Abstract
Background
Preclinical and clinical studies have shown for decades that tumor cells demonstrate significantly enhanced sensitivity to “fever range” hyperthermia (increasing the intratumoral temperature to 42-45°C) than normal cells, although it is unknown why cancer cells exhibit this distinctive susceptibility.
Methods
To address this issue, mammary epithelial cells and three malignant breast cancer lines were subjected to hyperthermic shock and microarray, bioinformatics, and network analysis of the global transcription changes was subsequently performed.
Results
Bioinformatics analysis differentiated the gene expression patterns that distinguish the heat shock response of normal cells from malignant breast cancer cells, revealing that the gene expression profiles of mammary epithelial cells are completely distinct from malignant breast cancer lines following this treatment. Using gene network analysis, we identified altered expression of transcripts involved in mitotic regulators, histones, and non-protein coding RNAs as the significant processes that differed between the hyperthermic response of mammary epithelial cells and breast cancer cells. We confirmed our data via qPCR and flow cytometric analysis to demonstrate that hyperthermia specifically disrupts the expression of key mitotic regulators and G2/M phase progression in the breast cancer cells.
Conclusion
These data have identified molecular mechanisms by which breast cancer lines may exhibit enhanced susceptibility to hyperthermic shock.
Similar content being viewed by others
Background
Although the effectiveness of standard therapies such as surgery, chemotherapy, and irradiation has steadily improved over the years, cancer remains one of the most challenging problems of modern medicine. Among the major issues that complicate cancer treatment is the fact that cancerous cells are very difficult to therapeutically target with any specificity as they are in many respects similar to normal cells and have an astonishing ability of “hiding” their peculiarities. It has been known for over three decades that tumor cells demonstrate significantly more sensitivity to mild hyperthermia in “fever-range” temperatures (41-45°C) than normal cells [1, 2]. Mild hyperthermia has been shown in a wealth of preclinical oncology studies to act as a dose modifying agent that increases the therapeutic ratio of conventional therapy, thus enhancing the effectiveness of a given dose without additional toxicity [3]. Furthermore, numerous clinical trials have combined hyperthermia with radiation therapy and/or chemotherapy for many types of carcinomas (including breast cancers) and sarcomas, and most studies have shown a significant reduction in tumor volume when hyperthermia is combined with standard treatments [4–7]. Various hyperthermia techniques have been developed to treat breast cancer, including focused ultrasound [8], focused microwaves [9, 10], and radiofrequency electric fields [11]. Despite these techniques, various factors including tumor size and depth greatly affect the homogenous distribution of heat specific to and throughout the entire tumor mass. With the recent and rapid progression of nanobiotechnology applications in medicine, the development of magnetic nanoparticles which can induce tumor hyperthermia through hysteresis loss in an alternating magnetic field has renewed great interest in reexamining this adjuvant therapy in tumor treatments [12]. Further development of this technology may have the potential to overcome the previous limitations associated with older modalities of inducing hyperthermia and lead to reduced morbidity and mortality for patients.
Hyperthermia over a short period (generally 30 minutes to 1 hour) has been shown to induce irreversible cell damage and subsequent death in tumor cells, yet normal cells are remarkably spared [1, 2]. These effects are often very rapid, with tumor apoptosis and necrosis occurring within a short time (3–6 hrs) post heating [13]. Several mechanisms have been proposed as to how hyperthermia kills tumor cells including disruption of plasma membrane protein and cytoskeletal distribution, altering mitochondrial membrane potential and cellular redox status, disrupting cell cycle progression, inducing tumor hypoxia, and affecting DNA damage repair mechanisms in the nucleus [14–16], yet despite several decades of research the definitive identification of mechanisms leading to the favorable clinical results of hyperthermia have not been established. It has been further hypothesized that the strong anti-tumor effect of hyperthermia may be due to the low blood flow rate (and thus reduced dispersant cooling following heating) found in the center of tumors due to a disorganized and often dysfunctional vascular system. Additionally, several reports indicate that hyperthermia induces a strong immunological response via activation of immune cells and sensitization of tumor cells to immune effector cells [17–19]. Several studies have elucidated the heat shock induced changes in global gene expression of tumor cell lines such as squamous cell carcinoma, lymphoma, and glioma and have commonly identified gene networks involved in apoptosis, cell cycle, and cell structure/maintanence [20–22]. However, none of these studies compared the gene expression profiles to that of hyperthermia treated normal cells, thus it remains unknown how the hyperthermic response of cancer cells differs from that of normal cells. Identification of the unique hyperthermia-induced gene expression changes between normal and cancer cells may not only shed light on the selective disadvantage of solid tumors in response to mild increases in temperature, but could also identify signaling targets and biological processes which potentially could be exploited to sensitize tumors to chemotherapy and radiation.
To address this issue, we analyzed the hyperthermia-induced global gene expression profiles of a panel of breast cancer and mammary epithelial cell lines and used bioinformatics analysis to identify the unique gene networks distinct between the normal and cancer lines following this treatment. Furthermore, we confirmed our identified gene expression changes using qPCR and utilized flow cytometry to verify that these transcriptional alterations indeed reflect breast cancer specific responses to hyperthermia.
Methods
Cell culture and hyperthermia treatment
MCF10A (ATCC #CRL10318) mammary epithelial cells and MCF7 luminal breast cancer cells (ATCC #HTB-22), MDA-MB-231 Basal B breast cancer cells (ATCC # HTB-26), and MDA-MB-468 Basal A breast cancer cells (ATCC #HTB-132) were purchased from ATCC and grown in standard culture conditions as previously reported [23–25]. For heat shock, cells were split into two groups: 37°C control (C and C’ for mammary epithelial and breast cancer cells, respectively) and 45°C hyperthermic treatment (H and H’ for mammary epithelial and breast cancer cells, respectively). The 37°C control was grown under standard culture conditions. For the hyperthermia treatment, 45°C prewarmed conditioned media was immediately added to each treatment group and continuously maintained at this temperature for 30 minutes. After this time, the 45°C media was completely removed and replaced with 37°C conditioned media. The cells were then grown under standard culture conditions and harvested at the time point indicated for each experiment.
Microarray analysis
Total RNA was collected from each cell line (triplicate biological replicates) 4 hours after completion of the hyperthermia treatment. RNA was amplified and biotin-labeled using Illumina TotalPrep RNA Amplification Kit (Ambion). 750 ng of biotinylated aRNA was then briefly heat-denatured and loaded onto expression arrays to hybridize overnight (triplicate technical replicates). Following hybridization, arrays were labeled with Cy3-streptavidin and imaged on the Illumina ISCAN. Intensity values were transferred to GeneSpring GX microarray analysis software (Agilent) and data was filtered based on quality of each call. Statistical relevance was determined using ANOVA with a Benjamini Hochberg FDR multiple testing correction (p-value < 0.05). Data were then limited by fold change analysis to statistically relevant data points demonstrating a 2-fold or more change in expression. The microarray data from this experiment is publically available on the Gene Expression Omnibus (GEO Accession #GSE48398). All heatmaps shown represent the combined average of all biological and technical replicates.
Bioinformatics analysis of microarray data
Pathway analysis to identify gene networks and biological processes affected by the gene expression changes was performed using Metacore software (Thomson Reuters). Protein-protein interaction networks were determined using String 9.05 (http://string-db.org).
Quantitative real time PCR analysis
RNA was isolated from cells 4 hours after the hyperthermia treatment using the Ambion Purelink Minikit according to the manufacturer’s directions. The RNA collected was from an independent biological experiment separate from the RNA collected for the microarray to minimize the discovery of false positives. qRT-PCR was performed on an ABI7900HT RT-PCR system using TaqMan Assays with predesigned primer sets for the genes of interest (Invitrogen). All RT-PCR experiments were performed in at least triplicate.
Flow cytometry
Cells were harvested 24 hours post treatment via trypsinization and stained with propidium iodide as previous reported [26]. Cell cycle profiles were independently obtained using either a BD LSRII flow cytometer or an Accuri C6 flow cytometer. Flow cytometry data was analyzed using FlowJo software (Tree Star) or CFlow Plus software (Accuri).
Results
Determination of the global transcriptional response of mammary epithelial and breast cancer cells to fever range hyperthermia
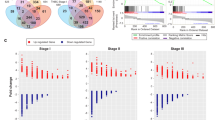
It remains to be determined how mild hyperthermia preferentially selects against breast cancer cells, yet largely spares normal tissue from collateral damage. To address this question, we first sought to elucidate how hyperthermia induces alterations in gene expression patterns in mammary epithelial and breast cancer cells. Mammary epithelial cells (MCF10A) and three malignant breast cancer lines from each of the known subtypes (MCF7 [luminal], MDA231 [Basal B], and MDA468 [Basal A]) were subjected to 30 minutes of fever range hyperthermic shock (or maintained at 37°C as a control) as described in the Materials and Methods section. To streamline identification of these treatment groups, cells grown at 37°C will be referred to as C and C’ (for mammary epithelial and breast cancer cells, respectively), while cells grown at 45°C will be referred to as H and H’ (for mammary epithelial and breast cancer cells, respectively). Total RNA was isolated 4 hours following hyperthermic treatment. We then performed microarray analysis of the global transcription changes using Illumina high density BeadArrays which measure the expression levels of more than 47,000 transcripts and known splice variants across the human transcriptome. Data was filtered based on quality of each call and statistical relevance was determined using ANOVA with a Benjamini Hochberg FDR multiple testing correction (p-value < 0.05). Data were then limited by fold change analysis to statistically relevant data points demonstrating a 2-fold or more change in expression. When comparing the expression changes based on the C vs H and C’ vs H’ analysis, we discovered that hyperthermia induced very dramatic changes in gene expression in all cell lines tested as reflected by 7252 two-fold or greater statistically significant gene expression changes (p < 0.05) occurring in at least one of the four cell lines (Figure 1A). Specifically, hyperthermia significantly altered the expression of 2670 genes in the MCF10A line (1810 genes upregulated and 860 genes downregulated), 442 genes in MCF7 (72 genes upregulated and 370 genes downregulated), 615 genes in MDA231 (244 genes upregulated and 371 downregulated), and 4458 genes in MDA468 (1744 genes upregulated and 2714 genes downregulated). A list of the top and bottom most regulated genes for each cell line can be found in Table 1. The complete gene expression dataset has been freely and publically deposited in Gene Expression Omnibus for ease of access and meta-analysis (GEO Accession #48398). These data suggest that mild hyperthermia induces large-scale alterations in gene expression profiles across normal and breast cancer cell lines.
Fever range hyperthermic shock induces large-scale changes in gene expression in breast cancer and mammary epithelial cells. (A) Heatmap depicting the 7252 two-fold or greater changes in gene expression (p < 0.05) occurring in the C vs H and C’ vs H’ comparisons. Hierarchical clustering based on cell lines shows the degree of similarity with respect to gene expression clustering for each indicated cell line (red?=?overexpressed, green?=?underexpressed). (B) Venn diagram illustrating common and unique 2-fold or greater gene expression changes (p < 0.05) between each of the cell lines in the C vs H and C’ vs H’ comparison. (C) Profile plot of the normalized intensity values for each two-fold or greater gene expression change (p < 0.05) showing relative expression for each cell line in the in the C vs H and C’ vs H’ comparison.
Hierarchical clustering of the gene expression changes based on each cell line indicates that the breast cancer lines responded to hyperthermia more similarly to each other than to the mammary epithelial line (Figure 1A). Using a Venn diagram that strictly eliminated any genes with less than a 2-fold expression change (p < 0.05), we compared the gene expression profiles that were shared and unique between each cell line in response to hyperthermia, revealing that while many gene expression changes were common between one or more of the breast cancer lines, not a single 2-fold or greater gene expression change was shared between the mammary epithelial line and all three breast cancer lines (Figure 1B). This data strongly suggested that the hyperthermic response of breast cancer cells is truly distinct from that of mammary epithelial cells. As an independent assessment, we generated profile plots depicting the changes in normalized intensity values between the four cell lines, revealing that many of the statistically significant gene expression alterations we identified were largely shared between the three breast cancer lines and distinctly unique from that of the MCF10A line (Figure 1C). Using Metacore network analysis of the microarray data, we identified key signaling pathways that were unique to the mammary epithelial line and the three breast cancer lines. The hyperthermic response of MFC10A was strongly indicative of statistically significant gene expression alterations in a large number of genes involved in cell cycle regulation, apoptosis, heat shock response, and DNA damage response, (Figure 2A-D, Table 2) and changes in the expression of genes involved in these biological pathways were not observed in the three breast cancer lines. Network analysis indicated that signaling pathways with the highest statistical significance amongst the three breast cancer lines responding to hyperthermia (but not in the MCF10A line) included genes involved in Ras and Rab5A G-protein regulation (GAPVD1, RASA1, RABEP1, CALM1, GMFB, PTPN11) (Figure 2E, Table 2) and survival/apoptosis (MAP2K4, BIRC2) (Figure 2B, Table 2).
Mammary epithelial cells respond to fever range hyperthermia through transcriptional alterations in gene networks unique from that of breast cancer cells. Hierarchical clustered heatmaps depicting the transcriptional expression changes for genes involved in cell cycle (A), apoptosis (B), heatshock (C), DNA damage (D), and Ran/Rab (E) regulation in the C vs H and C’ vs H’ comparison (red?=?overexpressed, green?=?underexpressed).
Identification of hyperthermia induced genes that differentiate the heat shock response of mammary epithelial cells from that of breast cancer cells
Our previous analysis compared the hyperthermic response of each individual cell line to its transcriptional expression baseline at the normal growth temperature (C vs H and C’ vs H’). Though this analysis provides us with information on how each individual cell line responds to hyperthermia relative to its normal growth temperature, it does not provide absolute comparisons of the transcriptome response of breast cancer cells relative to mammary epithelial cells following the elevated temperature. To better understand what provides breast cancer cells the selective disadvantage over mammary epithelial cells in response to hyperthermia we must identify those genes that are differentially expressed in breast cancer cell lines following hyperthermia from those of the mammary epithelial cell line following hyperthermia. To perform this analysis, we directly compared the gene expression changes that occurred for H’ vs H and identified genes whose expression was truly distinct between the breast cancer and mammary epithelial cell lines following hyperthermia.
When comparing H’ (MCF7) vs H we identified 2708 genes whose expression was distinct at statistically significant levels (≥2 fold, p < 0.05). H’ vs H comparisons of the MDA231 and MDA468 lines yielded 919 and 750 significant gene expression changes, respectively. Heatmap analysis indicated a strong trend in the gene expression profiles between each of the three breast cancer lines following hyperthermia (Figure 3A). Using a Venn diagram that strictly eliminated any genes with less than a 2-fold expression change (p < 0.05), we compared the gene expression alterations that were uniquely shared between all three cancer lines (H’) relative to the mammary epithelial line (H) (Figure 3B). This interpretation uncovered 393 genes whose 2-fold or greater changes in gene expression were differentially expressed in common amongst the three breast cancer lines following mild hyperthermic shock when compared to MCF10A cells following the same treatment (Table 3). These are the core genes that differentiate the hyperthermic response of breast cancer cells from that of mammary epithelial cells. In these data potentially lay the mechanism that may help define how mild hyperthermia preferentially selects against tumor cells.
Identification of genes that differentiate the transcriptional response of breast cancer cells following fever range hyperthermia. (A) Heatmap depicting the two-fold or greater changes in gene expression (p < 0.05) occurring in at least one of the three breast cancer cell lines (MCF7, MDA231, MDA468) relative to the mammary epithelial cells in the H’ vs H comparison (red?=?overexpressed, green?=?underexpressed). (B) Venn diagram illustrating common and unique 2-fold or greater gene expression changes (p < 0.05) between each of the breast cancer cell lines relative to the mammary epithelial cells in the H’ vs H comparison.
We performed computational analysis on the 393 genes using String software to identify interaction networks that might help reveal functional nodes indicative of the biological response of these cells to fever range hyperthermia. Our analysis uncovered a remarkably dense interaction node centered on genes involved in mitotic progression (Figure 4). We performed Metacore analysis on the list of 393 genes, confirming that mitotic cell cycle regulatory networks exclusively dominated the top statistically significant pathway maps (Table 4 lists the top 20 identified networks). Figure 5 illustrates Metacore’s analysis of the interrelationships of the identified mitotic regulatory genes including STAG2, NEK2, KPNA4, IPO5, TNPO1, CCNB1, CDK1, CDK6, NCAPG, NCAPG2, TOP2A, NUF2, CENPE, CENPF, ZWILCH, PDS5A, WEE1, KIF11, CHUK, and PPP1CB. Of the 393 genes that were differentially expressed between the breast cancer and mammary epithelial cells following H’ to H analysis, approximately 80% of the top 60 most upregulated genes were histone clusters and non-protein coding RNAs such as small nucleolar-, ribosomal-, and micro-RNAs. These data cumulatively suggest that the selective disadvantage that breast cancer lines experience following mild hyperthermic shock may be due to an inability to correctly regulate their core biological processes and mitotic cell cycle machinery. The differential expression of genes involved in these processes for the H’ vs H analysis is shown in Figure 6.
Interaction network analysis of the differential response of the breast cancer cells to fever range hyperthermia reveals a strong node centered on mitotic cell cycle progression. The list of 393 genes identified as differentially expressed in the breast cancer lines following fever range hyperthermia in the H’ vs H comparison were queried using String 9.05. Lines illustrate known physical and functional associations derived from previously reported genomic context, high-throughput experiments, coexpression analysis, and Pubmed.
Interrelationship between the mitotic regulators that differentiate the hyperthermic response of breast cancer cells from mammary epithelial cells. Metacore analysis of the 393 genes that differentiate the hyperthermic response of breast cancer from mammary epithelial cells in the H’ vs H comparison identified mitotic cell cycle progression (and 20 associated mitotic regulatory genes) as the primary differential gene networks. We used Metacore to identify the interrelationship of the known physical and functional associations between these 20 genes (red markers).
Gene expression changes in histones, non-protein coding RNAs, and mitotic regulators differentiate the hyperthermic response of breast cancer cells from mammary epithelial cells. Heatmap depicting the two-fold or greater changes in RNA expression levels (p < 0.05) for histone, non-protein coding RNA, and mitotic regulatory genes in the H’ vs H comparison (red?=?overexpressed, green?=?underexpressed).
Altered expression of mitotic arrest genes differentiates the hyperthermic response of breast cancer cells from that of mammary epithelial cells
Our microarray analysis strongly suggests that the inability of breast cancer cells to regulate their mitotic cell cycle machinery may be a major contributing factor to their selective disadvantage following hyperthermia. Therefore we independently tested the expression levels of a panel of mitotic regulators that were identified as differentially expressed in the H’ vs H analysis. Quantitative real time PCR analysis of cDNA collected from the H’ vs H treatments for the steady state mRNA levels of several genes with core processes related to mitosis including KIF11, CDK6, STAG2, NEK2, CHUK, KPNA4, CENPF, and NCAPG correlated well with our microarray data, revealing differential expression of these genes for each cell line in the hyperthermia treatment relative to the normal temperature (Figure 7A). A comparison of the qPCR and microarray data for each of these selected genes for the H’ to H comparisons is depicted in Table 5. To confirm the hyperthermia-induced mitotic defect in the breast cancer lines, we subjected all four cell lines to 30 minutes of fever range hyperthermia (H and H’) or normal control temperature (C and C’) and collected the cells after 24 hours for cell cycle analysis using flow cytometry. The cells were collected 24 hrs after treatment as this is sufficient time to see the phenotypic effects on the cell cycle that would be induced by altered RNA expression. Propidium iodide staining of cells from each condition clearly revealed that a G2/M phase accumulation as a common event across all three breast cancer lines following hyperthermia even after 24 hours following the treatment, but did not occur in the mammary epithelial lines (Figure 7B). Collectively, these data provide evidence to suggest that the selective disadvantage of breast cancer cells in response to hyperthermia could be due, in part, to altered regulation of mitotic machinery following heat shock.
Biological confirmation of gene expression analysis. (A) qPCR analysis measuring the fold change of mitotic regulators following hyperthermia treatment. RQ values are represented as the hyperthermia-induced change in gene expression for each gene relative to the expression of the same gene in the normal temperature condition (RQ?=?1). The data shown are the median of at least 3 replicates, plus or minus the standard deviation, and presented in log scale. (B) The panel of mammary epithelial and breast cancer cells were grown under standard growth conditions or treated with 30 minutes fever range hyperthermic shock. Cells were harvested after 24 hours and cell cycle analysis was performed using flow cytometric detection of propidium iodide intensity.
Discussion
While hyperthermic treatment of tumors has been utilized since the time of the ancient Greeks and modern medicine has implemented hyperthermia as an adjuvant treatment in various settings, use of this technique has been marred with limitations including the inability to target heat to the tumor without collateral damage to the neighboring cells, homogenous heat dispersion throughout the entire tumor, and intrinsic problems with targeting undetectable micrometastases. In recent years, advances in nanoparticle-enabled thermal therapy hold the promise to overcome many of these issues, thus a strong interest in treatment of tumors with hyperthermia has been renewed. While it has been established for decades that normal tissues exhibit enhanced thermotolerance relative to cancer cells [1, 2], the mechanisms controlling this are largely unknown. Studies on the heat shock response of cancer cells have revealed changes in apoptosis, cell cycle regulation, and cell structure/maintenance [3], yet very little has been reported critically comparing the heat shock responses of cancer cells to their non-diseased cellular counterparts. Thus it is currently unknown at the molecular level how thermotolerance is maintained in normal cells, but lost or deregulated in cancer cells. To address this, we utilized a genomics approach to address two areas: 1) identify the global transcriptional response to hyperthermia of a panel of breast cancer and mammary epithelial cells using a C vs H and C’ vs H’ analysis and 2) compare the hyperthermia-induced changes in global gene expression patterns of the breast cancer cell lines to the mammary epithelial cells using a H’ vs H analysis. As a result of these studies, we identified several gene networks that reflect the hyperthermic response of breast cancer and mammary epithelial cells (including cell cycle, heat shock, survival/apoptosis, DNA damage and Rab/Ran regulation) and that clearly differentiate the response of breast cancer cells from that of mammary epithelial cells (including mitotic regulation and expression of histone and non-protein coding RNAs).
Evaluation of the hyperthermic response of breast cancer and mammary epithelial cells
C vs H and C’ vs H’ comparative analysis of the gene expression profiles of each cell line revealed that the mammary epithelial cells responded to increased temperature distinctly from the breast cancer lines, with altered regulation of gene networks controlling DNA damage response, cell cycle progression, apoptosis, and heat shock characterizing the mammary epithelial cell response. In contrast, the three breast cancer lines commonly altered gene networks encoding Rab and Ran G-protein regulators in response to hyperthermia.
Arguably the most studied response of cells to hyperthermia is that of heat shock protein activation and expression and one might guess that heatshock-protein mediated responses are likely responsible for the selective disadvantage of solid tumors to fever range hyperthermia. Numerous cell stresses have been shown to induce heat shock proteins, which act as molecular chaperones inside cells to modulate thermotolerance and protect cells from stress-induced death [27–29]. MCF10A cells exhibited significantly increased expression of HSP90AA1, CARHSP1, HSPA12A and decreased expression of HSPB1, HSPBL2, HSPA6, and HSPA7 (C vs H), while the three breast cancer lines showed no significant 2-fold or greater alterations in the expression of these genes (C’ vs H’). Despite this finding, we provide evidence that suggests the ability of mammary epithelial cells to properly modulate their heat shock response does not contribute to the selective disadvantage of breast cancer cells to hyperthermia. For instance, comparison of heat shock protein expression in the H’ vs H analysis revealed no significant difference in the relative abundance of these heat shock protein genes regardless of the cell type. As elevated expression of heat shock proteins has been observed in various types of cancers [30–32], hyperthermic shock may simply bring the heat shock protein expression in MCF10A cells to the baseline levels in the breast cancer lines.
Hyperthermia has long been known as an effective radio- and chemo-sensitizing agent and it would be an attractive hypothesis that hyperthermia may impart a selective disadvantage to breast cancer cells via upregulation of DNA damage or reduction in its repair. Indeed, hyperthermia has been shown to induce chromosomal damage during S-phase [33] and inhibit homologous recombination repair via a heat shock protein/Brca1/2 pathway [34–36]. Furthermore, hyperthermia induces signaling pathways that overlap with those activated by ionizing radiation-induced DNA damage including histone H2Ax phosphorylation and enhanced ataxia-telangiectasia mutated protein (ATM) activity [37]. Analysis of C vs H and C’ vs H’ revealed a number of genes involved in DNA damage response whose expression was altered in the MCF10A cells, and similar changes were not observed in the three breast cancer lines. Despite this, no statistically significant changes in gene expression for these genes were observed in our H’ vs H comparison, suggesting that (similar to the heat shock proteins) this pathway may not clearly distinguish the selective disadvantage of breast cancer cells to hyperthermia.
Gene networks that distinguish the hyperthermic response of breast cancer cells from mammary epithelial cells
As our initial analysis compared only the heat shock response of each individual cell line relative to its transcriptional expression at normal growth temperature, we extended our analysis by directly comparing the transcriptional response of the H’ vs H treatments to identify the unique gene networks that clearly differentiate the gene expression changes unique to the breast cancer cells following heat treatment. This comparative analysis identified cell cycle networks preferentially involved in mitotic progression as well as large scale changes in the expression of histones and non-protein coding RNAs as the major distinctions between the hyperthermic responses between the breast cancer lines and the MCF10A cells. 80% of the top 60 genes commonly expressed at higher levels in the three breast cancer lines following heat shock relative to the mammary epithelial line following heat shock were histones and non-coding RNA. This effect was due primarily to decreased expression of these genes in the MCF10A cells with no change or only a small upregulation in expression in the breast cancer lines, suggesting that mammary epithelial cells are repressing many of their core processes (chromatin condensation, transcription, translation, etc.) following hyperthermic shock, while the breast cancer cells may continue performing these processes as normal. Similar findings have been reported following other cellular stresses whereby oxidative damage significantly decreases the expression of histones and ribosomal proteins [38]. In addition to histone gene expression, heat shock induces an array of chromatin post-translational modifications. For instance, HSP70 has been shown to enhance the phosphorylation of histone H3 following heat shock [39], and histone variant H3.3 has been shown to stimulate heat shock induced HSP70 transcription [40], suggesting that heat shock response and histone activity are tightly regulated.
Our data revealed that a number of small nucleolar RNAs, which play key roles in ribosomal biosynthesis, were differentially regulated between the mammary epithelial and breast cancer cells following hyperthermia. Several small nucleolar RNAs are reportedly critical mediators of oxidative stress and their overexpression has been associated with reduced resistance to oxidative stress [41, 42]. Small nucleolar RNAs have been shown to bind to the mature RNA of heat shock cognate protein (HSC70) [43] and inhibition of Hsp90 prevents the accumulation of U3 and U4 small nuclear ribonucleoproteins via a process that involves Pih1/Nop17 and R2Tp complexes [44–46].
Changes in cell cycle progression (particularly mitotic catastrophe) have been repeatedly shown to characterize the hyperthermic response of numerous cell types [47–49], though it is largely unstudied as to how cell cycle changes differ between normal and tumor cell lines following this treatment. Major distinctions in cell cycle networks involved in mitotic progression clearly distinguished the H’ vs H analysis of our data. The genes that were differentially expressed at statistically significant levels included those involved in spindle assembly and chromosome separation, chromosome condensation in prometaphase, metaphase checkpoint, sister chromatid cohesion, and initiation of mitosis. Our confirmatory experiments using flow cytometry further revealed that hyperthermia treated breast cancer cells stalled in the G2/M phase of the cell cycle within 24 hours post-treatment, while the cell cycle profiles of heat-shocked mammary epithelial cells were similar to those grown at normal temperatures. Interestingly, it has been shown that cells vary in their susceptibility to heat in accordance to their phase in the cell cycle, with the highest heat sensitivity observed during mitosis due to damage to the mitotic apparatus, leading to inefficient mitosis and polyploidy. M- and S-phase arrested cells show increased susceptibility to heat-induced damage, while G1-phase cells are relatively heat resistance [50–53].
This study has been the first to shed light on the comparisons of transcriptome-level fever range hyperthermic responses of mammary epithelial cells to breast cancer cells. While this data points to a number of areas that potentially contribute to the selective advantage of normal breast epithelium over its malignant counterparts following hyperthermia, our studies were simplistic in that they utilized a cell culture monolayer system solely consisting of cells derived from normal or tumor breast tissue. We have gained solid insight into the responses of these particular cell types to fever range hyperthermia, however a tumor is a very complex entity. For instance, solid tumors are not only composed of the tumor cells, but also consist of endothelial, fibroblast, and immune cells which will each respond to hyperthermia in their own fashion and potentially affect the response of the tumor as a whole. Moreover, heterogenous heat distribution and dissipation due to a faulty tumor vascular system may induce uneven heating in the tumor itself, thus affecting some areas distinctly and differentially altering the tumor’s response to hyperthermia. Future studies should be undertaken to address these issues.
Conclusion
Collectively, our data suggest that fever range hyperthermia affects breast cancer cells distinctly from mammary epithelial cells. These differences are largely attributed to alterations in the expression of genes involved in mitotic cell cycle progression, histones, and non-coding RNAs. Considering the hyperthermia induced G2/M cell cycle defects observed in the breast cancer cells but not the mammary epithelial cells, these data pose the question as to whether hyperthermia may function in a synergistic manner when combined with drugs that specifically target mitosis such as taxols and vinca alkaloid derivatives.
Abbreviations
- C:
-
37°C treatment of mammary epithelial cells
- C’:
-
37°C of breast cancer cells
- H:
-
45°C of mammary epithelial cells
- H’:
-
45°C of breast cancer cells
- RNA:
-
Ribonucleic acid
- DNA:
-
Deoxyribonucleic acid
- aRNA:
-
Amplified RNA
- cDNA:
-
Complementary DNA
- mRNA:
-
Messenger RNA
- G1:
-
Gap 1
- S:
-
Synthesis
- G2:
-
Gap 2
- M:
-
Mitosis
- RT PCR:
-
Real time polymerase chain reaction.
References
Habash RW, Bansal R, Krewski D, Alhafid HT: Thermal therapy, part 2: hyperthermia techniques. Crit Rev Biomed Eng. 2006, 34 (6): 491-542. 10.1615/CritRevBiomedEng.v34.i6.30.
Habash RW, Bansal R, Krewski D, Alhafid HT: Thermal therapy, part 1: an introduction to thermal therapy. Crit Rev Biomed Eng. 2006, 34 (6): 459-489. 10.1615/CritRevBiomedEng.v34.i6.20.
Horsman MR, Overgaard J: Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol). 2007, 19 (6): 418-426. 10.1016/j.clon.2007.03.015.
van der Zee J: Heating the patient: a promising approach?. Ann Oncol. 2002, 13 (8): 1173-1184. 10.1093/annonc/mdf280.
Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM: Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3 (8): 487-497. 10.1016/S1470-2045(02)00818-5.
Falk MH, Issels RD: Hyperthermia in oncology. Int J Hyperthermia. 2001, 17 (1): 1-18. 10.1080/02656730150201552.
Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW: Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005, 23 (13): 3079-3085. 10.1200/JCO.2005.05.520.
Moroz P, Jones SK, Gray BN: Magnetically mediated hyperthermia: current status and future directions. Int J Hyperthermia. 2002, 18 (4): 267-284. 10.1080/02656730110108785.
Hand JW, Vernon CC, Prior MV: Early experience of a commercial scanned focused ultrasound hyperthermia system. Int J Hyperthermia. 1992, 8 (5): 587-607. 10.3109/02656739209037995.
Gardner RA, Vargas HI, Block JB, Vogel CL, Fenn AJ, Kuehl GV, Doval M: Focused microwave phased array thermotherapy for primary breast cancer. Ann Surg Oncol. 2002, 9 (4): 326-332. 10.1007/BF02573866.
Abe M, Hiraoka M, Takahashi M, Egawa S, Matsuda C, Onoyama Y, Morita K, Kakehi M, Sugahara T: Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer. 1986, 58 (8): 1589-1595. 10.1002/1097-0142(19861015)58:8<1589::AID-CNCR2820580802>3.0.CO;2-B.
Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, Kobayashi T: Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J Biosci Bioeng. 2003, 96 (4): 364-369.
Moroi J, Kashiwagi S, Kim S, Urakawa M, Ito H, Yamaguchi K: Regional differences in apoptosis in murine gliosarcoma (T9) induced by mild hyperthermia. Int J Hyperthermia. 1996, 12 (3): 345-354. 10.3109/02656739609022523.
Roti Roti JL: Cellular responses to hyperthermia (40–46 degrees C): cell killing and molecular events. Int J Hyperthermia. 2008, 24 (1): 3-15. 10.1080/02656730701769841.
Kampinga HH, Dynlacht JR, Dikomey E: Mechanism of radiosensitization by hyperthermia (> or?=?43 degrees C) as derived from studies with DNA repair defective mutant cell lines. Int J Hyperthermia. 2004, 20 (2): 131-139. 10.1080/02656730310001627713.
Koutcher JA, Barnett D, Kornblith AB, Cowburn D, Brady TJ, Gerweck LE: Relationship of changes in pH and energy status to hypoxic cell fraction and hyperthermia sensitivity. Int J Radiat Oncol Biol Phys. 1990, 18 (6): 1429-1435. 10.1016/0360-3016(90)90318-E.
Atanackovic D, Nierhaus A, Neumeier M, Hossfeld DK, Hegewisch-Becker S: 41.8 degrees C whole body hyperthermia as an adjunct to chemotherapy induces prolonged T cell activation in patients with various malignant diseases. Cancer Immunol Immunother. 2002, 51 (11–12): 603-613.
Multhoff G: Heat shock protein 72 (HSP72), a hyperthermia-inducible immunogenic determinant on leukemic K562 and Ewing's sarcoma cells. Int J Hyperthermia. 1997, 13 (1): 39-48. 10.3109/02656739709056428.
Milani V, Frankenberger B, Heinz O, Brandl A, Ruhland S, Issels RD, Noessner E: Melanoma-associated antigen tyrosinase but not Melan-A/MART-1 expression and presentation dissociate during the heat shock response. Int Immunol. 2005, 17 (3): 257-268. 10.1093/intimm/dxh203.
Tabuchi Y, Wada S, Furusawa Y, Ohtsuka K, Kondo T: Gene networks related to the cell death elicited by hyperthermia in human oral squamous cell carcinoma HSC-3 cells. Int J Mol Med. 2012, 29 (3): 380-386.
Furusawa Y, Tabuchi Y, Wada S, Takasaki I, Ohtsuka K, Kondo T: Identification of biological functions and gene networks regulated by heat stress in U937 human lymphoma cells. Int J Mol Med. 2011, 28 (2): 143-151.
Tabuchi Y, Takasaki I, Wada S, Zhao QL, Hori T, Nomura T, Ohtsuka K, Kondo T: Genes and genetic networks responsive to mild hyperthermia in human lymphoma U937 cells. Int J Hyperthermia. 2008, 24 (8): 613-622. 10.1080/02656730802140777.
Boopalan T, Arumugam A, Damodaran C, Rajkumar L: The anticancer effect of 2'-3'-dehydrosalannol on triple-negative breast cancer cells. Anticancer Res. 2012, 32 (7): 2801-2806.
Mitchell DC, Abdelrahim M, Weng J, Stafford LJ, Safe S, Bar-Eli M, Liu M: Regulation of KiSS-1 metastasis suppressor gene expression in breast cancer cells by direct interaction of transcription factors activator protein-2alpha and specificity protein-1. J Biol Chem. 2006, 281 (1): 51-58. 10.1074/jbc.M506245200.
Meng L, Hunt C, Yaglom JA, Gabai VL, Sherman MY: Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene. 2011, 30 (25): 2836-2845. 10.1038/onc.2011.5.
Stiles JM, Amaya C, Rains S, Diaz D, Pham R, Battiste J, Modiano JF, Kokta V, Boucheron LE, Mitchell DC, et al: Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013, 8 (3): e60021-10.1371/journal.pone.0060021.
Ito A, Honda H, Kobayashi T: Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunol Immunother. 2006, 55 (3): 320-328. 10.1007/s00262-005-0049-y.
Samali A, Cotter TG: Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996, 223 (1): 163-170. 10.1006/excr.1996.0070.
Beere HM: “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004, 117 (Pt 13): 2641-2651.
Ciocca DR, Calderwood SK: Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005, 10 (2): 86-103. 10.1379/CSC-99r.1.
Gyrd-Hansen M, Nylandsted J, Jaattela M: Heat shock protein 70 promotes cancer cell viability by safeguarding lysosomal integrity. Cell Cycle. 2004, 3 (12): 1484-1485. 10.4161/cc.3.12.1287.
Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR: Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1998, 79 (5): 468-475. 10.1002/(SICI)1097-0215(19981023)79:5<468::AID-IJC4>3.0.CO;2-Z.
Mackey MA, Morgan WF, Dewey WC: Nuclear fragmentation and premature chromosome condensation induced by heat shock in S-phase Chinese hamster ovary cells. Cancer Res. 1988, 48 (22): 6478-6483.
Genet SC, Fujii Y, Maeda J, Kaneko M, Genet MD, Miyagawa K, Kato TA: Hyperthermia inhibits homologous recombination repair and sensitizes cells to ionizing radiation in a time and temperature dependent manner. J Cell Physio. 2012, 228 (7): 1473-1481.
Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, Zelensky A, van Bree C, Stalpers LJ, Buist MR, et al: Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci U S A. 2011, 108 (24): 9851-9856. 10.1073/pnas.1101053108.
Eppink B, Krawczyk PM, Stap J, Kanaar R: Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int J Hyperthermia. 2012, 28 (6): 509-517. 10.3109/02656736.2012.695427.
Hunt CR, Pandita RK, Laszlo A, Higashikubo R, Agarwal M, Kitamura T, Gupta A, Rief N, Horikoshi N, Baskaran R, et al: Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res. 2007, 67 (7): 3010-3017. 10.1158/0008-5472.CAN-06-4328.
Shin H, Lee H, Fejes AP, Baillie DL, Koo HS, Jones SJ: Gene expression profiling of oxidative stress response of C. elegans aging defective AMPK mutants using massively parallel transcriptome sequencing. BMC Res Notes. 2011, 4: 34-10.1186/1756-0500-4-34.
Gupta A, Cooper ZA, Tulapurkar ME, Potla R, Maity T, Hasday JD, Singh IS: Toll-like receptor agonists and febrile range hyperthermia synergize to induce heat shock protein 70 expression and extracellular release. J Biol Chem. 2013, 288 (4): 2756-2766. 10.1074/jbc.M112.427336.
Kim H, Heo K, Choi J, Kim K, An W: Histone variant H3.3 stimulates HSP70 transcription through cooperation with HP1gamma. Nucleic Acids Res. 2011, 39 (19): 8329-8341. 10.1093/nar/gkr529.
Michel CI, Holley CL, Scruggs BS, Sidhu R, Brookheart RT, Listenberger LL, Behlke MA, Ory DS, Schaffer JE: Small nucleolar RNAs U32a, U33, and U35a are critical mediators of metabolic stress. Cell Metab. 2011, 14 (1): 33-44. 10.1016/j.cmet.2011.04.009.
Cohen E, Avrahami D, Frid K, Canello T, Levy Lahad E, Zeligson S, Perlberg S, Chapman J, Cohen OS, Kahana E, et al: Snord 3A: a molecular marker and modulator of prion disease progression. PLoS One. 2013, 8 (1): e54433-10.1371/journal.pone.0054433.
Stepanov GA, Semenov DV, Kuligina EV, Koval OA, Rabinov IV, Kit YY, Richter VA: Analogues of Artificial Human Box C/D Small Nucleolar RNA As Regulators of Alternative Splicing of a pre-mRNA Target. Acta Nat. 2012, 4 (1): 32-41.
Zhao R, Kakihara Y, Gribun A, Huen J, Yang G, Khanna M, Costanzo M, Brost RL, Boone C, Hughes TR, et al: Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP complex activity that regulates snoRNA accumulation. J Cell Biol. 2008, 180 (3): 563-578. 10.1083/jcb.200709061.
Boulon S, Marmier-Gourrier N, Pradet-Balade B, Wurth L, Verheggen C, Jady BE, Rothe B, Pescia C, Robert MC, Kiss T, et al: The Hsp90 chaperone controls the biogenesis of L7Ae RNPs through conserved machinery. J Cell Biol. 2008, 180 (3): 579-595. 10.1083/jcb.200708110.
Eckert K, Saliou JM, Monlezun L, Vigouroux A, Atmane N, Caillat C, Quevillon-Cheruel S, Madiona K, Nicaise M, Lazereg S, et al: The Pih1-Tah1 cochaperone complex inhibits Hsp90 molecular chaperone ATPase activity. J Biol Chem. 2010, 285 (41): 31304-31312. 10.1074/jbc.M110.138263.
Roti Roti JL: Heat-induced alterations of nuclear protein associations and their effects on DNA repair and replication. Int J Hyperthermia. 2007, 23 (1): 3-15. 10.1080/02656730601091759.
Mackey MA, Ianzini F: Enhancement of radiation-induced mitotic catastrophe by moderate hyperthermia. Int J Radiat Biol. 2000, 76 (2): 273-280. 10.1080/095530000138925.
Debec A, Marcaillou C: Structural alterations of the mitotic apparatus induced by the heat shock response in Drosophila cells. Biol Cell. 1997, 89 (1): 67-78. 10.1016/S0248-4900(99)80082-3.
Westra A, Dewey WC: Variation in sensitivity to heat shock during the cell-cycle of Chinese hamster cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1971, 19 (5): 467-477. 10.1080/09553007114550601.
Dewey WC: Failla memorial lecture. The search for critical cellular targets damaged by heat. Radiat Res. 1989, 120 (2): 191-204. 10.2307/3577707.
Coss RA, Dewey WC, Bamburg JR: Effects of hyperthermia on dividing Chinese hamster ovary cells and on microtubules in vitro. Cancer Res. 1982, 42 (3): 1059-1071.
Vidair CA, Dewey WC: Two distinct modes of hyperthermic cell death. Radiat Res. 1988, 116 (1): 157-171. 10.2307/3577486.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/81/prepub
Acknowledgements
This research was supported by startup funding to BB from TTUHSC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CA contributed to experimental concept and design, cultured and treated cells, purified RNA, performed analysis and interpretation of microarray data, carried out network analysis, drafted and revised manuscript, gave final approval for version to be published. VK contributed to interpretation of network analysis, assisted with drafting and revising manuscript, gave final approval for version to be published. JS cultured and treated cells, purified RNA, drafting and revising manuscript, gave final approval for version to be published. AN performed flow cytometric cell cycle analysis and interpretation of data, gave final approval for version to be published. AA cultured cells, gave final approval for version to be published. RL contributed to experimental concept and design, drafted and revised manuscript, gave final approval for version to be published. CB contributed to concept and design, drafted and revised manuscript, gave final approval for version to be published. DM performed microarray experiments, performed bioinformatics analysis and interpretation of data, drafted and revised manuscript, gave final approval for version to be published. BB contributed to concept and design, acquisition of data, analysis and interpretation of data, drafted and revised manuscript, gave final approval for version to be published.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Amaya, C., Kurisetty, V., Stiles, J. et al. A genomics approach to identify susceptibilities of breast cancer cells to “fever-range” hyperthermia. BMC Cancer 14, 81 (2014). https://doi.org/10.1186/1471-2407-14-81
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-14-81