Abstract
Background
Fundamental etiologic differences have been suggested to cause earlier onset of breast cancer in less developed countries (LDCs) than in more developed countries (MDCs). We explored this hypothesis using world-wide breast cancer incidence data.
Methods
We compared international age-standardized incidence rates (ASR) of pre- (<50 years) and postmenopausal (≥50 years) breast cancers as well as temporal trends in ASRs of pre-and postmenopausal breast cancer among selected countries during 1975–2008. We used joinpoint log-linear regression analysis to estimate annual percent changes (APC) for premenopausal and postmenopausal breast cancer in the northern Europe and in Black and White women population in the US.
Results
Premenopausal breast cancers comprised a substantially higher proportion of all incident breast cancers in LDCs (average 47.3%) compared to MDCs (average 18.5%). However, the ASR of premenopausal breast cancer was consistently higher in MDCs (29.4/100,000) than LDCs (12.8/100,000). The ASR of postmenopausal cancer was about five-fold higher in the MDCs (307.6/100,000) than the LDCs (65.4/100,000). The APC of breast cancer in Denmark was substantially higher in postmenopausal (1.33%) than premenopausal cancer (0.98%). Higher incidence of breast cancer among the white than black women in the US was pertained only to the postmenopausal cancer.
Conclusion
The substantial and consistent lower age-specific incidence of breast cancer in LDCs than in MDCs contradicts the theory of earlier onset. Demographic differences with fewer old women in LDCs and lower prevalence of risk factors of postmenopausal cancer are the most likely explanation to the lower mean age at diagnosis in these countries.
Similar content being viewed by others
Background
Breast cancer is the most common malignancy among women and the fifth cause of death due to cancer both in the less developed (LDCs) and more developed (MDCs) countries worldwide [1]. However, among women living in MDCs (except Japan), world-age-standardized incidence rates (ASR) are still more than 4-fold higher than in LDCs. In 2008, ASR was estimated to be 89.9 per 100,000 women-years in Western Europe, but only 19.3 in Eastern Africa [1, 2]. Although the incidence rate of breast cancer has decreased in the US and many other developed countries since early 2000s [3], it has increased 50-100% in some Asian countries including India and China during the last two decades [4, 5].
Epidemiologic studies often distinguish premenopausal from postmenopausal breast cancer [6, 7]. Some reproductive risk factors have stronger associations with risk of postmenopausal breast cancers [8–10] and obesity increases the risk of postmenopausal but decreases the risk of premenopausal breast cancer [11, 12]. Therefore, it is conceivable that a higher prevalence of known and unknown causal factors in LCDs could increase chiefly the incidence of early onset disease.
In LDCs, the average age of women diagnosed with breast cancer is about 10 years lower than in MDCs [13–15]. Although the younger age structure of the population in the LDCs may explain this finding [5, 16], it could also reflect a birth cohort phenomenon arising due to a higher prevalence of risk factors for breast cancer – such as low parity and late age at first birth in the younger compared to the older generation [17–19]. In addition, however, researchers and clinicians have proposed that earlier onset of breast cancer reflects more fundamental etiologic differences between women in LDCs and MDCs that remain to be discovered [14, 20–23]. We aimed to investigate these rival interpretations of the difference in mean age at diagnosis of breast cancer in LDCs compared to MDCs.
Methods
We used data published in the GLOBOCAN 2008 by the International Agency for Research on Cancer (IARC) to compare the proportion and truncated world age-standardized rate (ASR) of premenopausal (i.e. <50 years) and postmenopausal (i.e. ≥50 years) breast cancer in different countries in 2008 [24]. Based on GLOBOCAN, we classified the countries into less developed and more developed. In each group, we estimated weighted truncated ASR (world population) for the five countries with the highest coverage of cancer registration in the latest version of the “Cancer in Five Continents CI5 Volume IX” [25]. Data from the United Nations Population Division [26] were used to demonstrate the age structure and difference in the proportion of women aged 20–49 and ≥50 years from total women population (0 to ≥100 years) in the selected countries.
In order to illustrate changes in the incidence rate of breast cancer over time, we graphed the annual proportions and ASRs of pre- and postmenopausal breast cancer for Denmark, Finland, Norway and Sweden because these countries have had high quality nationwide cancer registration for more than 50 years. Because the Danish cancer registry provided the oldest data, we used it to demonstrate temporal trends in the pre and post-menopausal breast cancer ratio and ASR from 1943 to 2008 in Denmark. Data for Denmark, Finland, Norway and Sweden was obtained from the NORDCAN project (version 5.2) [27].
We also compared temporal trends of pre- and postmenopausal breast cancer incidences among Black and White population in the US. Data for the US Black and White populations was obtained from the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute [28].
We hypothesized that the incidence of premenopausal breast cancer would be stable over time and that changes in lifestyle factor would mainly affect the postmenopausal cancers. We used joinpoint log-linear regression analysis [29] to estimate annual percent changes (APC) for premenopausal and postmenopausal breast cancer. For trend analysis, the joinpoint regression allows to more accurately interpret changes over time, and also to determine if those changes are statistically significant. We used Monte Carlo permutation test to select the best fitting model. Joinpoint program (version 4.0) was used for statistical analyses [30]. All ASRs were standardized using the world standard population [31], and the weights were taken from the population distribution of the world standard population. The age group < 50 years and the age group ≥ 50 years defined as premenopausal and postmenopausal respectively [32].
Results
The proportion of all breast cancer cases diagnosed before age 50 was substantially higher (47.3% in average) among the LDCs compared to the MDCs (18.5% in average). Specifically, 45.7%, 48.4% and 56.9% of breast cancer patients were diagnosed before age 50 in China, India and Algeria, whilst corresponding proportions were 21.5%, 19.1% and 15.9% in Australia, UK and Denmark. However, the average ASR for premenopausal breast cancer was lower in the LDCs (12.8 per 100,000) compared to the MDCs (29.4 per 100,000). Particularly, ASRs of premenopausal breast cancer were 12.1, 12.2 and 17 per 100,000 in China, India and Algeria, while corresponding ASRs in Australia, UK and Denmark were 30, 31.7 and 31.5 per 100,000 respectively (Table 1).
The age-specific incidence rate of premenopausal breast cancer was almost similar in the LDCs and MDCs, being slightly higher in MDCs. However, incidence rate of postmenopausal breast cancer was substantially higher in the MDCs compared to the LDCs (Figure 1). The proportion of postmenopausal women was higher in MDCs compared to LDCs (Figure 2).
Estimated proportions and age standardized incidence rate (ASR) of pre- and postmenopausal breast cancer on a log scale for selected countries in 2008 (GLOBOCAN 2008)[16].
Estimated proportion of Women population in age groups 20–49 and ≥50 from total women population in selected countries in 2005 (United Nations 2012) [[26]].
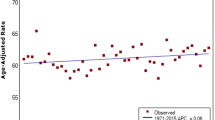
We found a dramatic increase in the ASR of postmenopausal compared to premenopausal breast cancer in Denmark from 1943 to 2008 (Figure 3). The annual percentage change for postmenopausal cancer was 1.33% (Table 2), and ASR increased 155% from 149.8 in 1943 to 382.4 per 100,000 in 2008. During the same period premenopausal breast cancer increased 91% from 15.3 per 100,000 in 1943 to 29.2 per 100,000 in 2008 (APC 0.98). Meanwhile, the proportion of premenopausal to all breast cancers decreased from 29% to 16% (APC -0.74).
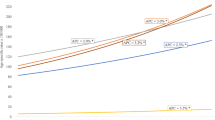
Although the incidence rates of premenopausal breast cancer increased slightly in all selected countries from 1975 through 2008, more significant changes happened in postmenopausal breast cancer incidence rate over time (Table 3). Furthermore, difference in the incidence rate of breast cancer between countries was considerable for postmenopausal breast cancer (Figure 4). Likewise, the difference in the risk of breast cancer among the black and white women pertained only to the postmenopausal women and the incidence of premenopausal women was equally low among them from 1975 to 2008.
Discussion
We showed that the incidence rate of premenopausal breast cancer in the LDCs is indeed invariably lower than that in the MDCs. However, the proportion of premenopausal breast cancer from total breast cancers is substantially higher in these countries compared to MDCs. In addition, our findings reveal that the global variation and increase in the incidence rate pertains mostly to postmenopausal breast cancers.
In LDCs, the majority of breast cancers occur among premenopausal women and the mean age of patients is around 50 years. Some investigators have concluded that breast cancer occurs about one decade earlier in several populations including Iran [23], Mexico [14], Africa [20] and blacks in the UK [21]. Some have also suggested the existence of specific genetic and environmental risk factors in LDCs [4, 14] which would call for specific prevention and early detection strategies in these countries such as starting mammography screening already at age 40 [13]. However, these conclusions were based on comparison of median age and proportion of breast cancer in different age groups rather than a comparison of age-specific incidence rates in LDCs and MDCs.
International variations in coverage and accuracy of cancer registries and diagnostic/screening strategies might explain part of the dramatic difference in the incidence rate of postmenopausal breast cancer worldwide. About one fourth of postmenopausal breast cancer in MDCs with national mammography screening is attributed to overdiagnosis [33, 34]. In a comparative study between Sweden and Singapore, both with nationwide cancer registries, difference in incidence of breast cancer was shown to be unrelated to differences in registration systems. Hence, cohort effects due to changes in lifestyle and reproductive factors in these countries as well as the screening impact need to be taken into account [18, 35]. Variations in lifestyle and reproductive risk factors seem to play a more important role in the global differences in the incidence of postmenopausal breast cancer. In several studies [19, 35–37], the increasing incidence of breast cancer follows changes in the reproductive and lifestyle factors.
Some established risk factors including age at menarche and age at menopause, include hormonal mechanisms, which are involved in the development of breast cancer [38]. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), are the tumor markers that have been widely studied in relation to the etiology, prognosis and treatment of breast cancer subtypes [8, 38, 39]. Some epidemiological studies have reported heterogeneity of breast cancer risk factors with hormone receptors and the expression of HER2 [40]. Reproductive factors and BMI were shown to be associated with ER + and PR + breast cancer, compared with ER- and PR- tumors [8, 41]. The prevalence of hormone receptor negative cancer is higher among premenopausal women than among postmenopausal women, while ER + and PR + breast cancer in more prevalent after menopause and its incidence increases with age [42, 43].
The relatively stable incidence rate of premenopausal breast cancer during several decades [44, 45] suggest a predominant role of genetic and constant environmental risk factors. Moreover, several studies showed that only postmenopausal breast cancer risk increased among women who moved from low risk to high-risk countries [10, 46–48]. In a recent study among migrant women in Sweden, risk of breast cancer increased only at postmenopausal ages [10]. A study of migration history and risk of breast cancer showed a significant increase in the trend of postmenopausal breast cancer risk in the second and third generation of Hispanic immigrants, but not in premenopausal breast cancer, after adjusting for lifestyle and reproductive factors [49]. In addition, a study of racial/ethnic differences in the incidence of postmenopausal breast cancer in the Women’s Health Initiative revealed significant ethnic differences, with lower incidence among Asian/Pacific Islanders, African American and Hispanics compared with Whites. However, after adjustment for established risk factors, the observed differences were attenuated and statistically not significant [45].
Conclusions
The global variation in the incidence rate of breast cancer is due chiefly to the differences in the risk of postmenopausal breast cancer and variation in exposure to the reproductive and lifestyle risk factors. Aging of the population and adaptation of western lifestyle in the LDCs would soon lead to a higher incidence of breast cancer in these countries. While the prevention and policy making at the time being should be focused on the bulk of young breast cancer patients in LDCs, it is essential to take a closer look and monitor the transition to higher incidence of postmenopausal breast cancer in the future and adapt the preventive and cancer control policies accordingly.
Abbreviations
- ASR:
-
Age-standardized rate
- LDCs:
-
Less developed countries
- MDCs:
-
More developed countries
- APC:
-
Annual percent change
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM: Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010, 127 (12): 2893-2917. 10.1002/ijc.25516.
Kamangar F, Dores GM, Anderson WF: Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006, 24 (14): 2137-2150. 10.1200/JCO.2005.05.2308.
Jemal A, Center MM, DeSantis C, Ward EM: Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010, 19 (8): 1893-1907. 10.1158/1055-9965.EPI-10-0437.
Huang C-S, Lin C-H, Lu Y-S, Shen C-Y: Unique features of breast cancer in Asian women-Breast cancer in Taiwan as an example. J Steroid Biochemistry Molecular Biology. 2010, 118 (4–5): 300-303.
Parkin DM, Fernández LMG: Use of statistics to assess the global burden of breast cancer. Breast J. 2006, 12 (s1): S70-S80. 10.1111/j.1075-122X.2006.00205.x.
Anderson WF, Matsuno R: Breast cancer heterogeneity: a mixture of at least two main types?. J Natl Cancer Inst. 2006, 98 (14): 948-951. 10.1093/jnci/djj295.
Bertucci F, Birnbaum D: Reasons for breast cancer heterogeneity. J Biol. 2008, 7 (2): 6-10.1186/jbiol67.
Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME: Etiology of hormone receptor–defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004, 13 (10): 1558-1568.
Rose DP, Vona-Davis L: Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas. 2010, 66 (1): 33-38. 10.1016/j.maturitas.2010.01.019.
Hemminki K, Försti A, Sundquist J, Mousavi S: Preventable breast cancer is postmenopausal. Breast Cancer Res Treat. 2011, 125: 163-167. 10.1007/s10549-010-0926-8.
Sonnenschein E, Toniolo P, Terry MB, Bruning PF, Kato I, Koenig KL, Shore RE: Body fat distribution and obesity in pre- and postmenopausal breast cancer. Int J Epidemiol. 1999, 28: 1026-1031. 10.1093/ije/28.6.1026.
Peacock SL, White E, Daling JR, Voigt LF, Malone KE: Relation between obesity and breast cancer in young women. Am J Epidemiol. 1999, 149 (4): 339-346. 10.1093/oxfordjournals.aje.a009818.
Nagi SES, Mazen KK, Toufic E, El Abdul Rahman K, Maya C, Fady G, Muhieddine S, Ali IS: Trends in epidemiology and management of breast cancer in developing Arab countries: a literature and registry analysis. Int J Surg. 2007, 5 (4): 225-233. 10.1016/j.ijsu.2006.06.015.
Rodríguez Cuevas S, Macías CG, Franceschi D, Labastida S: Breast carcinoma presents a decade earlier in Mexican women than in women in the United States or European countries. Cancer. 2001, 91 (4): 863-868. 10.1002/1097-0142(20010215)91:4<863::AID-CNCR1074>3.0.CO;2-Y.
Mousavi M, Montazeri A, Mohagheghi MA, Mousavi Jarrahi A, Harirchi I, Ebrahimi M: Breast Cancer in Iran: An Epidemiological Review. Breast J. 2007, 13 (4): 383-391. 10.1111/j.1524-4741.2007.00446.x.
Jack RH, Davies EA, Møller H: Breast cancer and age in Black and White women in South East England. Int J Cancer. 2012, 130 (5): 1227-1229. 10.1002/ijc.26088.
Adami H-O, Hunter D, Trichopoulos D: Breast cancer. Textbook of Cancer Epidemiology. 2008, NY: Oxford University Press, 403-445.
Ghiasvand R, Bahmanyar S, Zendehdel K, Tahmasebi S, Talei A, Adami HO, Cnattingius S: Postmenopausal breast cancer in Iran; risk factors and their population attributable fractions. BMC cancer. 2012, 12 (1): 414-10.1186/1471-2407-12-414.
Brown S, Morrison D, Cooke T: Increasing incidence of breast cancer: distinguishing between the effects of birth cohort and a national breast screening programme. Breast Cancer Res Treat. 2009, 116 (3): 603-607. 10.1007/s10549-008-0205-0.
Akarolo-Anthony S, Ogundiran T, Adebamowo C: Emerging breast cancer epidemic: evidence from Africa. Breast Cancer Res. 2010, 12 (Suppl 4): S8-10.1186/bcr2737.
Bowen RL, Duffy SW, Ryan DA, Hart IR, Jones JL: Early onset of breast cancer in a group of British black women. Br J Cancer. 2008, 98 (2): 277-281. 10.1038/sj.bjc.6604174.
Karami S, Young HA, Henson DE: Earlier age at diagnosis: another dimension in cancer disparity?. Cancer Detect Prev. 2007, 31 (1): 29-34. 10.1016/j.cdp.2006.11.004.
Harirchi I, Ebrahimi M, Zamani N, Jarvandi S, Montazeri A: Breast cancer in Iran: a review of 903 case records. Public Health. 2000, 114: 143-145.
Ferlay J, Shin HR, Bray F, Forman D, MC DMP: GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon. 2010, France: International Agency for Research on Cancer, Available from: http://globocan.iarc.fr
Ferlay J, Parkin DM, Curado MP, Bray F, Edwards B, HR S: Cancer Incidence in Five Continents, Volumes I to IX: IARC CancerBase No. 9 [Internet]. 2010, Lyon: France International Agency for Research on Cancer, Available from: http://ci5.iarc.fr
United Nations, Department of Economic and Social Affairs, Population Division: World Population Prospects: the 2012 Revision. 2013, DVD: Edition
Engholm G, Ferlay J, Christensen N, Johannesen TB, Klint A, Køtlum JE, Milter MC, Olafsdóttir E, Pukkala E, Storm HH: NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries. Version 5.2. 2012, Association of the Nordic Cancer Registries. Danish Cancer Society, Available from http://www.ancr.nu, December
Howlader NNA, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK: SEER Cancer Statistics Review. 1975, Bethesda (MD), based on November 2010 SEER data submission, posted to the SEER web site. Available from: http://seer.cancer.gov/csr/1975_2008/, 2011, –2008.National Cancer Institute
Kim H-J, Fay MP, Feuer EJ, Midthune DN: Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000, 19 (3): 335-351. 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z.
Research S, Applications NCI: Joinpoint Regression Program, In., 4.0 edn. 2012, Branch Surveillance Research Program, National Cancer Institute.: Bethesda, MD
Doll R, Payne P, Waterhouse JAH: Cancer Incidence in five Continents. 1966, Berlin Springer
Morabia A, Flandre P: Misclassification Bias Related to Definition of Menopausal Status in Case–control Studies of Breast Cancer. Int J Epidemiol. 1992, 21 (2): 222-228. 10.1093/ije/21.2.222.
Zahl P-H, Strand BH, Maahlen J: Incidence of breast cancer in Norway and Sweden during introduction of nationwide screening: prospective cohort study. BMJ. 2004, 328 (7445): 921-924. 10.1136/bmj.38044.666157.63.
Kalager M, Adami H-O, Bretthauer M, Tamimi RM: Overdiagnosis of invasive breast cancer due to mammography screening: results from the Norwegian screening program. Ann Intern Med. 2012, 156 (7): 491-499. 10.7326/0003-4819-156-7-201204030-00005.
Chia KS, Reilly M, Tan CS, Lee J, Pawitan Y, Adami HO, Hall P, Mow B: Profound changes in breast cancer incidence may reflect changes into a Westernized lifestyle: A comparative population‒based study in Singapore and Sweden. Int J Cancer. 2005, 113 (2): 302-306. 10.1002/ijc.20561.
Swerdlow AJ, dos Santos SI, Reid A, Qiao Z, Brewster DH, Arrundale J: Trends in cancer incidence and mortality in Scotland: description and possible explanations. Br J Cancer. 1998, 77 (Suppl 3): 1-54.
dos Santos SI, Swerdlow AJ: Recent trends in incidence of and mortality from breast, ovarian and endometrial cancers in England and Wales and their relation to changing fertility and oral contraceptive use. Br J Cancer. 1995, 72 (2): 485-492. 10.1038/bjc.1995.360.
Brenton JD, Carey LA, Ahmed AA, Caldas C: Molecular classification and molecular forecasting of breast cancer: ready for clinical application?. J Clin Oncol. 2005, 23 (29): 7350-7360. 10.1200/JCO.2005.03.3845.
Dolle JM, Daling JR, White E, Brinton LA, Doody DR, Porter PL, Malone KE: Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009, 18 (4): 1157-1166. 10.1158/1055-9965.EPI-08-1005.
Potter JD, Cerhan JR, Sellers TA, McGovern PG, Drinkard C, Kushi LR, Folsom AR: Progesterone and estrogen receptors and mammary neoplasia in the Iowa Women's Health Study: how many kinds of breast cancer are there?. Cancer Epidemiol Biomarkers Prev. 1995, 4 (4): 319-326.
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V: Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer. 2007, 109 (9): 1721-1728. 10.1002/cncr.22618.
Anderson WF, Chu KC, Chang S, Sherman ME: Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2004, 13 (7): 1128-1135.
Leung GM, Thach TQ, Lam TH, Hedley AJ, Foo W, Fielding R, Yip PS, Lau EM, Wong CM: Trends in breast cancer incidence in Hong Kong between 1973 and 1999: an age-period-cohort analysis. Br J Cancer. 2002, 87: 982-988. 10.1038/sj.bjc.6600583.
Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA: The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007, 356 (16): 1670-1674. 10.1056/NEJMsr070105.
Glass AG, Lacey JV, Carreon JD, Hoover RN: Breast Cancer Incidence, 1980–2006: Combined Roles of Menopausal Hormone Therapy, Screening Mammography, and Estrogen Receptor Status. J Natl Cancer Inst. 2007, 99 (15): 1152-1161. 10.1093/jnci/djm059.
Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF: Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993, 85: 1819-1827. 10.1093/jnci/85.22.1819.
Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA: Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005, 97 (6): 439-448. 10.1093/jnci/dji064.
Nelson NJ: Migrant studies aid the search for factors linked to breast cancer risk. J Natl Cancer Inst. 2006, 98 (7): 436-438. 10.1093/jnci/djj147.
John EM, Phipps AI, Davis A, Koo J: Migration history, acculturation, and breast cancer risk in hispanic women. Cancer Epidemiol Biomarkers Prev. 2005, 14 (12): 2905-2913. 10.1158/1055-9965.EPI-05-0483.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/343/prepub
Acknowledgement
We would like to acknowledge the Cancer Research Center of the Cancer Institute of I.R. Iran that provided funding for the publication cost of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
RG participated in the study design, performed statistical analysis and drafted the manuscript. HOA contributed in the interpretation of data and critically revised the manuscript for important intellectual concept. IH participated in the study design and interpretation of data. RA participated in the study design and interpretation of data. KZ conceived the study and contributed in the interpretation of data and helped draft the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ghiasvand, R., Adami, HO., Harirchi, I. et al. Higher incidence of premenopausal breast cancer in less developed countries; myth or truth?. BMC Cancer 14, 343 (2014). https://doi.org/10.1186/1471-2407-14-343
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-14-343