Abstract
Background
The prognostic value of circulating tumor cells (CTCs) in colorectal cancer (CRC) patients and their value in predicting tumor response to chemotherapy are controversial. The aim of this meta-analysis was to assess the prognostic and predictive value of CTCs in CRC patients treated with chemotherapy.
Methods
A comprehensive literature search for relevant studies was conducted in PubMed, Embase, the Cochrane Database, the Science Citation Index and the Ovid Database, and the reference lists of relevant studies were also perused for other relevant studies (up to April, 2014). Using the random-effects model in Stata software, version 12.0, the meta-analysis was performed using odds ratios (ORs), risk ratios (RRs), hazard ratios (HRs) and 95% confidence intervals (CIs) as effect measures. Subgroup and sensitivity analyses were also performed.
Results
Thirteen eligible studies were included. Our meta-analysis indicated that the disease control rate was significantly higher in CRC patients with CTC-low compared with CTC-high (RR = 1.354, 95% CI [1.002–1.830], p = 0.048). CRC patients in the CTC-high group were significantly associated with poor progression-free survival (PFS; HR = 2.500, 95% CI [1.746–3.580], p < 0.001) and poor overall survival (OS; HR = 2.856, 95% CI [1.959–4.164], p < 0.001). Patients who converted from CTC-low to CTC-high or who were persistently CTC-high had a worse disease progression (OR = 27.088, 95% CI [4.960–147.919], p < 0.001), PFS (HR = 2.095, 95% CI [1.105–3.969], p = 0.023) and OS (HR = 3.604, 95% CI [2.096–6.197], p < 0.001) than patients who converted from CTC-high to CTC-low.
Conclusions
Our meta-analysis indicates that CTCs are associated with prognosis in CRC patients treated with chemotherapy. Moreover, CTCs could provide additional prognostic information to tumor radiographic imaging and might be used as a surrogate and novel predictive marker for the response to chemotherapy.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in males and the second in females worldwide [1]. Approximately 50% of CRC patients will develop subsequent metastasis or recurrence, regardless of curative resection. Despite these outcomes, standard combined chemotherapy has been successfully used to increase the cure rate [2, 3]. In recent decades, significant improvements have been made in the response rate, disease control rate, progression-free survival (PFS) and overall survival (OS) of CRC patients [4, 5].
However, despite the improved efficacy of chemotherapy, only a fraction of patients respond to it [6, 7]. Furthermore, there are a lack of accurate markers for predicting tumor response that can be used to identify those patients who might safely discontinue prolonged treatment and those who should resume chemotherapy quickly. Such markers could reduce the use of chemotherapy in nonresponsive patients, reducing unnecessary costs and toxicity [8, 9].
Circulating tumor cells (CTCs) have been detected in the peripheral blood of patients with various cancers [10–12]. Several studies have reported that CTCs can be used as prognostic and predictive markers in patients with breast or prostate cancer [10, 12]. However, the clinical significance of CTCs in CRC patients treated with chemotherapy and targeted agents has not yet been confirmed consistently, and whether CTCs can be used as a predictive marker for response to chemotherapy is controversial.
The aim of our study was to use a meta-analysis to comprehensively summarize the prognostic and predictive significance of CTCs in evaluating the response to chemotherapy in CRC patients.
Methods
Search strategy
PubMed, Embase, the Science Citation Index, Cochrane Database and the Ovid Database were systematically searched for studies of the prognostic and predictive significance of CTCs in CRC patients treated with chemotherapy, with no restrictions on language, place of publication or date of publication (up to April, 2014). The reference lists of the retrieved studies and reviews were also perused manually to check for potentially relevant studies. The main search terms used were “circulating tumor cells”, “isolated tumor cells”, “occult tumor cells”, “peripheral blood”, “colorectal cancer”, “colon cancer”, “rectal cancer”, “gastrointestinal cancer”, “chemotherapy” and “targeted treatment/agent”.
Study eligibility criteria
Studies were considered eligible if they met all of the following criteria: (1) all enrolled patients (>20) were diagnosed with CRC; (2) prognostic and predictive significance of CTCs in patients treated with chemotherapy was assessed with at least one of the outcome measures of interest reported in the study or calculated from the published data; (3) tumor response to chemotherapy was assessed according to the Response Evaluation Criteria In Solid Tumors (RECIST) guidelines (complete response [CR], partial response [PR], stable disease [SD] or progressive disease [PD]) [13]; and (4) the samples were collected from peripheral blood. When several studies were based on the same patient population, only the most informative study was included.
Data extraction and quality assessment
Two reviewers (X. Z. Huang and P. Gao) independently extracted data from eligible studies. The following information was extracted: first author, year of publication, population characteristics, chemotherapy, sampling times (before the initiation of surgery and chemotherapy [“baseline”] or after the initiation of chemotherapy [“during chemotherapy”]), detection method, rate of CTC positivity, follow-up period, cut-off point, prognostic values (OS and PFS) and objective response to chemotherapy (CR, PR, SD or PD).
The quality of the included studies was assessed with the Newcastle-Ottawa Scale (NOS) criteria for cohort studies [14]. A funnel plot was used to assess publication bias. Any disagreements on data extraction and quality assessment of the included studies were resolved through comprehensive discussion. All written informed consents for participants have been described and obtained by the studies that were included in our meta-analysis.
Statistical analysis
Our meta-analysis was performed with Stata software, version 12.0 (2011) (Stata Corp, College Station, TX, USA), in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [15]. The odds ratios (ORs), risk ratios (RRs) and hazard ratios (HRs) were regarded as effect measures for summarizing prognosis and objective response to chemotherapy. If the HR and its 95% confidence interval (95% CI) were not provided directly, they were calculated from the available data using the method reported by Jayne F. Tierney [16].
To retain maximum information, if one study reported several results separately for different blood samples collected during chemotherapy, we combined multiple effect values into a pooled estimate for further meta-analysis. All relevant studies were included in the overall analysis. A subgroup analysis was performed simultaneously based on the sampling time (baseline or during chemotherapy) and CTC detection method. We assessed the correlation between the CTC level and the response to chemotherapy, assuming radiographic imaging to be the gold standard, and in this way, determined the sensitivity and specificity of CTC-high status in predicting the response rate (CR + PR) or the disease control rate (CR + PR + SD). Sensitivity, specificity, positive predictive value, negative predictive value and area under the receiver operating characteristic curve [ROC] were calculated using the binomial rendition of the bivariate mixed-effects regression model [17, 18].
A p value <0.05 was considered statistically significant and CI was set at 95%. In addition to the diagnostic accuracy test, the remaining analyses used a random-effects model, because it provided more conservative estimates and was more tailored to multicenter studies in which heterogeneity was usually present [19]. Heterogeneity among studies was evaluated using the Cochran Q test and I2 statistic [20]. Potential sources of heterogeneity were explored using a meta-regression analysis and Galbraith plot. To maintain the accuracy of the results of the meta-regression, the analysis was only conducted when the number of studies was greater than 10 [21, 22]. We used Egger’s and Begg’s tests to investigate publication bias [23, 24] and conducted trim-and-fill analysis to evaluate the effect of publication bias [25]. A sensitivity analysis was conducted to assess the quality and consistency of results using the leave-one-out approach.
Ethics statement
The study did not violate the local regulations, and was approved by the Research Ethics Committee of China Medical University, China.
Results
Baseline characteristics of the included studies
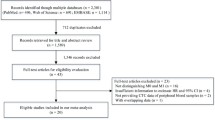
Seven hundred and twelve relevant studies were initially identified in the literature search. After screening titles and abstracts, 650 studies were excluded and 62 potential studies were reviewed further. An additional 49 studies were then excluded because they were redundant studies or lacked the outcomes of interest. Finally, 13 studies were identified as eligible for inclusion in the meta-analysis (Figure 1).
The 13 studies included 2388 eligible CRC patients (median sample size: 76 [38–735]; mean: 184). The studies were conducted in Asia, Europe, Oceania and North America, and were published between 2008 and 2014. In terms of sampling time, seven studies assessed the significance of CTCs detection at baseline and during chemotherapy separately [11, 26–31]: one study assessed the significance of CTCs combined at both time points [32], three assessed the significance of CTC detection only at baseline [33–35], one study assessed the significance of CTC detection only during chemotherapy [36] and one study did not report sampling times [37]. Six studies showed the relationship between clinical outcomes and CTC changes during chemotherapy [11, 26–29, 32]. Nine of the 13 studies only enrolled eligible patients with metastatic CRC (mCRC) [11, 26, 28–31, 33, 34, 37]. Table 1 summarizes the main baseline characteristics and study design variables. The quality of the included studies was evaluated with the NOS and is summarized in Table 2.
Correlation of CTCs with the objective response to chemotherapy
Our meta-analysis suggested that the disease control rate was significantly higher in CRC patients with CTC-low compared with CTC-high (RR = 1.354, 95% CI [1.002–1.830], p = 0.048), and patients with CTC-low tended to have a favorable response rate, although statistical significance was not reached (RR = 1.740, 95% CI [0.878–3.447], p = 0.113) (Figure 2). A similar tendency was obtained in the subgroup analysis based on sampling time: baseline (response rate: RR = 1.258, 95% CI [0.990–1.598], p = 0.060; disease control rate: RR = 1.375, 95% CI [0.961–1.967], p = 0.082) and during chemotherapy (response rate: RR = 2.315, 95% CI [1.242–4.316], p = 0.008; disease control rate: RR = 1.535, 95% CI [1.170–2.016], p = 0.002). After excluding studies containing stage II–III patients, the pooled results of the subgroup analysis of mCRC patients were still significant (disease control rate: RR = 1.354, 95% CI [1.065–1.721], p = 0.013). We also conducted a pooled analysis using the CellSearch system method, a standardized semiautomated quantification method system approved by the U.S. Food and Drug Administration, with similar results. These estimated results for the response to chemotherapy are summarized in Table 3.
For CTCs detected at baseline, sensitivity and specificity of CTC-high for imaging-based disease progression were 52.0% (95% CI [21.5–81.1%]) and 68.7% (95% CI [57.4–78.2%]), respectively. Positive and negative predictive values were 40.4% (95% CI [11.7–77.6%]) and 77.3% (95% CI [46.1–93.1%]), respectively, and the area under the ROC curve was 0.68 (95% CI [0.10–0.98]). Sensitivity and specificity of the CTCs detected during chemotherapy were 50.0% (95% CI [19.6–80.4%]) and 89.5% (95% CI [79.3–95.0%]), respectively. Positive and negative predictive values were 40.9% (95% CI [22.7–62.1%]) and 90.6% (95% CI [80.1–95.8%]), respectively, and the area under the ROC curve was 0.86 (95% CI [0.15–1.00]). For the imaging-based nonresponse (SD + PD), sensitivity and specificity of CTCs detected at baseline and during chemotherapy were 30.2% (95% CI [10.1–62.5%]) and 91.1% (95% CI [75.6–97.1%]), respectively. Positive and negative predictive values were 79.4% (95% CI [69.1–86.9%]) and 50.4% (95% CI [23.9–76.7%]), respectively, and the area under the ROC curve was 0.77 (95% CI [0.73–0.81]).
We analyzed three studies that suggested that CTC levels during chemotherapy could provide additional predictive information on survival outcomes when added to radiographic imaging [11, 29, 30]. The OS of CTC-low patients with disease control (median time: 21.694 months, 95% CI [18.871–24.517]) was significantly longer than that of CTC-low patients with PD (median time: 7.943 months, 95% CI [5.680–10.206]), CTC-high patients with disease control (median time: 9.613 months, 95% CI [6.090–13.137]), and CTC-high patients with PD (median time: 3.327 months, 95% CI [2.398–4.257]). Differences in median OS between CTC-low patients with disease control and patients in the other three groups were statistically significant (p < 0.0001 for all three comparisons) (Figure 3).
Results for the meta-analysis of overall survival (OS) time. The differences in median OS time based on the combination of CTCs and radiographic imaging, Group 1: patients with CTC-low during chemotherapy and disease control, Group 2: patients with CTC-low during chemotherapy and progression disease (PD), Group 3: patients with CTC-high during chemotherapy and disease control, Group 4: patients with CTC-high during chemotherapy and PD.
Conversion of CTC levels and prognosis
When we compared CTC levels of samples collected at baseline with those collected during chemotherapy, our results suggested that CRC patients who converted from CTC-low to CTC-high or who were persistently CTC-high during treatment had an unfavorable progressive disease compared with those CRC patients who converted from CTC-high to CTC-low (OR = 27.088, 95% CI [4.960–147.919], p < 0.001) (Figure 4). Therefore, it was understandable that CRC patients who became CTC-high or were persistently CTC-high were significantly associated with a poor PFS (HR = 2.095, 95% CI [1.105–3.969], p = 0.023) and a poor OS (HR = 3.604, 95% CI [2.096–6.197], p < 0.001). These results are summarized in Table 3.
The results for the relationship between CTCs conversion and prognosis. A: The estimated odds ratio (OR) was summarized for the correlation of disease control rate with CTCs conversion. B: The estimated hazard ratio (HR) was summarized for progression-free survival (PFS) with CTCs conversion. C: The estimated HR was summarized for overall survival (OS) with CTCs conversion.
Impact of CTCs on survival outcomes (PFS and OS)
HRs for PFS were available in eleven studies. The estimated pooled HRs indicated that CTC-high status was associated with a significantly decreased PFS (HR = 2.500, 95% CI [1.746–3.580], p < 0.001). Eleven studies provided HRs on OS and the pooled results indicated that CRC patients in the CTC-high group were significantly associated with a poor OS (HR = 2.856, 95% CI [1.959–4.164], p < 0.001; Figure 5). The pooled results of the subgroup analysis of mCRC patients were similar to the results of the overall analysis when we excluded the four studies containing stage II–III patients (PFS: HR = 2.237, 95% CI [1.485–3.370], p < 0.001; OS: HR = 2.642, 95% CI [1.716–4.067], p < 0.001). Interestingly, for the stage II–III subpopulation, CTCs were still a significant poor prognostic factor for survival: (PFS: HR = 3.226, 95% CI [2.193–4.745], p < 0.001; OS: HR = 3.735, 95% CI [1.710–8.158], p = 0.001). The estimated HRs for PFS and OS were similar in the subgroup analysis of the studies in which most mCRC patients underwent surgery (Table 3).
As shown in the subgroup analysis based on sampling time, CTCs were confirmed as a significant prognostic factor for survival: at baseline (PFS: HR = 1.853, 95% CI [1.405–2.445], p < 0.001; OS: HR = 2.356, 95% CI [1.957–2.836], p = 0.001) and during chemotherapy (PFS: HR = 2.386, 95% CI [1.454–3.917] p = 0.001; OS: HR = 3.292, 95% CI [1.611–6.726], p = 0.001). A similar trend was observed in the pooled analysis using the CellSearch system method (PFS: HR = 1.769, 95% CI [1.180–2.651], p = 0.006; OS: HR = 2.452, 95% CI [1.484–4.050], p < 0.001).
Evaluation of heterogeneity and publication bias
Neither the direction nor the magnitude of the estimated pooled results was obviously affected when each study was removed in the sensitivity analysis, indicating that no single study dominated our results (Additional file 1). The Galbraith plot showed that the study by Matsusaka et al. [28] contributed substantial heterogeneity to our meta-analysis. Exclusion of the study by Matsusaka et al. [28] could increase statistical power and reduce heterogeneity in the overall and subgroup analyses (Table 3). Begg’s and Egger’s tests were used to examine publication bias (Additional file 2). No significant publication bias was found in the results, except in the HRs for PFS. The trim-and-fill analysis indicated that there might be three unpublished or missing studies existing in the meta-analysis on PFS and the three studies might have an effect on the outcome, and the relationship of CTCs and PFS was still statistically significant if the three studies were published (HR = 2.069, 95% CI [1.505–2.842], p < 0.001). To explore the potential sources of heterogeneity, we conducted a meta-regression that considered the covariates of year, sample size, sampling time and detection method. In a univariate analysis, the explanatory variable that influenced the estimated HRs for PFS and OS was detection method (PFS: coefficient = 0.415, standard error = 0.104, p = 0.001; OS: coefficient = 0.341, standard error = 0.157, p = 0.046), while sampling time was also another potential source of heterogeneity (Additional file 3).
Discussion
In recent years, standard combined chemotherapy has been widely used for patients with operable or inoperable CRC, and the clinical prognosis of CRC patients has improved [3]. Unfortunately, chemotherapy does not always translate into survival benefits, and unnecessary toxicity can affect patient quality of life [38]. To date, there are no available predictive markers to resolve this problem. Recent studies have assessed whether CTCs can be used as a predictive marker for the response to chemotherapy, but the predictive role of CTCs is still controversial [28].
To our knowledge, this is the first meta-analysis to assess the prognostic and predictive value of CTCs in CRC patients treated with chemotherapy. Our meta-analysis indicated that CTCs detected at baseline and during chemotherapy was significantly associated with prognosis (PFS and OS) in CRC patients. Moreover, for the response to chemotherapy, CTCs detected during chemotherapy were significantly associated with response rate and disease control rate (p < 0.05), and CTCs detected at baseline trended towards an unfavorable response rate and disease control rate, although statistical significance was not reached. A sensitivity analysis, performed by omitting each study individually, confirmed the stability of our results. We also performed a pooled analysis to assess the clinical value of CTCs in mCRC patients only and in stage-II–III patients only, with similar results. These results were consistent with a previous meta-analysis, which suggested that CTC-high patients had unfavorable outcomes [39].
Increasing attention has been paid to the use of CTCs in predicting the efficacy of chemotherapy. Cristofanilli et al. reported that CTC levels could predict the efficacy of chemotherapy in patients with breast cancer earlier than traditional diagnostic methods (at 3–4 weeks versus 9–12 weeks, respectively) [10]. The time at which CTCs were first sampled (range: 1–5 weeks) was generally earlier than the time at which radiographic imaging was performed to assess tumor response (range: 6–25 weeks) in the included studies, when the authors assessed the correlation between CTCs and the response to chemotherapy.
Our results also suggested that the presence of CTCs before and during chemotherapy could be used as an early predictive marker of tumor response in CRC patients treated with chemotherapy. At 1–5 weeks after the initiation of chemotherapy, CTC-high patients had approximately 1.5 times the risk of radiographic PD than CTC-low patients. This could provide earlier opportunities for intervention or for the adjustment of chemotherapy by changing the chemotherapeutic regimen, intensity and/or period. Therefore, CTCs could be very valuable in resolving the problem in which an inefficient chemotherapy regimen is administered to a CRC patient for a prolonged period or a potentially effective chemotherapy regimen is interrupted prematurely.
However, we noted that the sensitivity and specificity of CTCs in predicting the tumor response determined by radiographic imaging were slightly limited. The most plausible explanation may be that the effects of targeted chemotherapy within a few CRC patients do not always translate into a change in tumor size. For example, angiogenesis inhibitors and antivascular therapies (i.e., bevacizumab and sorafenib) could cause necrosis and cavitation without tumor shrinkage [40, 41]. This was consistent with the imprecise correlation between the objective response and the survival outcome [42].
Indeed, several studies included in our analysis evaluated these targeted agents (i.e., bevacizumab or cetuximab) in CRC patients. Therefore, CTCs could provide additional relevant information on the efficacy of chemotherapy, especially in the context of the disadvantages of radiographic imaging. Limitations of CTC detection methods may also explain the limited sensitivity and specificity of CTCs in predicting the tumor response (i.e., moderate sensitivity of immunological techniques and low specificity of the RT-PCR technique). Therefore, the integration of CTC detection at baseline and during chemotherapy (or at different “during-chemotherapy” times) may more accurately predict the tumor response. Further studies are required to investigate ways to improve the accuracy of CTCs in predicting the response to chemotherapy and whether a combined analysis of CTCs and radiographic imaging will be more accurate in assessing the prognosis in CRC patients.
Interestingly, we analyzed the effect of CTCs as a predictive biomarker according to different sampling times (at baseline, 6–12 weeks, 6–12 weeks and 13–20 weeks after the initiation of chemotherapy) and the results suggested that the prognostic and predictive significance of CTCs was relevant to CTC sampling time. This finding concurred with the results of the meta-regression analysis suggesting that sampling time was an important factor. This may occur because of the interaction between CTCs and chemotherapeutic drugs. Furthermore, tumor proliferative activity and response to chemotherapeutic agents may also impact CTC status during chemotherapy [43]. However, the underlying reasons for the different effects of CTCs detected before and during chemotherapy were unclear and will need to be investigated in future studies. In the clinic, the relevance of CTCs as a predictive biomarker may have maximal value in a pre-treatment setting. Meanwhile, CTCs detected during chemotherapy may indicate that the drug given was not working and may not necessarily predict benefit from a higher dose (which would be toxic) or a different drug.
Recent studies have also demonstrated that fluctuations in CTC levels before and during chemotherapy were closely associated with the tumor response to chemotherapy and prognosis, and should be of value in individualizing chemotherapy [44]. Our meta-analysis indicated that there was a more pronounced association between the disease control rate/prognosis and changes in CTC levels than between the disease control rate/prognosis and CTC levels at baseline or during chemotherapy alone (Table 3).
This finding was supported by five of the included studies, which provided prognoses or responses to chemotherapy according to changes in CTC levels [11, 26, 27, 29, 32]. The main reason for this result may be that changes in CTC levels better reflect chemotherapeutic sensitivity and tumor proliferative activity [43]. This finding suggested that fluctuations in CTC levels compensated for the limited sensitivity and specificity of CTCs in predicting the tumor response. Therefore, our results indicated that “real-time” CTC levels detected at different times during chemotherapy, combined with radiographic imaging, should be helpful in guiding individual therapeutic decisions.
Heterogeneity was observed among the studies in our meta-analysis. In particular, the study by Matsusaka et al. [28] contributed substantial heterogeneity to our meta-analysis. A possible reason was that the patients included had better Eastern Cooperative Oncology Group (ECOG) performance scores (0–1 versus 0–2, respectively) and a younger median age than those in the other included studies. Excluding the study by Matsusaka et al. [28], heterogeneity could be decreased but not eliminated.
The meta-regression analysis showed that sampling time and detection method were sources of heterogeneity. Indeed, heterogeneity was reduced in the subgroup analysis based on sampling time and detection method, confirming the results of our meta-regression analysis (Table 3). Heterogeneity could have also been caused by differences in patient characteristics (i.e., age, sex and race), biological differences between colon cancer and rectal cancer, and differences in the chemotherapy strategies used. Furthermore, cytogenetically heterogeneous CTC populations could have contributed to the heterogeneity among the studies [45]. The studies included were all nonrandomized trials and cohort studies; therefore, differences in experimental design may have also given rise to heterogeneity.
Several limitations of this meta-analysis must be noted. First, our meta-analysis was not conclusive regarding when CTCs should be evaluated after the initiation of chemotherapy and what levels of CTCs would be useful for clinical prognostication. Second, as a retrospective study, our meta-analysis was based on published data from the studies included. Several studies did not provide HRs directly and we estimated them from the published data [11, 27, 28]. Third, although the meta-regression showed that sampling time and detection method were the sources of heterogeneity, heterogeneity could not be eliminated because of other factors mentioned above (i.e., patient characteristics, chemotherapy strategies and heterogeneous CTC populations). Therefore, the prognostic power of CTCs must be confirmed with large-scale multicenter studies in homogeneous patients. Fourth, we pooled the studies without separating them into their subcohorts of patients with colon cancer and patients with rectal cancer, because no study provided these data separately. Furthermore, most of the studies included did not comprehensively report patient status regarding surgery or neoadjuvant chemoradiotherapy, thus we could not conduct an in-depth subgroup analysis that adjusted for these confounding factors. The limited number of studies that were included in the subgroup and sensitivity analyses would have also influenced the statistical power of our results. Despite these limitations, our meta-analysis is the first study to assess the prognostic and predictive value of CTCs in CRC patients treated with chemotherapy.
Conclusions
Our meta-analysis indicates that CTCs could be useful as a surrogate marker for the response to chemotherapy providing additional prognostic information to tumor radiographic imaging. CTCs were also associated with the prognosis of CRC patients treated with chemotherapy. As a “liquid biopsy”, CTC detection is a less invasive way to evaluate the prognosis and response to chemotherapy of CRC patients than a biopsy of the primary tumor. Further high-quality, well-designed, large-scale multicenter studies are required to explore whether an individualized therapeutic decision based on CTC levels would improve the prognosis of CRC patients.
Abbreviations
- CIs:
-
Confidence intervals
- CR:
-
Complete response
- CRC:
-
Colorectal cancer
- CTCs:
-
Circulating tumor cells
- HR:
-
Hazard ratio
- mCRC:
-
Metastatic CRC
- NR:
-
Not reported
- OR:
-
Odds ratio
- OS:
-
Overall survival
- PB:
-
Peripheral blood
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- RECIST:
-
Response evaluation criteria in solid tumors
- ROC:
-
Receiver operating characteristic
- RR:
-
Relative risk
- SD:
-
Stable disease.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D: Global cancer statistics. CA Cancer J Clin. 2011, 61 (2): 69-90. 10.3322/caac.20107.
Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G, Loosveld OJ, van Bochove A, Sinnige HA, Creemers GJ, Tesselaar ME, Slee PH, Werter MJ, Mol L, Dalesio O, Punt CJ: Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 2007, 370 (9582): 135-142. 10.1016/S0140-6736(07)61086-1.
Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, Smith DB, Shepherd S, Maraveyas A, Ferry DR, Meade AM, Thompson L, Griffiths GO, Parmar MK, Stephens RJ: Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 2007, 370 (9582): 143-152. 10.1016/S0140-6736(07)61087-3.
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kröning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA: EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008, 26 (14): 2311-2319. 10.1200/JCO.2007.13.1193.
Zinser-Sierra JW, Rodriguez-Ramirez S, Villalobos-Valencia R, Ramirez-Marquez M: Use of bevacizumab in metastatic colorectal cancer: report from the Mexican opinion and analysis forum on colorectal cancer treatment with bevacizumab (September 2009). Drugs R&D. 2011, 11 (2): 101-111. 10.2165/11590440-000000000-00000.
Malapelle U, Carlomagno C, Salatiello M, De Stefano A, De Luca C, Bianco R, Marciano R, Cimminiello C, Bellevicine C, De Placido S, Troncone G: KRAS mutation detection by high-resolution melting analysis significantly predicts clinical benefit of cetuximab in metastatic colorectal cancer. Br J Cancer. 2012, 107 (4): 626-631. 10.1038/bjc.2012.275.
Vigano L, Capussotti L, De Rosa G, De Saussure WO, Mentha G, Rubbia-Brandt L: Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2013, 258 (5): 731-740. 10.1097/SLA.0b013e3182a6183e. discussion 741–732
Sorbye H, Dahl O: Carcinoembryonic antigen surge in metastatic colorectal cancer patients responding to oxaliplatin combination chemotherapy: implications for tumor marker monitoring and guidelines. J Clin Oncol. 2003, 21 (23): 4466-4467. 10.1200/JCO.2003.99.200.
Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC: Asco: ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006, 24 (33): 5313-5327. 10.1200/JCO.2006.08.2644.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF: Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004, 351 (8): 781-791. 10.1056/NEJMoa040766.
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ: Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008, 26 (19): 3213-3221. 10.1200/JCO.2007.15.8923.
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D: Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008, 14 (19): 6302-6309. 10.1158/1078-0432.CCR-08-0872.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J: New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009, 45 (2): 228-247. 10.1016/j.ejca.2008.10.026.
Stang A: Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010, 25 (9): 603-605. 10.1007/s10654-010-9491-z.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6 (7): e1000097-10.1371/journal.pmed.1000097.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR: Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007, 8: 16-10.1186/1745-6215-8-16.
Chu H, Cole SR: Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol. 2006, 59 (12): 1331-1332. 10.1016/j.jclinepi.2006.06.011. author reply 1332–1333
van Houwelingen HC, Arends LR, Stijnen T: Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002, 21 (4): 589-624. 10.1002/sim.1040.
Schmidt FL, Oh IS, Hayes TL: Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol. 2009, 62 (Pt 1): 97-128.
Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ. 2003, 327 (7414): 557-560. 10.1136/bmj.327.7414.557.
Schmid CH, Stark PC, Berlin JA, Landais P, Lau J: Meta-regression detected associations between heterogeneous treatment effects and study-level, but not patient-level, factors. J Clin Epidemiol. 2004, 57 (7): 683-697. 10.1016/j.jclinepi.2003.12.001.
Higgins J, Thompson S, Deeks J, Altman D: Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002, 7 (1): 51-61. 10.1258/1355819021927674.
Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997, 315 (7109): 629-634. 10.1136/bmj.315.7109.629.
Begg CB, Mazumdar M: Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994, 50 (4): 1088-1101. 10.2307/2533446.
Duval S, Tweedie R: Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000, 56 (2): 455-463. 10.1111/j.0006-341X.2000.00455.x.
Sastre J, Maestro ML, Gomez-Espana A, Rivera F, Valladares M, Massuti B, Benavides M, Gallen M, Marcuello E, Abad A, Arrivi A, Fernandez-Martos C, Gonzalez E, Tabernero JM, Vidaurreta M, Aranda E, Diaz-Rubio E: Circulating tumor cell count is a prognostic factor in metastatic colorectal cancer patients receiving first-line chemotherapy plus bevacizumab: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. Oncologist. 2012, 17 (7): 947-955. 10.1634/theoncologist.2012-0048.
de Albuquerque A, Kubisch I, Stolzel U, Ernst D, Boese-Landgraf J, Breier G, Stamminger G, Fersis N, Kaul S: Prognostic and predictive value of circulating tumor cell analysis in colorectal cancer patients. J Transl Med. 2012, 10: 222-10.1186/1479-5876-10-222.
Matsusaka S, Suenaga M, Mishima Y, Kuniyoshi R, Takagi K, Terui Y, Mizunuma N, Hatake K: Circulating tumor cells as a surrogate marker for determining response to chemotherapy in Japanese patients with metastatic colorectal cancer. Cancer Sci. 2011, 102 (6): 1188-1192. 10.1111/j.1349-7006.2011.01926.x.
Tol J, Koopman M, Miller MC, Tibbe A, Cats A, Creemers GJ, Vos AH, Nagtegaal ID, Terstappen LW, Punt CJ: Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol. 2010, 21 (5): 1006-1012. 10.1093/annonc/mdp463.
Barbazan J, Muinelo-Romay L, Vieito M, Candamio S, Diaz-Lopez A, Cano A, Gomez-Tato A, de Los Angeles Casares de Cal M, Abal M, Lopez-Lopez R: A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer. International journal of cancer Journal international du cancer. 2014, 135 (11): 2633-2643. 10.1002/ijc.28910.
Staritz P, Kienle P, Koch M, Benner A, von Knebel DM, Rudi J, Weitz J: Detection of disseminated tumour cells as a potential surrogate-marker for monitoring palliative chemotherapy in colorectal cancer patients. J Exp Clin Cancer Res. 2004, 23 (4): 633-639.
Yalcin S, Kilickap S, Portakal O, Arslan C, Hascelik G, Kutluk T: Determination of circulating tumor cells for detection of colorectal cancer progression or recurrence. Hepatogastroenterology. 2010, 57 (104): 1395-1398.
Konigsberg R, Gneist M, Jahn-Kuch D, Pfeiler G, Hager G, Hudec M, Dittrich C, Zeillinger R: Circulating tumor cells in metastatic colorectal cancer: efficacy and feasibility of different enrichment methods. Cancer Lett. 2010, 293 (1): 117-123. 10.1016/j.canlet.2010.01.003.
Kuboki Y, Matsusaka S, Minowa S, Shibata H, Suenaga M, Shinozaki E, Mizunuma N, Ueno M, Yamaguchi T, Hatake K: Circulating tumor cell (CTC) count and epithelial growth factor receptor expression on CTCs as biomarkers for cetuximab efficacy in advanced colorectal cancer. Anticancer Res. 2013, 33 (9): 3905-3910.
Iinuma H, Watanabe T, Mimori K, Adachi M, Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M, Mori M: Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol. 2011, 29 (12): 1547-1555. 10.1200/JCO.2010.30.5151.
Lu CY, Tsai HL, Uen YH, Hu HM, Chen CW, Cheng TL, Lin SR, Wang JY: Circulating tumor cells as a surrogate marker for determining clinical outcome to mFOLFOX chemotherapy in patients with stage III colon cancer. Br J Cancer. 2013, 108 (4): 791-797. 10.1038/bjc.2012.595.
Yen LC, Yeh YS, Chen CW, Wang HM, Tsai HL, Lu CY, Chang YT, Chu KS, Lin SR, Wang JY: Detection of KRAS oncogene in peripheral blood as a predictor of the response to cetuximab plus chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res. 2009, 15 (13): 4508-4513. 10.1158/1078-0432.CCR-08-3179.
Oye RK, Shapiro MF: Reporting results from chemotherapy trials. Does response make a difference in patient survival?. JAMA. 1984, 252 (19): 2722-2725. 10.1001/jama.1984.03350190024013.
Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Buchler MW, Koch M, Weitz J: Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010, 138 (5): 1714-1726. 10.1053/j.gastro.2010.01.008.
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM: Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007, 356 (2): 125-134. 10.1056/NEJMoa060655.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA: A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003, 349 (5): 427-434. 10.1056/NEJMoa021491.
Buyse M, Thirion P, Carlson RW, Burzykowski T, Molenberghs G, Piedbois P: Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: a meta-analysis. Meta-Analysis Group in Cancer. Lancet. 2000, 356 (9227): 373-378. 10.1016/S0140-6736(00)02528-9.
Molnar B, Sipos F, Galamb O, Tulassay Z: Molecular detection of circulating cancer cells. Role in diagnosis, prognosis and follow-up of colon cancer patients. Dig Dis. 2003, 21 (4): 320-325. 10.1159/000075355.
Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, Raghavan D, Heller G: Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10 (3): 233-239. 10.1016/S1470-2045(08)70340-1.
Steinert G, Scholch S, Niemietz T, Iwata N, Garcia SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, Rahbari NN, Buchler MW, Stoecklein NH, Weitz J, Koch M: Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 2014, 74 (6): 1694-1704. 10.1158/0008-5472.CAN-13-1885.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/976/prepub
Acknowledgements
We thank the department of Surgical Oncology of First Hospital of China Medical University and the College of China Medical University for technical assistance. This work was supported by National Science Foundation of China (No.81201888, 81372549 and No. 81172370), Specialized Research Fund for the Doctoral Program of Higher Education (No.20122104110009) and the Project of Science and Technology of Shenyang (F12-193-9-08).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Xuanzhang Huang, Peng Gao, Yongxi Song, Jingxu Sun, Xiaowan Chen, Junhua Zhao, Huimian Xu, Zhenning Wang declare that they have no competing interest.
Authors’ contributions
XH and PG contributed equally to this work. XH, PG, and ZW were responsible for conception and design of the study. XH and PG did the studies selection, data extraction, statistical analyses and the writing of report. YS, JS and XC participated in studies selection and data extraction and provided statistical expertise. JZ contributed to the literature search, studies selection, and figures. HX and ZW provided clinical expertise and interpretation of data. The report was drafted, revised, and approved by all investigators.
Xuanzhang Huang, Peng Gao contributed equally to this work.
Electronic supplementary material
12885_2014_5151_MOESM3_ESM.pdf
Additional file 3: Table S2: Results of meta-regression analysis exploring source of heterogeneity (univariate analysis). (PDF 60 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Huang, X., Gao, P., Song, Y. et al. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: a meta-analysis. BMC Cancer 14, 976 (2014). https://doi.org/10.1186/1471-2407-14-976
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-14-976