Abstract
Background
The purpose of this study was to use insurance claims and tumor registry data to examine determinants of breast conserving surgery (BCS) in women with early stage breast cancer.
Methods
Breast cancer cases registered in the Hawaii Tumor Registry (HTR) from 1995 to 1998 were linked with insurance claims from a local health plan. We identified 722 breast cancer cases with stage I and II disease. Surgical treatment patterns and comorbidities were identified using diagnostic and procedural codes in the claims data. The HTR database provided information on demographics and disease characteristics. We used logistic regression to assess determinants of BCS vs. mastectomy.
Results
The linked data set represented 32.8% of all early stage breast cancer cases recorded in the HTR during the study period. Due to the nature of the health plan, 79% of the cases were younger than 65 years. Women with early stage breast cancer living on Oahu were 70% more likely to receive BCS than women living on the outer islands. In the univariate analysis, older age at diagnosis, lower tumor stage, smaller tumor size, and well-differentiated tumor grade were related to receiving BCS. Ethnicity, comorbidity count, menopausal and marital status were not associated with treatment type.
Conclusions
In addition to developing solutions that facilitate access to radiation facilities for breast cancer patients residing in remote locations, future qualitative research may help to elucidate how women and oncologists choose between BCS and mastectomy.
Similar content being viewed by others
Background
In 1990 the National Institutes of Health Consensus Development Conference [1] concluded that breast conserving surgery (BCS) followed by radiation is an appropriate method of primary treatment for the majority of women with early stage breast cancer (AJCC stages [2] I, IIA, and IIB). Numerous clinical studies have shown that survival after BCS followed by radiation therapy is equivalent to survival following mastectomy for women in these stages [3–5]. Some absolute indicators for mastectomy remain [6, 7], in particular widespread malignant-type microcalcifications, previous radiotherapy, and a relation of tumor size to breast size that would not allow a cosmetically satisfactory result. Although there has been an increase in the use of BCS since the early 1990s, an apparent under-utilization of BCS among women for whom such treatment was not contraindicated has been documented [8]. Geographic location, type of hospital and health plan, and personal preferences have been investigated as possible factors to explain the continued use of mastectomy [9–12]. Comorbidity [13] has been found to be an important predictor among older women.
The Surveillance, Epidemiology and End Results (SEER) cancer registries do not collect sufficient detail to examine some of these issues and medical records reviews are time consuming. Insurance claims data are a cost-effective alternative; they have already been collected and put into an electronic format by the insurance carriers. They cover large segments of the population, allow follow-up, use standardized codes [14, 15], and do not rely on subject recall. In cancer research, insurance claims data have been used to estimate the effectiveness of cervical cancer screening [16], to assess the role of screening practices in the incidence of prostate cancer [17], and to estimate mammography participation [18]. Medicare enrollees in Seattle and San Francisco who received care from a Health Maintenance Organization (HMO) were found to receive more BCS than women in Fee-For-Service (FFS) plans [19]. Whereas a Medicare/SEER registry linked data set has been used extensively to examine treatment and screening issues in individuals 65 years and older [20], linking of insurance claims data from private health plans in younger populations has been more difficult because of the large number of health plans in most geographic areas. However, Hawaii provides unique opportunities for insurance claims research because the majority of medical care is received within the state and more than 90% of the population [21] are covered by a limited number of health plans. As a pilot project, we were able to link data from the Hawaii Tumor Registry and from a health plan in Hawaii. The objectives of this analysis were to describe breast cancer treatment and comorbid conditions using the insurance claims data and to examine possible determinants of BCS vs. mastectomy for breast cancer patients with stage I and II disease.
Methods
The study protocol was reviewed and approved by the University of Hawaii's Committee on Human Studies and by the Hawaii Tumor Commission that oversees the Hawaii Tumor Registry (HTR). Before linking of databases was initiated, a memorandum of agreement between all parties involved in this research project was signed. The resulting agreement safeguarded patients' privacy at the highest possible level. All names and identifying information were deleted from the datasets and replaced with arbitrary numbers to be used for the data analyses.
Data Sources
The HTR has maintained a database of all cases of cancer diagnosed in the State of Hawaii since 1960 and became part of the SEER program in 1973. The HTR record contains demographic characteristics such as age, ethnicity, marital status, island of residence, as well as information on tumor size, extent of disease, lymph node involvement, and tumor grade. From medical records in hospitals and physicians' offices, information is collected on the initial course (six months in the Hawaii Tumor Registry) of cancer-directed treatment following diagnosis. Quality control reviews have shown that case-ascertainment through HTR has been virtually complete [22]. Over 99% of cancer cases reported to the registry are histologically confirmed. The HTR also maintains a link with Hawaii Department of Health, which allows for death information to be captured in the HTR database.
Linking
The insurance claims for this study were obtained from a local insurer who was a party to the memorandum of agreement. The linked data set contained cancer cases diagnosed from 1995 through 1998. During the linking process, a list of health plan members who had at least two cancer diagnostic codes in their claims history were matched against the HTR using a probabilistic method. For each matched record, the health plan furnished all claims data for that period. They also indicated whether the individual was enrolled in a Fee-For-Service (FFS) or a capitated (HMO) plan, a choice provided by the insurer. However, the majority of linked cancer cases belonged to the FFS plan and the HMO plan provided by this insurer differs considerably from a typical HMO. Individuals aged 65 years and older were included only if they were still working and had primary coverage through the health plan or if they were covered under Medicare but also had secondary coverage through the health plan. The dataset furnished by the health insurer contained all claims processed for cancer patients in this study. The data elements included: date of service, International Classification of Disease, version 9 (ICD-9) diagnosis codes [14], Current Procedural Terminology (CPT) codes [15], and the provider specialty code. The dataset contained claims for services from physicians, laboratories, freestanding facilities, as well as outpatient services provided by hospital facilities.
Data set
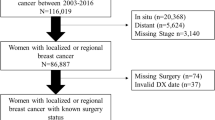
Breast cancer cases accounted for 16.6% of all cancer cases recorded in the HTR during the period from 1995 to 1998. In the linked dataset, 27.7% of the cases were breast cancer cases. Using the ICD-9-CM code range [14] for breast cancer (174.0 to 174.9), we identified 1,377 female breast cancer cases. We then excluded 265 cases diagnosed after June 30, 1998 because we would not have a complete history of claims data covering at least six months of treatment. We also identified 30 women who had been recorded twice in the linked dataset because breast cancer was diagnosed in both breasts, as identified by the laterality codes. For 24 women, the diagnosis date was identical, in which case we considered the two diagnoses as one case. For the six cases with different diagnoses dates, we retained both records because there was a separate completed course of treatment for the cancer in each breast. We eliminated one case that was in the linked dataset and had inpatient data but no outpatient data, resulting in a dataset containing 1,088 breast cancer cases.
Staging
We used the HTR information in the Extent of Disease (EOD-10) field [23] to determine the American Joint Committee on Cancer (AJCC) TNM stage [2], where T represents the primary tumor size, N refers to lymph node involvement, and M refers to presence of metastases. In cases where the tumor size was unknown, we assigned a stage only if the size was not a determinant in the TNM grouping. If the extension was unknown, we equated it to extension 10 (confinement to breast tissue and fat). For the small number of cases (N = 8) for whom lymph node involvement code was unknown, we considered this the same as no lymph node involvement because the tumor size was so small for these cases that a lymph node dissection was not considered necessary at the time of this study. The results of the TNM staging agreed well with the SEER summary stage codes.
Cancer treatment
We used the CPT codes [15] in the claims dataset to identify the type of surgery performed. For mastectomies, we included codes for simple and subcutaneous mastectomies (19180 and 19182), radical mastectomies (19200 and 19220) and modified radical mastectomy (19240). BCS was identified by the CPT codes for partial mastectomies (19160, 19162). We also included codes for excision of breast cysts or lesions (19120 and 19125) as these codes meet the definition of a lumpectomy, although we recognize that some surgeons may have billed these codes for excisional biopsies as a diagnostic procedure. If BCS was initially performed, but followed by a subsequent mastectomy, the subject was classified in the mastectomy group. This situation would have occurred when the BCS code was actually used for a diagnostic biopsy and the subsequent surgical treatment was a mastectomy or in situations where a lumpectomy was first selected, but a mastectomy became necessary because pathological examination revealed cancerous cells in the margins of the excised tissue. CPT codes 77261 to 77799 were selected to determine whether radiation therapy was received. Several CPT codes and also a number of other codes used by the health plan for billing were considered evidence of chemotherapy treatment.
Comorbidities
Information on the existence of comorbidities was extracted from the claims data using the occurrence of ICD-9 codes associated with comorbid conditions included in the Charlson Index [24]. We also analyzed data for conditions that were not included in that index, but occurred at a high enough frequency to warrant examination as possible comorbidities, in particular hypertension and lipid disorders. We included the following twelve comorbid conditions into our index: diabetes with complications, diabetes w/o complications, hypertension, heart disease, cerebrovascular disease, chronic pulmonary disease, peripheral vascular disease, kidney disease, liver disease, rheumatological diseases, hypothyroidism, and lipid metabolism disorders. Comorbidities were based on physicians' claims with the respective ICD-9 code [14] during the 12-months period preceding the month in which the cancer was diagnosed. The month of diagnosis was excluded to avoid identifying as comorbidities any complications or conditions directly resulting from cancer treatment. Laboratory, radiology, and other diagnostic services were excluded from the comorbidity identification process because tests may have been done to rule out the condition. Each subject was assigned a comorbidity score of 0 (no condition), 1 (one condition), or 2 (two or more conditions).
Other variables
In addition to stage at diagnosis and size of tumor, we examined several other variables that were possible determinants in the utilization of BCS vs. mastectomy in early stage breast cancer, including age at diagnosis, ethnicity, island of residence, and marital status. For ethnicity, we used the five major groups in Hawaii (Japanese, Caucasian, Hawaiian, Filipino, and Chinese). All other ethnicities were grouped into an "Other" category. The specific island of residence was coded in the HTR, but since close to 80% of the population resides on Oahu [25], we classified residence as either Oahu or non-Oahu. For marital status, we grouped all women who were identified as single, separate, divorced or widowed in the unmarried category. Women under age 50 were considered pre-menopausal and women 50 years and older were classified as postmenopausal. Tumor grade information from the HTR record was grouped into grade I (well-differentiated cells) vs. all other grades (II, III, IV and unknown).
Statistical analysis
All analyses were performed with SAS version 8.00 for Windows (SAS Institute, Cary, NC). Simple Kappa statistics (κ) was calculated to validate the treatment information from the insurance claims and the HTR [26, 27]. BCS was used as reference group throughout the analysis. It was defined as the dependent (outcome) variable and coded as a dichotomous variable, with 1 indicating BCS received and 0 indicating mastectomy received. Age was entered in units of ten years and tumor size was grouped into units of ten millimeters (one centimeter). For analyses of ethnicity as a predictor of treatment selection, Caucasian was used as the control group and indicator variables for all other ethnic groups were created. Logistic regression [28] was used to explore the influence of each variable on the use of BCS vs. mastectomy. First, we considered each independent variable by itself in a model and then we entered all variables simultaneously in a logistic regression model. Odds Ratios (OR) [29] with corresponding 95% Confidence Intervals (CI) were calculated to measure the degree of influence of variables on the utilization of BCS vs. mastectomy.
Results
Based on the TNM stages, we identified 722 women who had stage I, IIA, or IIB breast cancer (66.4% of the 1,088 breast cancer cases in the linked data set). These 722 cases represented 32.8% (722 of 2,203 cases) of all early stage breast cancer cases recorded in the HTR during the study period. Approximately two-thirds of the women in the study population (Table 1) were diagnosed in stage I of the disease and only 24% and 12% in stage IIA and IIB, respectively. Approximately 30% of the study subjects were aged less than 50 years, close to half were 50 to 64 years old, and 21% were 65 years and older. Three out of four women resided on the island of Oahu. Overall, 52.8% of the women had received BCS. Of the cases diagnosed at stage I, 57% of the women had received BCS and 47% a mastectomy. This decreased to 50% for stage IIA and to 34% for stage IIB. Among women, 65 years and older, 56% had BCS, compared to 50% of those under 50 years of age and 53% of the 50–64 year old women. While 56% of women on Oahu underwent BCS, only 43% of the women residing on the outer islands received BCS. We observed no statistically significant differences between the BCS and mastectomy group in terms of ethnicity, comorbidity count, menopausal status, marital status, and insurance plan. We found very high agreement between HTR data and claims data in identifying BCS [κ = 0.91 (95% CI 0.88, 0.94)], only 32 women were misclassified. For the majority of women who received BCS (92.1%), the lumpectomy was followed by radiation therapy. Additional chemotherapy was given to 34.1% and 42.5% of the women who had a lumpectomy or a mastectomy, respectively.
In the univariate models (Table 1), we found that tumor size and grade, island of residence, age, and stage at diagnosis were predictors of breast cancer treatment. Women residing on Oahu were considerably more likely to have BCS than women living on all other islands in the state. For each one-centimeter increase in tumor size, there was a 3% lesser chance of undergoing BCS. Since size of tumor correlates to breast cancer stage, this also decreased the likelihood for women in stage IIA and IIB to receive BCS. Women with well-differentiated tumor grades were 50% more likely to undergo BCS as compared to women with all other grades. We found that for each ten-year increment in age, the chances of having BCS increased by 1%. We also observed that average tumor size was inversely related to age. Mean tumor sizes (in cm) with standard deviations were 1.82 ± 1.1, 1.59 ± 1.2, 1.39 ± 0.94 for women younger than 50 years, 50 to 64 years, and 65 years and older, respectively. Therefore, the smaller tumor sizes may account for the greater likelihood of BCS among older women.
In a combined model with all independent variables in a logistic regression, island of residence remained the only significant predictor of BCS in this population. Women living on Oahu were 67% more likely to have BCS than women on the outer islands. Although all other variables lost their statistical significance, associations for age, TNM stage, tumor size, and tumor grade remained similar in magnitude as in the univariate models. Although none of the ethnicity variables was significant, it appeared that Filipino women were less likely to receive BCS than women from all other groups. Residence on outer islands did not explain this observation. Women with a TNM stage of IIB were still 40% less likely to receive BCS than women diagnosed at stage I, but the relation lost its statistical significance due to the small number of cases. Menopausal or marital status, type of health plan, or the number of comorbidities were not related to the type of surgery.
Discussion
The place of residence at the time of diagnosis was the most important predictor for receiving BCS in this dataset of health plan members. Women with early stage breast cancer living on Oahu were 70% more likely to have BCS than women living on outer islands. Age at diagnosis and tumor size were also related to breast cancer treatment although they were not statistically significant in the combined model. Contrary to our expectation and a previous publication [13], the number of comorbid conditions did not effect treatment in this analysis. The importance of geographic location can be explained by the availability of radiation facilities on the different Hawaiian Islands. Whereas on Oahu treatment facilities can be reached within one hour, distances on the outer islands are much farther. Only the Island of Hawaii and Maui have a radiation facility, but they are hard to reach from many parts of the islands. Women residing on Kauai, Molokai, and Lanai have to fly to Honolulu daily to receive the course of treatment or they have to remain there temporarily. Because this may be not be economically feasible for some women, as well as physically and psychologically challenging, it is possible that many women choose to undergo a mastectomy instead. Alternatively, patients and physicians in rural areas may favor mastectomy because of differences in education or cultural attitudes toward medical advances. We do not have information to explain why a small proportion of women who underwent BCS (6.9%) did not receive radiation. However, compared to other reports [13, 30, 31], the proportion of women who received radiation is very high. An interview study with cancer patients [32] showed that some patients decide against their physicians' recommendation for radiation because they fear the treatment or hold certain beliefs about cancer. For other patients, radiation may have been contraindicated due to comorbid conditions.
In 1994, a national BCS rate of 42.6% was reported [33] with a rate of 46.7% for the Pacific region (Hawaii, California, Alaska, Washington, and Oregon). Our results for 1995 to 1998 indicate a slightly higher rate for Hawaii (52.8%). Geographic differences in BCS use have repeatedly been described in the literature. In western Washington [34], women residing outside the Seattle area were less likely to have BCS than women residing inside the county, in particular if radiation therapy facilities were not available in their county. Comparing rates across the country showed that BCS was much more common in the Northeast than in the South [33]. Low BCS rates were also found in rural areas, such as North and South Carolina [10], Minnesota [35], and the Southwest [36]. An analysis of 1991–1992 SEER data [37] demonstrated a strong association between BCS and distance to the next radiotherapy facility. The likelihood of undergoing BCS was 50% lower among women living more than 15 miles from the next radiotherapy facility.
In contrast to our results, a number of studies have shown that the likelihood of BCS decreases with increasing age [11, 30, 33], possibly due to less concern about cosmetic results. Because of the relatively small proportion (21%) of women 65 years and older and the fact that the women 65 years and older in our study had a health plan other than Medicare, they were not directly comparable to the populations in the published literature. Therefore, our ability to observe an effect among older women was limited. In particular, we were not able to examine the treatment of women 80 years and older who have been described as receiving more BCS than women age 65 to 79 years [13]. Our findings of lower BCS in smaller and well differentiated tumors agree with previous studies [33], but the absence of an effect of comorbid conditions disagrees with a study in older women [13] that showed a higher rate of BCS in women with comorbidities. Type of health plan did not predict type of treatment in our study probably because the majority of women in this data set had a FFS plan. In previous analyses with a larger variety of health plans, HMO members were considerably more likely to receive BCS in San Francisco and in Seattle [19]. Medicare and Medicaid patients received more BCS in a North Carolina hospital [7]. However, a study using administrative data [38] described lower BCS use among Medicaid patients and treatment was similar by health plan [39] in Northern California.
Our study had several limitations, including the possibility of incomplete claims histories if patients changed health plans during the course of treatment, but the high agreement with HTR information indicates that the validity of insurance claims information was high. Continuing efforts by the health plan through feedback loops has improved diagnostic coding [40] in Hawaii, but as documented for Medicare claims, problems remain [41]. As discussed above, our study sample of breast cancer patients does not represent the population of the state, excluding in particular Medicare and Medicaid recipients, members of a typical HMO, and the relatively small number of uninsured patients. The exclusion of Medicare claims lead to a younger population, whereas the lack of Medicaid claims biased the cases toward a higher socioeconomic status. On the other hand, the study population represented the population quite well in terms of ethnicity (Hawaii Department of Business 1998 1705 /id). Because our data set included 42.4% of all breast cancer cases under 65 years diagnosed during the study period, the results represents this population much better than the population of older women. Also, we were unable to measure some factors that have been found to be important in other reports, in particular, influence of physician age [11], physician specialty [42], type of hospital (teaching hospitals and hospitals with radiation facilities, private, county and public) [36, 38, 39, 43], socio-economic status and education [42], tumor-breast ratio and the expected cosmetic results [7, 9], physician/patient interactions before surgery [42], and psychological factors, such as fear of radiation or cancer [9]. The strengths of using of insurance claims data for this analysis were twofold. First, we had more detailed treatment information available than collected by the tumor registry. Second, the diagnostic codes in the claims data allowed us to estimate the number of comorbid conditions before the cancer diagnosis.
The results of this pilot study combining tumor registry and insurance claims data raises an important issue for cancer practice in the State of Hawaii and elsewhere. Health care providers and insurance plans need to work with other agencies to develop viable solutions to facilitate access to radiation facilities for women residing in remote locations, such as islands with no radiation facility. Attitudes of patients and physicians living in rural areas may be important in the choice of treatment, but could not be investigated in this project. Future research that includes interviews with patients and physicians may investigate attitudes toward certain types of treatment and identify additional barriers to BCS, such as psychological problems. In order to understand how treatment decisions were made in practice, qualitative information from physicians and breast cancer patients should be collected and examined in detail. Further validation of treatment and comorbidity from insurance claims data would strengthen this type of research in the future. The inclusion of a larger proportion of breast cancer cases is needed to establish the validity of our findings for the population at-large.
References
NIH Consensus Development Conference on the Treatment of Early-Stage Breast Cancer: Bethesda, Maryland, June 18–21, 1990. J Natl Cancer Inst Monogr. 1992, 1-187.
American Joint Committee on Cancer: Manual for staging of cancer. Philadelphia: Lippincott;. 1992
Fisher B, Redmond C, Poisson R, Margolese R, Wolmark N, Wickerham L, et al: Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989, 320: 822-828.
Lichter AS, Lippman ME, Danforth DN, d'Angelo T, Steinberg SM, deMoss E, et al: Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the National Cancer Institute. J Clin Oncol. 1992, 10: 976-983.
Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, del Vecchio M, et al: Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer. 1995, 31A: 1574-1579. 10.1016/0959-8049(95)00271-J.
National Cancer Institute: PDQ® – NCI's Comprehensive Cancer Database. 2001, [http://cancernet.nci.nih.gov/pdqfull.html]
Kotwall C, Covington D, Churchill P, Brinker C, Weintritt D, Maxwell JG: Breast conservation surgery for breast cancer at a regional medical center. Am J Surg. 1998, 176: 510-514. 10.1016/S0002-9610(98)00254-2.
Patton ML, Moss BE, Kraut JD, Germain TJ, Haith LR, Shotwell BA, et al: Underutilization of breast-conservation surgery with radiation therapy for women with stage Tis, I or II breast cancer. Int Surg. 1996, 81: 423-427.
Nold RJ, Beamer RL, Helmer SD, McBoyle MF: Factors influencing a woman's choice to undergo breast-conserving surgery versus modified radical mastectomy. Am J Surg. 2000, 180: 413-418. 10.1016/S0002-9610(00)00501-8.
Benedict S, Cole DJ, Baron L, Baron P: Factors influencing choice between mastectomy and lumpectomy for women in the Carolinas. J Surg Oncol. 2001, 76: 6-12. 10.1002/1096-9098(200101)76:1<6::AID-JSO1002>3.0.CO;2-F.
Kotwall CA, Covington DL, Rutledge R, Churchill MP, Meyer AA: Patient, hospital, and surgeon factors associated with breast conservation surgery. A statewide analysis in North Carolina. Ann Surg. 1996, 224: 419-426. 10.1097/00000658-199610000-00001.
Sainsbury R, Haward B, Rider L, Johnston C, Round C: Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet. 1995, 345: 1265-1270. 10.1016/S0140-6736(95)90924-9.
Ballard-Barbash R, Potosky AL, Harlan LC, Nayfield SG, Kessler LG: Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst. 1996, 88: 716-726.
International Classification of Disease: ICD-9-CM. Salt Lake City: Medicode;. 1995
American Medical Association: Physician's current procedural terminology – cpt '95. Chicago, IL: AMA. 1994
Cohen MM: Using administrative data for case-control studies: the case of the Papanicolaou smear. Ann Epidemiol. 1993, 3: 93-98.
Potosky AL, Miller BA, Albertsen PC, Kramer BS: The role of increasing detection in the rising incidence of prostate cancer. JAMA. 1995, 273: 548-552. 10.1001/jama.273.7.548.
Maskarinec G, Wilkens L, Meng L: Mammography screening and the increase in breast cancer incidence in Hawaii. Cancer Epidemiol Biomarkers Prev. 1997, 6: 201-208.
Potosky AL, Merrill RM, Riley GF, Taplin SH, Barlow W, Fireman BH, et al: Breast cancer survival and treatment in health maintenance organization and fee-for-service settings. J Natl Cancer Inst. 1997, 89: 1683-1691. 10.1093/jnci/89.22.1683.
Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG: Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993, 31: 732-748.
Hawaii Medical Service Association Foundation: Health Trends in Hawaii. Honolulu: HMSA;. 1997
Goodman MT, Yoshizawa CN, Kolonel LN: Descriptive epidemiology of thyroid cancer in Hawaii. Cancer. 1988, 61: 1272-1281.
Shambaugh EM, Ries LG, Young JLJ, Kruse MA, Platz CE, Ryan RF, et al: SEER extent of disease – 1988 codes and coding instructions. National Cancer Institute. NIH Publication No. 92-2313. 1992
Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992, 45: 613-619.
Hawaii Department of Business: State of Hawaii data book 1997: a statistical abstract. Honolulu: State of Hawaii;. 1998
Cooper GS, Yuan Z, Stange KC, Dennis LK, Amini SB, Rimm AA: Agreement of Medicare claims and tumor registry data for assessment of cancer-related treatment. Med Care. 2000, 38: 411-421. 10.1097/00005650-200004000-00008.
Du X, Freeman JL, Warren JL, Nattinger AB, Zhang D, Goodwin JS: Accuracy and completeness of Medicare claims data for surgical treatment of breast cancer. Med Care. 2000, 38: 719-727. 10.1097/00005650-200007000-00004.
Stokes M, Davis C, Koch G: Categorical data analysis using the SAS System,. Cary, NC: SAS Institute Inc. 2000, 2
Breslow NE, Day NE: Statistical methods in cancer research. The design and analysis of cohort studies. Lyon: International Agency for Research on Cancer;. 1987, II:
Du X, Freeman JL, Goodwin JS: Information on radiation treatment in patients with breast cancer: the advantages of the linked Medicare and SEER data. Surveillance, Epidemiology and End Results. J Clin Epidemiol. 1999, 52: 463-470. 10.1016/S0895-4356(99)00011-6.
Silliman RA, Guadagnoli E, Weitberg AB, Mor V: Age as a predictor of diagnostic and initial treatment intensity in newly diagnosed breast cancer patients. J Gerontol. 1989, 44: M46-M50.
Shumay DM, Maskarinec G, Kakai H, Gotay CC: Why some cancer patients choose complementary and alternative medicine instead of conventional treatment. J Fam Pract. 2001, 50: 1067-
Morrow M, White J, Moughan J, Owen J, Pajack T, Sylvester J, et al: Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol. 2001, 19: 2254-2262.
Lazovich DA, White E, Thomas DB, Moe RE: Underutilization of breast-conserving surgery and radiation therapy among women with stage I or II breast cancer. JAMA. 1991, 266: 3433-3438. 10.1001/jama.266.24.3433.
Guadagnoli E, Weeks JC, Shapiro CL, Gurwitz JH, Borbas C, Soumerai SB: Use of breast-conserving surgery for treatment of stage I and stage II breast cancer. J Clin Oncol. 1998, 16: 101-106.
Kelemen JJ, Poulton T, Swartz MT, Jatoi I: Surgical treatment of early-stage breast cancer in the Department of Defense Healthcare System. J Am Coll Surg. 2001, 192: 293-297. 10.1016/S1072-7515(00)00803-6.
Nattinger AB, Kneusel RT, Hoffmann RG, Gilligan MA: Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. 2001, 93: 1344-1346. 10.1093/jnci/93.17.1344.
Young WW, Marks SM, Kohler SA, Hsu AY: Dissemination of clinical results. Mastectomy versus lumpectomy and radiation therapy. Med Care. 1996, 34: 1003-1017. 10.1097/00005650-199610000-00003.
Lee-Feldstein A, Feldstein PJ, Buchmueller T, Katterhagen G: The relationship of HMOs, health insurance, and delivery systems to breast cancer outcomes. Med Care. 2000, 38: 705-718. 10.1097/00005650-200007000-00003.
Worth RM, Mytinger RE: Medical insurance claims as a source of data for research: accuracy of diagnostic coding. Hawaii Med J. 1996, 55: 9-11.
Fisher ES, Whaley FS, Krushat WM, Malenka DJ, Fleming C, Baron JA, et al: The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992, 82: 243-248.
Keating NL, Weeks JC, Landrum MB, Borbas C, Guadagnoli E: Discussion of treatment options for early-stage breast cancer: effect of provider specialty on type of surgery and satisfaction. Med Care. 2001, 39: 681-691. 10.1097/00005650-200107000-00005.
Nattinger AB, Gottlieb MS, Hoffman RG, Walker AP, Goodwin JS: Minimal increase in use of breast-conserving surgery from 1986 to 1990. Med Care. 1996, 34: 479-489. 10.1097/00005650-199605000-00009.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/2/3/prepub
Acknowledgements
We are very grateful to the employees of the health plan for their time and willingness to assist with this project. Special thanks go to Andrew White, PhD for his long-term support of our research efforts. The help of Marc Goodman, PhD and the staff of the Hawaii Tumor Registry is greatly appreciated. This research was funded by a special study grant from the National Cancer Institute, Surveillance, Epidemiology, and End Results program under contract number N01-PC67001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Maskarinec, G., Dhakal, S., Yamashiro, G. et al. The use of breast conserving surgery: linking insurance claims with tumor registry data. BMC Cancer 2, 3 (2002). https://doi.org/10.1186/1471-2407-2-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-2-3