Abstract
Background
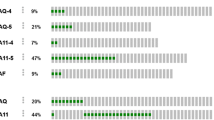
Mucosal melanomas represent about 1% of all melanoma cases and classically have a worse prognosis than cutaneous melanomas. Due to the rarity of mucosal melanomas, only limited clinical studies with metastatic mucosal melanoma are available. Mucosal melanomas most commonly contain mutations in the gene CKIT, and treatments have been investigated using targeted therapy for this gene. Mutations in mucosal melanoma are less common than in cutaneous or uveal melanomas and occur in descending order of frequency as: CKIT (20%), NRAS (5%) or BRAF (3%). Mutations in G-alpha proteins, which are associated with activation of the mitogen-activated protein kinase pathway, have not been reported in mucosal melanomas. These G-alpha protein mutations occur in the genes GNAQ and GNA11 and are seen at a high frequency in uveal melanomas, those melanomas that begin in the eye.
Case presentation
A 59-year old Caucasian male was diagnosed with a mucosal melanoma after evaluation for what was thought to be a hemorrhoid. Molecular analysis of the tumor revealed a GNAQ mutation. Ophthalmologic exam did not disclose a uveal melanoma.
Conclusion
Here we report, to our knowledge, the first known case of GNAQ mutation in a patient with metastatic mucosal melanoma.
Similar content being viewed by others
Background
The incidence and the mortality of melanoma have increased over the last several decades with more than 76,000 estimated new cases and more than 9,000 estimated deaths in the USA in 2013 [1]. Although most of melanoma is cutaneous in origin, it can arise from extra-cutaneous sites such as the uveal tract and mucosal surfaces where melanocytes exist. The mucosal origin melanomas which mostly arise from the mucosal membrane of the head and neck, the anorectal mucosa and the vulvovaginal mucosa have distinct biologic and clinical features compared with cutaneous melanomas. Mucosal melanomas are rare but very aggressive. The rate of mucosal melanoma is 2.2 cases per million per year, and the five-year survival is a mere 25% compared to over 200 cases per million and over 80% five-year survival for cutaneous melanoma [2, 3]. The poor prognosis is likely due to the obscured anatomic sites and the rich lymphovascular supply of the mucosa [2]. Although the discovery of BRAF gene mutation and the advancement of immunotherapy in melanoma have led to the development of highly effective targeted therapy such as vemurafenib, dabrafenib, and trametinib and durable immunotherapy such as interleukin-2 and ipilimumab, the efficacy of these treatments in metastatic mucosal melanoma is not clear due to limited number of these patients included in clinical trials. Recently, several clinical trials reported promising results with targeting of CKIT mutation in mucosal melanoma [4–6]. CKIT mutations are reported in 21% of mucosal melanoma, and only patients with mucosal melanoma harboring a special subset of CKIT mutations such as L576P and K642E in exon 11 and 13 may have a clinical benefit from c-KIT inhibitors [7]. The role of amplification of CKIT and response to c-KIT inhibitors has also been studied [6, 8]. Despite these advances, further workup is necessary to define the standard of care for mucosal melanoma.
GNAQ and GNA11 are alpha subunits of heterotrimeric G proteins, which couple seven transmembrane domain receptors to intracellular signaling pathways [9]. Mutations in the genes GNAQ and GNA11 are critical for development and progression of uveal melanoma and are associated with activation of the mitogen-activated protein kinase (MAPK) pathway [10, 11]. This same pathway is activated by oncogenic BRAF mutations in cutaneous melanoma [12]. Approximately, 80% of primary uveal melanomas have GNAQ or GNA11 mutations. However, GNAQ or GNA11 mutations have not been reported in mucosal melanoma. Here, we present a patient with metastatic mucosal melanoma harboring a classic GNAQ mutation.
Case presentation
A 59-year-old otherwise healthy Caucasian man was diagnosed with a mucosal melanoma during hemorrhoid evaluation in August of 2009. Histopathological examination revealed a polypoid tumor occupying lamina propria and submucosa of the anal canal with intraepithelial lentiginous component in the center of the lesion. The tumor cells were epithelioid and showed clear cell change. Immunohistochemical studies showed the tumor cells to be positive for S100 and Melan-A. A diagnosis of a 15-mm thick mucosal melanoma with ulceration, 6 mitotic figures per mm2 and perineural invasion in the anal canal was made (Figures 1, 2 and 3). Molecular analysis showed the melanoma harbored a GNAQ mutation with wild-type BRAF, KIT and NRAS genes. The GNAQ gene mutation of the patient was the substitution of glutamine to proline in codon 209 (Q209P) which has been reported in uveal melanoma at a frequency of 20.8% but not in cutaneous melanoma or other subtypes of the disease [10, 13].
The patient underwent a wide local excision of the primary melanoma with subsequent adjuvant radiation therapy. He was without relapse until January of 2010, when he had locally recurrent disease for which he underwent another wide local excision. He again remained free of disease until July of 2010 when he was found to have metastatic lesions in the perinephric lymph nodes, the liver, and lung, for which he received 2 doses of ipilimumab (3 mg/kg intravenous Day 1) and temozolomide (200 mg/m2 by mouth Days 1–4) [14] with further disease progression and new metastatic lesions in hilar and mediastinal lymph nodes and in the right adrenal gland. Subsequently, he received two cycles of the combination of carboplatin, paclitaxel and bevacizumab before he had further disease progression in January of 2011. He started imatinib at 400 mg twice a day in February of 2011. Due to further disease progression with imatinib, ipilimumab (3 mg/kg) was re-introduced in April of 2011 and he completed 4 cycles of ipilimumab. However, his disease progressed further with multiple metastatic lesions and he expired in August of 2011.
Conclusions
GNAQ and GNA11 mutations, which are potential drivers of MAPK activation, have been reported in blue nevi and in up to 85% of cases of uveal melanoma [10, 11, 15]. Mutations occur in a mutually exclusive fashion and affect codons 209 (95%) and 183 (5%) in both genes. Although GNAQ and GNA11 mutations have been reported in a cutaneous melanoma case and a cell line from cutaneous melanoma [10, 16], there are no data to demonstrate GNAQ or GNA11 mutations in mucosal melanoma. It is possible that our patient may have had an undetected or spontaneously regressed primary uveal melanoma since his melanoma harbored the GNAQ mutation and metastasized to liver which is the most common metastatic site of uveal melanoma [17]. However, spontaneous regression of primary uveal melanoma is extremely rare in comparison to cutaneous melanoma [18]; furthermore, anal canal is not a common metastatic site as a single and the first metastatic lesion of uveal melanoma [17], and our patient did not have any evidence of uveal melanoma in multiple magnetic resonance image (MRI) of the brain. In addition, he had had frequent ophthalmology exams with his retina specialist due to multiple retinal detachments 3 years before he was diagnosed with melanoma. These follow-ups included slit-lamp examinations. Another possible explanation is that our patient might have an undetected or regressed primary cutaneous melanoma, since GNAQ mutation has been reported in one case of cutaneous melanoma [9]. However, it is less likely since our patient did not have any suspected cutaneous lesions during multiple thorough and detailed clinical examinations, and the predominant metastatic site of melanoma in the gastrointestinal tract is not the anal canal but the small bowel [19]. The melanoma seen in the anal canal, although predominantly involving lamina propria and submucosa, was interpreted to be most likely primary due to presence of intraepidermal (in situ) melanoma showing lentiginous pattern of growth (as mucosal melanoma frequently does), along with the lack of clinical evidence of tumor elsewhere at the time of diagnosis. Since GNAQ mutations are potential drivers of MAPK activation similar to oncogenic BRAF, and a recent clinical study demonstrated a significant clinical benefit of selumetinib (a selective MEK inhibitor) in metastatic uveal melanoma with GNAQ or GNA11 mutations [20], our patient might have achieved clinical response with selumetinib. Unfortunately, he did not receive a MEK inhibitor as part of his treatment course.
To our knowledge, this is the first case report to demonstrate mucosal melanoma harboring a GNAQ mutation. This case suggests that molecular profiling may give us better understanding of genetic changes in mucosal melanoma and may afford actionable targets for therapy.
Consent
Written informed consent was obtained from the patient’s next of kin for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
References
Biermann JS, Adkins DR, Agulnik M, Benjamin RS, Brigman B, Butrynski JE, Cheong D, Chow W, Curry WT, Frassica DA, Frassica FJ, Hande KR, Hornicek FJ, Jones RL, Mayerson J, McGarry SV, McGrath B, Morris CD, O’donnell RJ, Randall LR, Santana VM, Satcher RL, Siegel HJ, Von Mehren M, Nergman MA, Sundar H: Bone cancer. JNCCN. 2013, 11 (6): 688-723.
Chang AE, Karnell LH, Menck HR: The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998, 83 (8): 1664-1678.
McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW: Incidence of noncutaneous melanomas in the U.S. Cancer. 2005, 103 (5): 1000-1007.
Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonzalez R, Weber JS, Gajewski TF, O’day SJ, Kim KB, Lawrence D, Flaherty KT, Luke JJ, Collichio FA, Ernstoff MS, Heinrich MC, Beadling C, Zukotynski KA, Yap JT, Van den Abbeele AD, Demetri GD, Fisher DE: Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013, 31 (26): 3182-3190.
Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, Panageas KS, Busam KJ, Chmielowski B, Lutzky J, Pavlick AC, Fusco A, Cane L, Takebe N, Vemula S, Bouvier N, Bastian BC, Schwartz GK: KIT as a therapeutic target in metastatic melanoma. JAMA. 2011, 305 (22): 2327-2334.
Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, Corless CL, Li L, Li H, Sheng X, Cui C, Chi Z, Li S, Han M, Mao L, Lin X, Du N, Zhang X, Li J, Wang B, Qin S: Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011, 29 (21): 2904-2909.
Woodman SE, Davies MA: Targeting KIT in melanoma: a paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol. 2010, 80 (5): 568-574.
Kluger HM, Dudek AZ, McCann C, Ritacco J, Southard N, Jilaveanu LB, Molinaro A, Sznol M: A phase 2 trial of dasatinib in advanced melanoma. Cancer. 2011, 117 (10): 2202-2208.
Neves SR, Ram PT, Iyengar R: G protein pathways. Science. 2002, 296 (5573): 1636-1639.
Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, Simpson EM, Barsh GS, Bastian BC: Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009, 457 (7229): 599-602.
Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, Sozen MM, Baimukanova G, Roy R, Heguy A, Dolgalev I, Khanin R, Busam K, Speicher MR, O'Brien J, Bastian BC: Mutations in GNA11 in uveal melanoma. New Engl J Med. 2010, 363 (23): 2191-2199.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, et al: Mutations of the BRAF gene in human cancer. Nature. 2002, 417 (6892): 949-954.
Lamba S, Felicioni L, Buttitta F, Bleeker FE, Malatesta S, Corbo V, Scarpa A, Rodolfo M, Knowles M, Frattini M, Marchetti A, Bardelli A: Mutational profile of GNAQQ209 in human tumors. PLoS One. 2009, 4 (8): e6833-
Patel SP, Hwu W-J, Kim KB, Papadopoulos NE, Hwu P, Bedikian AY: Phase II study of the frontline combination of ipilimumab and temozolomide in patients with metastatic melanoma. http://meetinglibrary.asco.org/content/93186-114,
Ambrosini G, Pratilas CA, Qin LX, Tadi M, Surriga O, Carvajal RD, Schwartz GK: Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin Cancer Res. 2012, 18 (13): 3552-3561.
Dutton-Regester K, Irwin D, Hunt P, Aoude LG, Tembe V, Pupo GM, Lanagan C, Carter CD, O’Connor L, O’Rourke M, Scolyer RA, Mann GJ, Schmidt CW, Herington A, Hayward NK: A high-throughput panel for identifying clinically relevant mutation profiles in melanoma. Mol Cancer Ther. 2012, 11 (4): 888-897.
Collaborative Ocular Melanoma Study Group: Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol. 2001, 119 (5): 670-676.
Nathanson: Spontaneous regression of malignant melanoma: a review of the literature on incidence, clinical features, and possible mechanisms. Natl Cancer Inst Monogr. 1976, 44: 67-76.
Washington K, McDonagh D: Secondary tumors of the gastrointestinal tract: surgical pathologic findings and comparison with autopsy survey. Mod Pathol. 1995, 8 (4): 427-433.
Carvajal RD, Sosman JA, Quevedo JF, Milhem MM, Joshua AM, Kudchadkar RR, Linette GP, Gajewski TF, Lutzky J, Lawson DH, Lao CD, Flynn PJ, Albertini MR, Sato T, Lewis K, Doyle A, Ancell K, Panageas KS, Bluth M, Hedvat C, Erinjeri J, Ambrosini G, Marr B, Abramson DH, Dickson MA, Wolchok JD, Chapman PB, Schwartz GK: Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014, 311 (23): 2397-2405.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/516/prepub
Acknowledgements
The authors would like to extend their gratitude to Agop Bedikian and Kristin Simar for their care of this patient and acquisition of data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CK, DK, KK, and SP drafted the manuscript. JC and CT performed the histological review and immunoassays. SP conceived of the manuscript and carried out interpretation of molecular findings. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kim, CY., Kim, D.W., Kim, K. et al. GNAQmutation in a patient with metastatic mucosal melanoma. BMC Cancer 14, 516 (2014). https://doi.org/10.1186/1471-2407-14-516
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-14-516