on the same day during the second week of the menstrual cycle in premenopausal women. When indicated, additional investigation with fine-needle aspiration or core biopsy was performed.
Data collection and statistics
All data regarding family and individual characteristics, surveillance program, follow-up, additional investigations, and the final outcome of each examination from the years 1992 to 2005 were collected in a database. A person-year approach was used to evaluate the BC incidence. Person-years of risk were calculated from the baseline visit to the BC diagnosis (at surveillance or in the interval between two examinations) or for those without diagnosis, to the end of the study period (December 31, 2005). Detection rates were expressed as the number of events per 1,000 person-years of follow-up, and confidence intervals (CI) were calculated using the exact method [30]. Expected cancer incidence for consultants and for all first-degree and second-degree relatives in the lineage at risk older than 18 years was based on age-specific Modena Cancer Registry (MCR) rates from 1998 through 2002 in 5-year age groups, beginning at age 25 years and ending with age 85 years or older [31]. The observed women-years at risk were then multiplied by expected cancer incidence obtained from the MCR database to estimate the total number of cancers expected. Standardized incidence ratios (SIRs) were determined by calculating the ratio of observed to expected numbers of cancers. An "interval cancer" was defined as any cancer presenting between two regular screening rounds. Interval cancer rate was defined as the number of women with a diagnosis of an interval cancer per 1,000 person-years at risk. Sensitivity of the screening test was calculated as the ratio of BCs detected by surveillance divided by the total number of BC (screen-detected plus interval cancers). The chi-square test was used to calculate P values. All statistical tests were two-sided.
Results
Clinical characteristics
After a median follow-up of 55 months (range 1 to 151 months), a total of 44 breast tumors including 28 infiltrating (64%) and 16 ductal carcinomas in situ (DCIS) (36%) were found in women belonging to different risk categories. Five cancers occurred in BRCA carriers (4 infiltrating and 1 DCIS), 23 in the high risk group (14 infiltrating, 9 DCIS), 11 in the intermediate risk group (8 infiltrating and 3 DCIS), and 5 in the slightly increased risk group (2 infiltrating and 3 DCIS). Among the 28 invasive tumors, 21 were ductal, 6 lobular, and one was a tubular carcinoma. All patients were surgically treated. Of the 44 patients, 30 (68%) received conservative surgery, 14 (32%) had a mastectomy of which 6 were bilateral. At the time of diagnosis, 17 patients were diagnosed with stage I (63%), 7 with stage II, 2 with stage III, and 2 with stage IV. Eight (29%) tumors were less then 10 mm in diameter, 10 (36%) were between 10 and 15 mm, 9 (32%) were greater than 15 mm (range 16 to 50 mm) and one was an inflammatory BC. Ten (36%) were node-positive. Five patients were treated with hormonal therapy, 8 patients received chemotherapy, 11 patients were treated with chemotherapy and hormonal therapy, and 7 received surgery only. In 8 cases, chemotherapy was anthracycline-based. After a median follow-up of 55 months, 4 recurrences and 3 deaths were observed, 2 for disease progression and one due to heart failure. The actuarial 5 year survival rate was 93% (Fig. 1).
Efficacy of screening
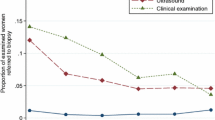
With a total number of follow-up years of 6,066, the BC detection rate (invasive and in situ BC) was 7.3 per 1000 person-years and 4.6 per 1000 person-years excluding DCIS. Of the 44 patients who developed BC, 11 (25%) were palpable and 33 (75%) were nonpalpable tumors. Thirty-six of the 44 tumors were detected at screening (5 during the first round and 31 at a subsequent round) making the rate of screen-detected cancers 5.9 per 1000. Eight cancers, all identified by CBE, were diagnosed in the interval between screening events (interval cancer rate 1.3 per 1000). The diagnosis was made only by CBE in 4 cases, (one inflammatory carcinoma, 2 nipple bleeding, and one axillary metastasis), by CBE plus ultrasonography in 3 cases, and by CBE plus ultrasound plus mammography in one case. The time interval from the last negative screen until diagnosis ranged from 1 to 14 months. The characteristics of patients with interval cancers are shown in Table 4.
Among the 36 screen-detected BC, 3 were palpable and 33 were nonpalpable cancers. Twenty-eight were diagnosed with mammography (78%), 18 with ultrasound (50%), 35 with mammography plus ultrasound (97%), and 4 by MRI (100%) (Table 5). An MRI was performed only in BRCA carriers and one BC was detected only by this imaging technique.
Eight DCIS were detected in women aged less than 50 years and 8 in women older than or equal to 50 years. The screening sensitivity increased with age with a low rate in the age group <50 (65%) and a very high rate (93%) in the oldest age group, with an overall sensitivity of 82%.
The detection rates of BC were 31.6 per 1000 person-years in BRCA1/2 gene mutation carriers, 6.9 per 1000 in the high risk-group, 9.9 per 1000 person-years in the intermediate risk group, and 3.5 per 1000 in the slightly increased risk-group. The incidence of BC in the entire study population was significantly higher than expected (SIR = 4.9; 95% confidence interval (CI) = 1.6 to 7.6; p < 0.001). The incidence was significantly higher among BRCA1 and BRCA2 carriers (SIR = 20.3; 95% CI = 3.1 to 83.9; P < 0.001) and amongst subjects classified at high (SIR = 4.5; 95% CI = 1.5 to 8.3; P < 0.001) or intermediate risk (SIR = 7.0, 95% CI = 2.0 to 17.1; P = 0.0018). However the incidence of BC was not higher than expected in the group classified at slightly increased risk (SIR = 2.4, 95% CI = 0.9 to 8.3; P = 0.74) (Table 6). Finally the SIR of BC was 14.4 (95% CI 6.7–26.5, P < 0.001) in the group of women aged less than 50 years and 3.3 (95% CI 1.9–5.5 P < 0.001) among women aged 50 years or older.
Discussion
Our clinical and radiological imaging surveillance program led to the detection of 44 breast cancers, including 28 infiltrating and 16 in situ. The SIR for BC was very high overall for the women in the study (4.9, P < 0.001) and BRCA carriers (20.3, P < 0.001) compared to that expected in the general age-matched population. In the high and intermediate risk groups the SIR reached statistical significance (4.5, P < 0.001 and 7.0, P = 0.0018, respectively) confirming the effectiveness of our current approach in the identification of women at increased risk. On the other hand, a low SIR (2.4, P = 0.76) was detected in the slightly increased risk group. Interestingly, a much higher proportion of DCIS (36%) was detected in our screening compared with patients from an age-matched population not considered at increased risk (9%). Furthermore, the performance of our screening compares favourably with the recommendations of the European Commission for quality assurance in mammography screening [32] which indicates a good detection rate in the first round where it was more than 3.5 fold the incidence rate before screening (2.6‰) and more than 1.5 fold in the subsequent rounds. The combination of mammographic and ultrasound screening in women with a family history was further investigated. As in other studies, where ultrasound was useful for bridging the relatively long time interval between the annual surveillance rounds [33, 34], adding ultrasound to mammography improved the sensitivity of screening from 78% (28/36) to 97% (35/36). In all age groups and risk categories, ultrasound showed a very high sensitivity in addition to mammography with the exception of the slightly increased risk group where no cancer was detected by this modality. As expected, the major advantage of ultrasound was seen in women aged less than 50 years where the sensitivity was up to 100% (from 7 to 11 of 11 cancers). Finally, although performed in a limited number of cases, breast MRI screening showed high sensitivity in women with a genetic predisposition for BC. In fact, an interval cancer in a BRCA carrier patient, had already appeared on an MRI, but was considered an intramammary lymph node. A retrospective evaluation of the preceding mammograms was performed for all interval cancers, with the exception of one 32 year old woman (Table 4) in the slightly increased risk group who had never had a mammography before diagnosis. An interval cancer in a 51 year old woman at high risk was considered a missed cancer because the previous mammogram identified a mammary sprain at the left upper outer quadrant, although a fine needle aspiration was negative for atypical cells (Table 4). Also a DCIS in a 45 year old woman at intermediate risk must be considered a missed cancer, since the patient had a nipple discharge that was negative for atypical cells. Excluding these cases, the real detection rate of interval cancer was 0.8 per 1,000. All the invasive interval cancers, except for 1 DCIS, had a tumor grading of 3. Nevertheless, the 5 years disease-free survival and overall survival for interval cancers was 100%, suggesting the high compliance of women followed at our institution. The overall percentage of tumors with positive lymph nodes was 36% (10 of 28) with no difference in age or risk group. The mean number of lymph nodes removed was 24. Other studies have reported a lower 10 – 35% node positivity. The higher rate in our study may be due to different patient population characteristics, such as age or screening schemes and modalities, or to our more stringent node sampling.
The Saetersdal study [35] reported a detection rate of 15 per 1,000 on 537 women at risk for BC, who were selected on the basis of autosomal dominant inheritance with DCIS accounting for 11% of all cancers. Kollias [36] performed a screening on 1371 women less than 50 years old with a family history of BC; 23 invasive cancers were detected during a mean follow-up of 22 months. The incidence for invasive breast cancer was 7.9 per 1000 women-years, with a SIR of 5 when compared with an age-matched female population in the U.K. Six carcinomas in situ (21%) were detected, suggesting that young women at risk of BC due to family history may benefit from regular breast screening for the early detection of in situ lesions. Lalloo [37] selected 1259 women under the age of 50 with a positive family history and a lifetime risk of BC of 1 in 6 or greater. In this population, 12 cancers were detected giving a SIR of 1.42, 95% CI 0.73–2.48. The percent of node-positive tumors (45%) was very high. Chart [38] identified 24 tumors (invasive and in situ) in 1044 women distributed in three categories (high, moderate, and slightly increased risk). All screen-detected tumors were in situ or stage I, suggesting that surveillance of women at increased risk for breast cancer may be useful in detecting disease at an early stage. Finally, Brekelmans [39], who has enrolled 1198 women characterized by BRCA1/2 mutations or by a BC risk over 15% between 21–70 years of age in a screening program, found 35 cancers (31 invasive and 4 DCIS) after a median follow-up of 3 years. The SIR for invasive cancers was 7. Furthermore he had a 74% screening sensitivity.
Both the Kollias and Lalloo studies analyzed young women aged less than 50 years. In our study, 897 women were under 50 years of age and 306 were older. The SIR of BC in women aged <50 years was higher than that observed in the U.K. screening program (14.4 vs.5), while this ratio decreased in women aged more than 50 years.
The significance of detecting DCIS in mass screening programs is unknown. It is estimated that the risk of invasive cancer following untreated DCIS in the general population is 30–50% and this usually occurs within 10 years. In the context of a family history, several investigators believe detection of these non-invasive lesions may become more important. The meaning of lobular carcinoma in situ (LCIS) is debatable. This lesion was recently considered as a high risk premalignant lesion such as atypical ductal hyperplasia, papillomatosis, and so on [40].
In conclusion the detection rate and the observed versus expected ratio found in our surveillance program were in accordance with the risk group, with a statistically significant value for BRCA carriers, and for the high and intermediate risk groups. Also, the screening sensitivity was improved in the above groups, but fell in the slightly increased risk group. In this latter group, no significant differences in detection rates and SIR were found with respect to the expected number of BCs based on age-specific Modena Cancer Registry (MCR) rates from 1998 through 2002. As already reported by other authors [41], early breast cancer screening does not seem to be cost-effective in women belonging to a slightly increased risk group. Newer imaging technologies, such as MRI, may offer a better technique for the early diagnosis of breast cancer, especially in BRCA1/2 gene carriers. Furthermore, with the objective of reducing the number of interval cancers, randomized trials should be designed utilizing MRI in the high and intermediate risk groups, as already proposed by other authors [23, 33].
Finally, our operational criteria seemed effective in identifying people at increased risk of developing breast cancer, and are currently being evaluated in a larger group of individuals from families followed at Institutions of the Italian Network on Hereditary Breast and Ovarian Cancer.
Conclusion
In conclusion, our data show that the proposed screening program was able to select individuals at risk, in agreement with the Italian Network on Hereditary Breast and Ovarian Cancer. This paper provides evidence based proof that an appropriate surveillance program can identify a relevant number of breast cancers at an early stage in a population at risk.
References
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, et al: A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994, 266: 66-71. 10.1126/science.7545954.
Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G: Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995, 378: 789-792. 10.1038/378789a0.
Hartmann LC, Sellers TA, Schaid DJ, Nayfield S, Grant CS, Bjoraker JA, Woods J, Couch F: Clinical options for women at high risk for breast cancer. Surg Clin North Am. 1999, 79: 1189-1206. 10.1016/S0039-6109(05)70068-2.
Meijers-Heijboer EJ, Verhoog LC, Brekelmans CT, Seynaeve C, Tilanus-Linthorst MM, Wagner A, Dukel L, Devilee P, van den Ouweland AM, van Geel AN, Klijn JG: Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet. 2000, 355: 2015-2020. 10.1016/S0140-6736(00)02347-3.
Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van 't Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E, Daly MB, Olopade OI, Weber BL: Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004, 22: 1055-1062. 10.1200/JCO.2004.04.188.
Eeles RA, Powles TJ: Chemoprevention options for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2000, 18: 93S-9S.
Chen S, Iversen ES, Friebel T, Finkelstein D, Weber BL, Eisen A, Peterson LE, Schildkraut JM, Isaacs C, Peshkin BN, Corio C, Leondaridis L, Tomlinson G, Dutson D, Kerber R, Amos CI, Strong LC, Berry DA, Euhus DM, Parmigiani G: Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006, 24: 863-871. 10.1200/JCO.2005.03.6772.
Claus EB, Risch N, Thompson WD: The calculation of breast cancer risk for women with a first degree family history of ovarian cancer. Breast Cancer Res Treat. 1993, 28: 115-120. 10.1007/BF00666424.
Claus EB, Risch N, Thompson WD: Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994, 73: 643-651. 10.1002/1097-0142(19940201)73:3<643::AID-CNCR2820730323>3.0.CO;2-5.
Gail MH, Benichou J: Validation studies on a model for breast cancer risk. J Natl Cancer Inst. 1994, 86: 573-575.
Wainberg S, Husted J: Utilization of screening and preventive surgery among unaffected carriers of a BRCA1 or BRCA2 gene mutation. Cancer Epidemiol Biomarkers Prev. 2004, 13: 1989-1995.
Kuhl CK, Kuhn W, Schild H: Management of women at high risk for breast cancer: new imaging beyond mammography. Breast. 2005, 14: 480-486. 10.1016/j.breast.2005.08.005.
Tilanus-Linthorst MM, Kriege M, Boetes C, Hop WC, Obdeijn IM, Oosterwijk JC, Peterse HL, Zonderland HM, Meijer S, Eggermont AM, de Koning HJ, Klijn JG, Brekelmans CT: Hereditary breast cancer growth rates and its impact on screening policy. Eur J Cancer. 2005, 41: 1610-1617. 10.1016/j.ejca.2005.02.034.
Venkitaraman AR: Functions of BRCA1 and BRCA2 in the biological response to DNA damage. J Cell Sci. 2001, 114: 3591-3598.
Yoshida K, Miki Y: Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004, 95: 866-871. 10.1111/j.1349-7006.2004.tb02195.x.
Kolb TM, Lichy J, Newhouse JH: Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002, 225: 165-175.
Crystal P, Strano SD, Shcharynski S, Koretz MJ: Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003, 181: 177-182.
Foxcroft LM, Evans EB, Porter AJ: The diagnosis of breast cancer in women younger than 40. Breast. 2004, 13: 297-306. 10.1016/j.breast.2004.02.012.
Stoutjesdijk MJ, Boetes C, Jager GJ, Beex L, Bult P, Hendriks JH, Laheij RJ, Massuger L, van Die LE, Wobbes T, Barentsz JO: Magnetic resonance imaging and mammography in women with a hereditary risk of breast cancer. J Natl Cancer Inst. 2001, 93: 1095-1102. 10.1093/jnci/93.14.1095.
Morris EA, Liberman L, Ballon DJ, Robson M, Abramson AF, Heerdt A, Dershaw DD: MRI of occult breast carcinoma in a high-risk population. AJR Am J Roentgenol. 2003, 181: 619-626.
Tilanus-Linthorst MM, Obdeijn IM, Bartels KC, de Koning HJ, Oudkerk M: First experiences in screening women at high risk for breast cancer with MR imaging. Breast Cancer Res Treat. 2000, 63: 53-60. 10.1023/A:1006480106487.
Kuhl CK, Schmutzler RK, Leutner CC, Kempe A, Wardelmann E, Hocke A, Maringa M, Pfeifer U, Krebs D, Schild HH: Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: preliminary results. Radiology. 2000, 215: 267-279.
Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, Manoliu RA, Kok T, Peterse H, Tilanus-Linthorst MM, Muller SH, Meijer S, Oosterwijk JC, Beex LV, Tollenaar RA, de Koning HJ, Rutgers EJ, Klijn JG: Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004, 351: 427-437. 10.1056/NEJMoa031759.
Warner E, Plewes DB, Hill KA, Causer PA, Zubovits JT, Jong RA, Cutrara MR, DeBoer G, Yaffe MJ, Messner SJ, Meschino WS, Piron CA, Narod SA: Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. Jama. 2004, 292: 1317-1325. 10.1001/jama.292.11.1317.
Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, Gilbert FJ, Griebsch I, Hoff RJ, Kessar P, Lakhani SR, Moss SM, Nerurkar A, Padhani AR, Pointon LJ, Thompson D, Warren RM: Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005, 365: 1769-1778. 10.1016/S0140-6736(05)66646-9.
Eccles DM, Evans DG, Mackay J: Guidelines for a genetic risk based approach to advising women with a family history of breast cancer. UK Cancer Family Study Group (UKCFSG). J Med Genet. 2000, 37: 203-209. 10.1136/jmg.37.3.203.
NICE: Guidelines for familial breast cancer. [http://www.nice.org.uk]
Contegiacomo A, Pensabene M, Capuano I, Tauchmanova L, Federico M, Turchetti D, Cortesi L, Marchetti P, Ricevuto E, Cianci G, Venuta S, Barbieri V, Silingardi V: An oncologist-based model of cancer genetic counselling for hereditary breast and ovarian cancer. Ann Oncol. 2004, 15: 726-732. 10.1093/annonc/mdh183.
Marroni F, Aretini P, D'Andrea E, Caligo MA, Cortesi L, Viel A, Ricevuto E, Montagna M, Cipollini G, Federico M, Santarosa M, Marchetti P, Bailey-Wilson JE, Bevilacqua G, Parmigiani G, Presciuttini S: Penetrances of breast and ovarian cancer in a large series of families tested for BRCA1/2 mutations. Eur J Hum Genet. 2004, 12: 899-906. 10.1038/sj.ejhg.5201256.
M.Woodward: Epidemiology study design and data analysis. 1999, Boca Raton, Chapman & Hall/CRC, 557-567.
AIRT: I tumori in Italia-Rapporto 2006. Epidemiologia & Prevenzione. Edited by: Inferenze . 2006, Milano, , 62-63.
M.Rosselli del Turco JHNP: Radiological guidelines for quality assurance in mammography screening. European guidelines for quality assurance in mammography screening. Edited by: N.Perry MBCWST. 2001, Bruxelles, European Commission office for ufficial pubblications, 143-158.
Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, Kuhn W, Schild HH: Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005, 23: 8469-8476. 10.1200/JCO.2004.00.4960.
Sim LS, Hendriks JH, Fook-Chong SM: Breast ultrasound in women with familial risk of breast cancer. Ann Acad Med Singapore. 2004, 33: 600-606.
Saetersdal A, Dorum A, Heimdal K, Helgerud P, Sager EM, Bohler P, Tretli S, Kvinnsland S, Moller P: Inherited predisposition to breast carcinoma. Results of first round examination of 537 women at risk. Anticancer Res. 1996, 16: 1989-1992.
Kollias J, Sibbering DM, Blamey RW, Holland PA, Obuszko Z, Wilson AR, Evans AJ, Ellis IO, Elston CW: Screening women aged less than 50 years with a family history of breast cancer. Eur J Cancer. 1998, 34: 878-883. 10.1016/S0959-8049(97)00365-1.
Lalloo F, Boggis CR, Evans DG, Shenton A, Threlfall AG, Howell A: Screening by mammography, women with a family history of breast cancer. Eur J Cancer. 1998, 34: 937-940. 10.1016/S0959-8049(98)00005-7.
Chart PL, Franssen E: Management of women at increased risk for breast cancer: preliminary results from a new program. Cmaj. 1997, 157: 1235-1242.
Brekelmans CT, Seynaeve C, Bartels CC, Tilanus-Linthorst MM, Meijers-Heijboer EJ, Crepin CM, van Geel AA, Menke M, Verhoog LC, van den Ouweland A, Obdeijn IM, Klijn JG: Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol. 2001, 19: 924-930.
Arpino G, Laucirica R, Elledge RM: Premalignant and in situ breast disease: biology and clinical implications. Ann Intern Med. 2005, 143: 446-457.
Jacobi CE, Nagelkerke NJ, van Houwelingen JH, de Bock GH: Breast cancer screening, outside the population-screening program, of women from breast cancer families without proven BRCA1/BRCA2 mutations: a simulation study. Cancer Epidemiol Biomarkers Prev. 2006, 15: 429-436. 10.1158/1055-9965.EPI-05-0223.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/6/210/prepub
Acknowledgements
This work was supported by AIRC (Associazione Italiana per la Ricerca sul Cancro) (Italian Consortium For Hereditary Breast And Ovarian Cancer), by COFIN-MURST 2003–2005, by Fondazione Cassa di Risparmio di Modena and by "Associazione Angela Serra per la ricerca sul Cancro".
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
LC participated in the design of the study and drafted the manuscript.
DT participated in the design of the study.
IM acquired the data of the center
AF acquired the data of the Modena Cancer Center and performed the statistical analysis.
BC performed the surveillance screening.
RB performed the surveillance screening
SR carried on the genetic counselling
AP participated the MRI study.
PT coordinated the radiological screening
MF designed the study and revised the final manuscript.
All authors read and approved the final manuscript
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cortesi, L., Turchetti, D., Marchi, I. et al. Breast cancer screening in women at increased risk according to different family histories: an update of the Modena Study Group experience. BMC Cancer 6, 210 (2006). https://doi.org/10.1186/1471-2407-6-210
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-6-210