Abstract
Background
Recent studies relating to the association between DNA repair-gene polymorphisms and colorectal cancer risk would, to the best of our knowledge, appear to be very limited. This study was designed to examine the polymorphisms associated with three DNA repair genes, namely: XRCC1 Arg399Gln, XRCC3 Thr241Met and XPD Lys751Gln, and investigate their role as susceptibility markers for colorectal cancer.
Methods
We conducted a case-control study including 727 cases of cancer and 736 hospital-based age- and sex-matched healthy controls to examine the role of genetic polymorphisms of three DNA-repair genes (XRCC1, XRCC3 and XPD) in the context of colorectal cancer risk for the Taiwanese population. Genomic DNA isolated from 10 ml whole blood was used to genotype XRCC1 Arg399Gln, XRCC3 Thr241Met and XPD Lys751Gln by means of polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis.
Results
The risk for colorectal cancer did not appear to differ significantly amongst individuals featuring the XRCC1 399Arg/Arg genotype (OR = 1.18; 95% CI, 0.96–1.45), the XRCC3 241Thr/Thr genotype (OR = 1.25; 95% CI, 0.88–1.79) or the XPD 751Gln allele (OR = 1.20; 95% CI, 0.90–1.61), although individuals featuring a greater number of risk genotypes (genotype with OR greater than 1) did experience a higher risk for colorectal cancer when compared to those who didn't feature any risk genotypes (Trend test P = 0.03). Compared with those individuals who didn't express any putative risk genotypes, individuals featuring all of the putative risk genotypes did experience a significantly greater cancer risk (OR = 2.43, 95% CI = 1.21–4.90), particularly for individuals suffering tumors located in the rectum (OR = 3.18, 95% CI = 1.29–7.82) and diagnosed prior to the age of 60 years (OR = 4.90, 95% CI = 1.72–14.0).
Conclusions
Our results suggest that DNA-repair pathways may simultaneously modulate the risk of colorectal cancer for the Taiwanese population, and, particularly for rectal cancer and younger patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Humans are routinely exposed to mutagenic and carcinogenic aromatic amines via smoking, well-cooked food and other sources [1]. These chemicals can form DNA adducts in vivo and thus lead to DNA damage [2]. The integrity of most of the so-damaged DNAs is typically restored as a consequence of the action of certain DNA-repairing enzymes, the normal function of which is important for maintaining genomic integrity and preventing cellular neoplastic transformation [3]. Since the genetic polymorphisms of DNA-repair enzymes might be able to influence DNA adduct levels [4–6], the particular degree of DNA-repair capacity has often been associated with the risk of human cancers [7–11].
Amongst the known genetic polymorphisms of the DNA-repair genes [12], the xeroderma pigmentosum group D (XPD, also known as ERCC2) and x-ray repair cross-complementing groups 1 and 3 (XRCC1 and XRCC3) have been studied most commonly [13]. The XPD gene encodes a helicase that is a component of the transcription factor TFIIH [14], this factor being an essential member of the nucleotide-excision repair (NER) pathway that is responsible for effecting repairs to bulky adducts and UV-induced DNA damage [15]. In 2002, Qiao et al. [16] reported that individuals featuring XPD 751Gln/Gln did demonstrate suboptimal DNA-repair capacity (DRC) in regard to its ability to remove UV photoproducts when compared to the XPD 751Lys/Lys and Lys/Gln genotypes. The XRCC1 protein is a scaffolding protein directly associated with polymerase beta, DNA ligase III and poly (ADP-ribose) polymerase (PARP) and functions in a complex to facilitate the base-excision repair (BER) and single-strand break-repair processes [17–19]. In 1999, Lunn et al. noted that individuals harboring the XRCC1 399Gln allele were associated, more significantly, with higher levels of both aflatoxin B1-DNA adducts and glycophorin A variants when compared to individuals who exhibited the Arg/Arg genotype [4]. XRCC3 participates in DNA double-strand break repair and is a member of an emerging family of Rad-51-related proteins that likely participate in homologous recombinational repair (HRR) in order to maintain chromosome stability [20].
To the best of our knowledge, studies pertaining to these DNA-repair genes focusing on colorectal cancer risk would appear to be limited and controversial. In 2000, Abdel-Rahman et al. [21] observed that the XRCC1 399Gln allele, compared to the XRCC1 399Arg/Arg genotype, was associated with an increased risk for developing colorectal cancer, especially amongst young urban residents, although in 2003, Mort et al. [22] failed to reveal any significant associations between colorectal cancer risk and any polymorphisms of four of the NER genes (XPD, XPF, XPG, ERCC1) or XRCC1. In the present paper, we conducted a hospital-based case-controlled study to examine the role of genetic polymorphisms of three DNA-repair genes (XRCC1, XRCC3 and XPD) in the context of colorectal cancer risk for the Taiwanese population.
Methods
Subjects
Detailed descriptions of the specific characteristics of the study participants have been published previously [23]. In brief, participants were recruited from the Chang Gung Memorial Hospital between January 1995 and January 1999 inclusively. The colorectal adenocarcinoma cancer cases (n = 776) participating in this study were newly diagnosed and histologically confirmed. Patients suffering from familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, or inflammatory bowel disease and other related malignancies were excluded from study participation. Seven hundred and twenty-seven of the original 776 cases (94%) were finally included in this study. Seven hundred and forty-seven age (same age) and sex-matched controls were recruited from the Physical Check-Up Department during the same period. All the participating controls had received comprehensive health examinations including colonoscopies. After excluding individuals diagnosed with other colorectal diseases, a history of other cancers or the existence of a family history of colorectal cancer, 736 controls (98%, 736/747) were finally included in this study. With informed consent, the socio-demographic characteristics of study participants were ascertained by means of the application of a structured questionnaire, at which time 10 ml of venous blood was collected.
Genotyping
Genomic DNA isolated from 10 ml whole blood was used to genotype XRCC1 Arg399Gln, XRCC3 Thr241Met and XPD Lys751Gln by means of polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis. All of the PCR reactions were carried out by a Mastercycler gradient thermocycler (Eppendorf, Hamburg, Germany) in a final volume of 25 ul containing 200 ng of each primer, 50 ng genomic DNA, 1.5 mM MgCl2, 200 ul dNTPs and 1.0 unit of Taq DNA Polymerase in the buffer provided by the manufacturer. In addition, all laboratory genotyping personnel were blind to the case-control status of the samples.
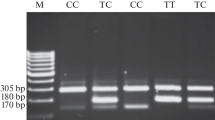
The 615 bp XRCC1 PCR products were amplified with the primers 5'-TTGTGCTTTCTCTGTGTCCA-3' (sense) and 5'-TCCTCCAGCCTTTTCTGATA-3' (antisense) and digested with MspI (New England BioLabs, Beverly, MA, USA). The Arg allele revealed 374 and 221 bp fragments following digestion and polyacrylamide gel electrophoresis, whilst the Gln allele was not digested by MspI [4]. The 136 bp XRCC3 PCR products were amplified with the primers 5'-GCCTGGTGGTCATCGACTC-3' (sense) and 5'-ACAGGGCTCTGGAAGGCACTGCTCAGCTCACGCACC-3' (antisense) and digested with NcoI (New England BioLabs). The Met allele revealed 97 and 39 bp fragments following digestion and polyacrylamide gel electrophoresis, while the Thr allele was not digested by NcoI [24]. The 734 bp XPD PCR products were amplified with the primers 5'-CCTCTCCCTTTCCTCTGTTC-3' (sense) and 5'-CAGGTGAGGGGGACATCT-3' (antisense) and digested with PstI (Takara, Japan). The Gln allele revealed 646 and 88 bp fragments following digestion and polyacrylamide gel electrophoresis, whilst the Lys allele was not able to be digested by PstI [25].
Statistical analysis
Tests for Hardy-Weinberg equilibrium amongst controls were conducted using observed genotype frequencies and a chi-square test featuring one degree of freedom. Baseline sociodemographic characteristics between cases and controls were analyzed using the chi-square test and two-sample Students' t-test. Multivariate unconditional logistic regressions were used to examine the association between the XRCC1, XRCC3 and XPD genotypes and the risk for colorectal cancer. Since the differences in the estimated risks between conditional logistical regression and unconditional logistical regression were small, unconditional logistical regression was used to estimate odds ratio (OR) and 95% confidence interval (CI) with the matching factors (age and gender) included in the model for estimation [26]. Other potential confounders (such as physical activity, cigarette smoking, alcohol use, coffee intake, and consumption of staple, meat, vegetable/fruit and fish/shrimp) would be also included if they have >10% effect on the gene main effects. However, none of these factors have met the inclusion criteria.
Based on the multiplicative scale, the likelihood ratio test was further used to evaluate the interaction between XRCC1, XRCC3 and XPD genes on the risk for the colorectal cancer. Since the associations between polymorphisms of DNA-repair gene, cancer susceptibility and DNA repair capacity are inconsistent [13], we defined the risk allele as the allele with OR>1 observed in the present study. Stratified analyses were also conducted to evaluate the differences between specific tumor sites (colon and rectum) and age groups (< = 60 years old and > 60 years old). All analyses were performed using the SAS statistical package (version 8.1 for windows; SAS Institute, Inc., Cary, NC, USA) and all tests were two-sided.
Results
More men (56%) than women participated in this study (Table 1). The mean age for both groups was 60 years. From the cancer cases, 352 patients suffered from colon cancer (48%) and 375 patients from rectal cancer (52%), with most such cases being deemed to be at stage II or stage III (both were 33%). Detailed analyses of the sociodemographic characteristics and potential risk factors associated with colorectal cancer amongst the study population have been published previously [23].
The genotypic distributions of the three DNA-repair genes for both cancer cases and controls are shown in Table 2. The frequencies for the XRCC1 399Gln, XRCC3 241Met and XPD 751Gln allele amongst the controls were, respectively, 0.27, 0.05 and 0.07, these genotype frequencies being in Hardy-Weinberg equilibrium. Due to the relatively low frequencies of variant alleles for these genes in the present study, any genotype featuring one or more variant alleles was combined for further analyses. The risk for colorectal cancer was not significantly different for individuals featuring the XRCC1 399Arg/Arg genotype (OR = 1.18; 95% CI, 0.96–1.45), the XRCC3 241Thr/Thr genotype (OR = 1.25; 95% CI, 0.88–1.79) or the XPD 751Gln allele (OR = 1.20; 95% CI, 0.90–1.61).
As revealed in Table 3, those individuals exhibiting a greater number of risk genotypes (genotype with OR greater than 1) faced a greater risk for colorectal cancer when compared to those individuals who did not display any risk genotypes (Trend test, P = 0.03). Subjects who demonstrated two or three of the putative risk genotypes did reveal a significantly greater risk for colorectal cancer (OR = 1.95; 95% CI, 1.08–3.52 and OR = 2.43; 95% CI, 1.21–4.90, respectively) as compared to those individuals who did not feature any putative risk genotypes, although it appears that no gene-gene interactions arose amongst these three genes (all P levels for interaction were >0.21). When stratified by tumor site and age at diagnosis, these combined gene effects upon cancer risk were observed for individuals who revealed that their tumor was located in the rectum (Trend test P = 0.03) and those individuals for whom their tumor was diagnosed prior to their being 60 years of age (Trend test P = 0.004; Table 4). The ORs for subjects with three putative risk genotypes were 3.18 (95% CI, 1.29–7.82) for rectal cancer and 4.90 (95% CI, 1.72–14.0) for those individuals diagnosed prior to 60 years of age, respectively.
Discussion
To the best of our knowledge, few studies have investigated the role of polymorphisms in DNA-repair genes for patients suffering colorectal cancer [21, 22]. In this hospital-based case-control study of colorectal cancer-suffering patients in Taiwan, we found polymorphisms in three DNA-repair genes associated with an elevated risk of colorectal cancer. In addition, a gene-dosage effect was found for rectal cancer and younger patients. These findings suggest that those genes involved in different DNA-repair pathways may act simultaneously in the process of carcinogenesis for colorectal cancer.
Although we didn't find any significant independent associations between these DNA-repair genes and colorectal cancer risk, the risk appeared to be slightly increased for individuals who featured the XRCC1 399Arg/Arg, XRCC3 241Thr/Thr genotypes and the XPD 751Gln allele. Our results do not appear to be entirely consistent with the results of some previous reports [21, 22], the former group reporting that the XRCC1 399Gln allele significantly increased the risk of colorectal cancer (OR = 3.98, 95% CI = 1.50–10.6). In 2003, Mort et al. [22] noted that the risk of suffering colorectal cancer was significantly heightened for individuals who featured the XRCC3 241Thr allele (OR = 1.52, 95% CI = 1.04–2.22) and only slightly increased for those individuals who revealed the XRCC1 399Gln and XPD 751Gln alleles. In 2001, Park et al. [27] reported that advanced colorectal cancer-suffering patients who revealed the XPD 751Gln/Gln genotype featured a poorer (positive) response rate to chemotherapy and also a shorter survival period compared with colorectal cancer-suffering individuals who belonged to either the 751Lys/Lys or the 751Lys/Gln group.
The apparent divergence between these studies and ours might be due to one of two reasons. Firstly, it may simply be that ethnic differences in allele frequency for the polymorphism might explain the controversial findings. The frequencies for the XRCC1 399Gln, XRCC3 241Met and XPD 751Gln alleles amongst the healthy controls in this study (0.27, 0.05 and 0.07, respectively) were similar to the results for other studies conducted in Taiwan [28, 29] and China [30, 31], but appeared to be much lower than those reported in 2003 by Mort et al. [22] (0.42 for XRCC1 399Gln, 0.45 for XRCC3 241Met and 0.36 for XPD 751Gln) for a British population. On the other hand, the XRCC1 399Gln allele frequency observed in the present study is greater than that observed by Abdel-Rahman et al. in 2000 (0.14 for XRCC1 399Gln) [21]. Abdel-Rahman et al. also found that urban residents have 9.97-fold increased risk of early-onset colorectal carcinoma than rural residents with the XRCC1 399Gln allele [21]. Therefore, it is possible that the divergence in results from different studies might be related to different levels of carcinogen exposure for different populations. Moreover, inadequate study design such as a too-small sample size and/or the inadequate controlling for certain confounders (such as age and gender) should also be considered as constituting the underpinning for such differing results.
Combined effects of polymorphisms of the XRCC1 Arg399Gln, XRCC3 Thr241Met and XPD Lys751Gln genes in regard to colorectal cancer risk were observed in the present study. With the complexity of detail of environmental exposures to various carcinogens, it is plausible that the effective repair of DNA damaged by chemical mixtures necessitates multiple DNA-repair pathways (including BER, HRR and NER pathways). The failure of, or the presence of deficient DNA repair capacity for each specific DNA-repair pathway may contribute to an increase in cancer risk. Additive or multiplicative effects of combined genetic variants for different DNA-repair pathways have been previously reported for lung cancer [32], melanoma [33] and breast cancer [34]. In 2003, Zhou et al. [35] found that the risk of lung cancer amongst nonsmokers increased progressively with the increase in the number of high-risk alleles of XRCC1 and XPD genes. Hu et al. [36] also observed that prolonged cell-cycle delay was significantly associated with the number of variant alleles of the APE1 and XRCC1 genes that were present. It would therefore appear reasonable to hypothesize that genetic polymorphism(s) for DNA-repair genes may simultaneously contribute to colorectal cancer susceptibility.
In this study, we found that the combined effect of multiple DNA-repair genes upon colorectal cancer risk was significant for our younger age group, but not so for the older age group. This finding appears to be similar to the results of most of the previous studies pertaining to colorectal cancer [21], basal-cell carcinoma [37], head-and-neck cancer [38], hepatocellular carcinoma [39], and lung cancer [6, 32, 35, 40] that we reviewed, although we did note that one study reported that such an elevated risk was also observed for old-aged head-and-neck cancer patients [41]. In addition, few studies that we reviewed failed to find a difference between age groups in regard to for cancer risk [31, 42–46]. In 2000, Duell et al. [47] noted that old healthy subjects who featured the XRCC1 399Gln allele appeared to be significantly associated with detectable DNA adducts in their blood mononuclear cells when compared to younger subjects who featured the 399Arg/Arg genotype. In 2001, Hemminki et al. [48] also found that old subjects who revealed the XPD 751Gln/Gln genotype exhibited a decreased DNA-repair rate for, specifically, UV-specific cyclobutane pyrimidine dimers in the skin. Therefore, it is possible that individuals who demonstrate a less-efficient DNA-repair capacity might develop tumors at a younger age than individuals who reveal an efficient DNA-repair capacity.
In our study, we also found that the combined genetic effect of these three DNA-repair genes in regard to cancer risk was more pronounced for the rectum than for the colon. This difference may reflect certain etiological differences between colon and rectal carcinogenesis. In fact, different epidemiological characteristics, etiology, pathogenesis and clinical behavior have been reported for different anatomical sites of colorectal cancer [49, 50], this latter group suggesting that carcinogenesis within the distal colon was associated with bulky-adduct-forming (BAF) agents and that these DNA lesions were repaired through NER pathways. In 2001, Hong et al. [51] also found that much more DNA-repair and apoptosis activity occurred in the distal rather than the proximal colon for the rat azoxymethane carcinogenesis model. Taken together, these observations support the notion that insufficient DNA-repair capacity could contribute to the risk of cancer associated with exogenous carcinogen exposure.
To the best of our knowledge, this study is the first to report on XRCC1, XRCC3 and XPD polymorphisms in relation to the risk of colorectal cancer for the Taiwanese population. Our results suggest that genetic polymorphisms of the XRCC1, XRCC3 and XPD genes, particularly in combination, may be associated with an individual's susceptibility to colorectal cancer. Acknowledging the relatively limited sample size in the subgroups for the low allelic frequencies, further studies incorporating a larger sample size and/or another ethnic population are needed to confirm the genetic role of DNA-repair mechanisms as regards colorectal cancer susceptibility.
Conclusions
Our results suggest that DNA-repair pathways may simultaneously modulate the risk of colorectal cancer for the Taiwanese population, and, particularly for rectal cancer and younger patients.
References
Vineis P: Epidemiology of cancer from exposure to arylamines. Environ Health Perspect. 1994, 7-10. Suppl 6
Vineis P, Talaska G, Malaveille C, Bartsch H, Martone T, Sithisarankul P, Strickland P: DNA adducts in urothelial cells: relationship with biomarkers of exposure to arylamines and polycyclic aromatic hydrocarbons from tobacco smoke. Int J Cancer. 1996, 65: 314-316.
Charames GS, Bapat B: Genomic instability and cancer. Curr Mol Med. 2003, 3: 589-596.
Lunn RM, Langlois RG, Hsieh LL, Thompson CL, Bell DA: XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Res. 1999, 59: 2557-2561.
Matullo G, Guarrera S, Carturan S, Peluso M, Malaveille C, Davico L, Piazza A, Vineis P: DNA repair gene polymorphisms, bulky DNA adducts in white blood cells and bladder cancer in a case-control study. Int J Cancer. 2001, 92: 562-567. 10.1002/ijc.1228.
Hou SM, Falt S, Angelini S, Yang K, Nyberg F, Lambert B, Hemminki K: The XPD variant alleles are associated with increased aromatic DNA adduct level and lung cancer risk. Carcinogenesis. 2002, 23: 599-603. 10.1093/carcin/23.4.599.
Ankathil R, Jyothish B, Madhavan J, Nair MK: Deficient DNA repair capacity: a predisposing factor and high risk predictive marker in familial colorectal cancer. J Exp Clin Cancer Res. 1999, 18: 33-37.
Cheng L, Spitz MR, Hong WK, Wei Q: Reduced expression levels of nucleotide excision repair genes in lung cancer: a case-control analysis. Carcinogenesis. 2000, 21: 1527-1530. 10.1093/carcin/21.8.1527.
Wei Q, Cheng L, Amos CI, Wang LE, Guo Z, Hong WK, Spitz MR: Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000, 92: 1764-1772. 10.1093/jnci/92.6.440.
Rajaee-Behbahani N, Schmezer P, Risch A, Rittgen W, Kayser KW, Dienemann H, Schulz V, Drings P, Thiel S, Bartsch H: Altered DNA repair capacity and bleomycin sensitivity as risk markers for non-small cell lung cancer. Int J Cancer. 2001, 95: 86-91. 10.1002/1097-0215(20010320)95:2<86::AID-IJC1015>3.0.CO;2-B.
Spitz MR, Wu X, Wang Y, Wang LE, Shete S, Amos CI, Guo Z, Lei L, Mohrenweiser H, Wei Q: Modulation of nucleotide excision repair capacity by XPD polymorphisms in lung cancer patients. Cancer Res. 2001, 61: 1354-1357.
Shen MR, Jones IM, Mohrenweiser H: Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 1998, 58: 604-608.
Goode EL, Ulrich CM, Potter JD: Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002, 11: 1513-1530.
Sung P, Bailly V, Weber C, Thompson LH, Prakash L, Prakash S: Human xeroderma pigmentosum group D gene encodes a DNA helicase. Nature. 1993, 365: 852-855. 10.1038/365852a0.
Weeda G, Hoeijmakers JH: Genetic analysis of nucleotide excision repair in mammalian cells. Semin Cancer Biol. 1993, 4: 105-117.
Qiao Y, Spitz MR, Shen H, Guo Z, Shete S, Hedayati M, Grossman L, Mohrenweiser H, Wei Q: Modulation of repair of ultraviolet damage in the host-cell reactivation assay by polymorphic XPC and XPD/ERCC2 genotypes. Carcinogenesis. 2002, 23: 295-299. 10.1093/carcin/23.2.295.
Caldecott KW, Aoufouchi S, Johnson P, Shall S: XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular 'nick-sensor' in vitro. Nucleic Acids Res. 1996, 24: 4387-4394. 10.1093/nar/24.22.4387.
Kubota Y, Nash RA, Klungland A, Schar P, Barnes DE, Lindahl T: Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J. 1996, 15: 6662-6670.
Cappelli E, Taylor R, Cevasco M, Abbondandolo A, Caldecott K, Frosina G: Involvement of XRCC1 and DNA ligase III gene products in DNA base excision repair. J Biol Chem. 1997, 272: 23970-23975. 10.1074/jbc.272.38.23970.
Tebbs RS, Zhao Y, Tucker JD, Scheerer JB, Siciliano MJ, Hwang M, Liu N, Legerski RJ, Thompson LH: Correction of chromosomal instability and sensitivity to diverse mutagens by a cloned cDNA of the XRCC3 DNA repair gene. Proc Natl Acad Sci U S A. 1995, 92: 6354-6358.
Abdel-Rahman SZ, Soliman AS, Bondy ML, Omar S, El-Badawy SA, Khaled HM, Seifeldin IA, Levin B: Inheritance of the 194Trp and the 399Gln variant alleles of the DNA repair gene XRCC1 are associated with increased risk of early-onset colorectal carcinoma in Egypt. Cancer Lett. 2000, 159: 79-86. 10.1016/S0304-3835(00)00537-1.
Mort R, Mo L, McEwan C, Melton DW: Lack of involvement of nucleotide excision repair gene polymorphisms in colorectal cancer. Br J Cancer. 2003, 89: 333-337. 10.1038/sj.bjc.6601061.
Yeh CC, Hsieh LL, Tang R, Chang-Chieh CR, Sung FC: Risk factors for colorectal cancer in Taiwan: a hospital-based case-control study. J Formos Med Assoc. 2003, 102: 305-312.
Matullo G, Palli D, Peluso M, Guarrera S, Carturan S, Celentano E, Krogh V, Munnia A, Tumino R, Polidoro S, Piazza A, Vineis P: XRCC1, XRCC3, XPD gene polymorphisms, smoking and (32)P-DNA adducts in a sample of healthy subjects. Carcinogenesis. 2001, 22: 1437-1445. 10.1093/carcin/22.9.1437.
Lunn RM, Helzlsouer KJ, Parshad R, Umbach DM, Harris EL, Sanford KK, Bell DA: XPD polymorphisms: effects on DNA repair proficiency. Carcinogenesis. 2000, 21: 551-555. 10.1093/carcin/21.4.551.
Breslow NE, Day NE: Statistical Methods in Cancer Research – The Analysis of Case-Control Studies. IARC scientific publications No. 32. 1980, Lyon: International Agency for Research on Cancer, 1:
Park DJ, Stoehlmacher J, Zhang W, Tsao-Wei DD, Groshen S, Lenz HJ: A Xeroderma pigmentosum group D gene polymorphism predicts clinical outcome to platinum-based chemotherapy in patients with advanced colorectal cancer. Cancer Res. 2001, 61: 8654-8658.
Lee JM, Lee YC, Yang SY, Yang PW, Luh SP, Lee CJ, Chen CJ, Wu MT: Genetic polymorphisms of XRCC1 and risk of the esophageal cancer. Int J Cancer. 2001, 95: 240-246. 10.1002/1097-0215(20010720)95:4<240::AID-IJC1041>3.0.CO;2-1.
Wu HC, Liao KM, Hung PW, Weng TH, Yang PC, Chen CJ: Interactive effects of exposures to tobacco smoke and cooking fume and genetic polymorphisms of DNA repair enzymes on female lung adenocarcinoma in northern Taiwan. Taiwan J Public Health. 2001, 20: 357-364.
Shen H, Wang X, Hu Z, Zhang Z, Xu Y, Hu X, Guo J, Wei Q: Polymorphisms of DNA repair gene XRCC3 Thr241Met and risk of gastric cancer in a Chinese population. Cancer Lett. 2004, 206: 51-58. 10.1016/j.canlet.2003.09.003.
Xing D, Qi J, Miao X, Lu W, Tan W, Lin D: Polymorphisms of DNA repair genes XRCC1 and XPD and their associations with risk of esophageal squamous cell carcinoma in a Chinese population. Int J Cancer. 2002, 100: 600-605. 10.1002/ijc.10528.
Chen S, Tang D, Xue K, Xu L, Ma G, Hsu Y, Cho SS: DNA repair gene XRCC1 and XPD polymorphisms and risk of lung cancer in a Chinese population. Carcinogenesis. 2002, 23: 1321-1325. 10.1093/carcin/23.8.1321.
Winsey SL, Haldar NA, Marsh HP, Bunce M, Marshall SE, Harris AL, Wojnarowska F, Welsh KI: A variant within the DNA repair gene XRCC3 is associated with the development of melanoma skin cancer. Cancer Res. 2000, 60: 5612-5616.
Smith TR, Miller MS, Lohman K, Lange EM, Case LD, Mohrenweiser HW, Hu JJ: Polymorphisms of XRCC1 and XRCC3 genes and susceptibility to breast cancer. Cancer Lett. 2003, 190: 183-190. 10.1016/S0304-3835(02)00595-5.
Zhou W, Liu G, Miller DP, Thurston SW, Xu LL, Wain JC, Lynch TJ, Su L, Christiani DC: Polymorphisms in the DNA repair genes XRCC1 and ERCC2, smoking, and lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2003, 12: 359-365.
Hu JJ, Smith TR, Miller MS, Mohrenweiser HW, Golden A, Case LD: Amino acid substitution variants of APE1 and XRCC1 genes associated with ionizing radiation sensitivity. Carcinogenesis. 2001, 22: 917-922. 10.1093/carcin/22.6.917.
Dybdahl M, Vogel U, Frentz G, Wallin H, Nexo BA: Polymorphisms in the DNA repair gene XPD: correlations with risk and age at onset of basal cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1999, 8: 77-81.
Sturgis EM, Castillo EJ, Li L, Zheng R, Eicher SA, Clayman GL, Strom SS, Spitz MR, Wei Q: Polymorphisms of DNA repair gene XRCC1 in squamous cell carcinoma of the head and neck. Carcinogenesis. 1999, 20: 2125-2129. 10.1093/carcin/20.11.2125.
Yu MW, Yang SY, Pan IJ, Lin CL, Liu CJ, Liaw YF, Lin SM, Chen PJ, Lee SD, Chen CJ: Polymorphisms in XRCC1 and glutathione S-transferase genes and hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2003, 95: 1485-1488.
Divine KK, Gilliland FD, Crowell RE, Stidley CA, Bocklage TJ, Cook DL, Belinsky SA: The XRCC1 399 glutamine allele is a risk factor for adenocarcinoma of the lung. Mutat Res. 2001, 461: 273-278.
Sturgis EM, Zheng R, Li L, Castillo EJ, Eicher SA, Chen M, Strom SS, Spitz MR, Wei Q: XPD/ERCC2 polymorphisms and risk of head and neck cancer: a case-control analysis. Carcinogenesis. 2000, 21: 2219-2223. 10.1093/carcin/21.12.2219.
Shen H, Xu Y, Qian Y, Yu R, Qin Y, Zhou L, Wang X, Spitz MR, Wei Q: Polymorphisms of the DNA repair gene XRCC1 and risk of gastric cancer in a Chinese population. Int J Cancer. 2000, 88: 601-606. 10.1002/1097-0215(20001115)88:4<601::AID-IJC13>3.0.CO;2-C.
Shen H, Sturgis EM, Dahlstrom KR, Zheng Y, Spitz MR, Wei Q: A variant of the DNA repair gene XRCC3 and risk of squamous cell carcinoma of the head and neck: a case-control analysis. Int J Cancer. 2002, 99: 869-872. 10.1002/ijc.10413.
Stern MC, Umbach DM, van Gils CH, Lunn RM, Taylor JA: DNA repair gene XRCC1 polymorphisms, smoking, and bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2001, 10: 125-131.
Kuschel B, Auranen A, McBride S, Novik KL, Antoniou A, Lipscombe JM, Day NE, Easton DF, Ponder BA, Pharoah PD, Dunning A: Variants in DNA double-strand break repair genes and breast cancer susceptibility. Hum Mol Genet. 2002, 11: 1399-1407. 10.1093/hmg/11.12.1399.
Olshan AF, Watson MA, Weissler MC, Bell DA: XRCC1 polymorphisms and head and neck cancer. Cancer Lett. 2002, 178: 181-186. 10.1016/S0304-3835(01)00822-9.
Duell EJ, Wiencke JK, Cheng TJ, Varkonyi A, Zuo ZF, Ashok TD, Mark EJ, Wain JC, Christiani DC, Kelsey KT: Polymorphisms in the DNA repair genes XRCC1 and ERCC2 and biomarkers of DNA damage in human blood mononuclear cells. Carcinogenesis. 2000, 21: 965-971. 10.1093/carcin/21.5.965.
Hemminki K, Xu G, Angelini S, Snellman E, Jansen CT, Lambert B, Hou SM: XPD exon 10 and 23 polymorphisms and DNA repair in human skin in situ. Carcinogenesis. 2001, 22: 1185-1188. 10.1093/carcin/22.8.1185.
Bufill JA: Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990, 113: 779-788.
Breivik J, Gaudernack G: Carcinogenesis and natural selection: a new perspective to the genetics and epigenetics of colorectal cancer. Adv Cancer Res. 1999, 76: 187-212.
Hong MY, Chapkin RS, Morris JS, Wang N, Carroll RJ, Turner ND, Chang WC, Davidson LA, Lupton JR: Anatomical site-specific response to DNA damage is related to later tumor development in the rat azoxymethane colon carcinogenesis model. Carcinogenesis. 2001, 22: 1831-1835. 10.1093/carcin/22.11.1831.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/5/12/prepub
Acknowledgments
This study was supported by Grant number NSC 89-2314-B-002-373, NSC 90-2320-B-002-123 and NSC 91-2320-B-002-121 provided by the National Science Council and Grant number DOH 85-HR-516, DOH 86-HR-516, and DOH 87-HR-516 provided by the National Health Research Institute, Department of Health, The Executive Yuan, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
CCY carried out the genotyping analysis, performed the statistical analysis and drafted the manuscript. FCS participated in the design of the study. RT and CRCC participated in the design of the study and provided clinical biospecimens. LLH conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yeh, CC., Sung, FC., Tang, R. et al. Polymorphisms of the XRCC1, XRCC3, & XPDgenes, and colorectal cancer risk: a case-control study in Taiwan. BMC Cancer 5, 12 (2005). https://doi.org/10.1186/1471-2407-5-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-5-12