Abstract
Background
Cancer vaccine is one of the attractive treatment modalities for patients with castration-resistant prostate cancer (CRPC). However, because of delayed immune responses, its clinical benefits, besides for overall survival (OS), are not well captured by the World Health Organization (WHO) and Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Several surrogate markers for evaluation of cancer vaccine, including prostate-specific antigen doubling time (PSADT), are currently sought. The purpose of this study was to assess prospectively the PSA kinetics and immune responses, as well as the efficacy, safety, and biomarkers of personalized peptide vaccination (PPV) in progressive CRPC.
Methods
One hundred patients with progressive CRPC were treated with PPV using 2–4 positive peptides from 31 candidate peptides determined by both human leukocyte antigen (HLA) class IA types and the levels of immunoglobulin G (IgG) against each peptide. The association between immune responses and PSADT as well as overall survival (OS) was studied.
Results
PPV was safe and well tolerated in all patients with a median survival time of 18.8 months. Peptide-specific IgG and T-cell responses strongly correlated with PSADT (p < 0.0001 and p = 0.0007, respectively), which in turn showed correlation with OS (p = 0.018). Positive IgG responses and prolongation of PSADT during PPV were also significantly associated with OS (p = 0.001 and p = 0.004) by multivariate analysis.
Conclusions
PSADT could be an appropriate surrogate marker for evaluation of the clinical benefit of cancer vaccine. Further randomized trials are needed to confirm these results.
Trial registration
Similar content being viewed by others
Background
Changes in serum prostate-specific antigen (PSA) can reflect the burden of disease and clinical benefit in patients with castration-resistant prostate cancer (CRPC) with cytotoxic chemotherapy or hormonal agents known to kill tumor cells; these changes can have practical utility by providing and updating prognostic information on an individual patient over time [1–4]. As observed in many clinical trials, however, immunotherapy can induce novel patterns of antitumor responses distinct from those of chemotherapy [5]. For example, an autologous dendritic-cell-based vaccine (sipuleucel-T) is known to improve survival without having an impact on early PSA decline [6], whereas docetaxel's improvement in overall survival (OS) correlates for the most part with a PSA decline within the first 3 months of therapy [7, 8]. Thus, interpreting PSA decline in the context of novel immunotherapy must be carried out with caution on the basis of the mechanism of action, and may also depend on the time of sampling [9].
Personalized peptide vaccine (PPV) uses multiple peptides based on the pre-existing immunity. Under PPV treatment, each patient with human leukocyte antigen (HLA)-class IA types positive was tested for their immunological reactivity to 31 different peptides capable of inducing T-cell responses. The 31 peptides were derived from a number of tumor associated antigens: PSA, prostatic acid phosphatase (PAP), prostate-specific membrane antigen (PSMA), multidrug resistance protein and a variety of other epithelial tumor antigens. We previously demonstrated that PPV was safe and improved OS with immune responses in phase I, I/II, and II clinical trials in patients with CRPC [10–16]. However, it was not addressed whether PSADT could be an appropriate surrogate marker for evaluation of the clinical benefit of cancer vaccine. To address this, we evaluated data from a phase II clinical trial for CRPC using PPV.
Methods
Patient Eligibility
Eligibility required a histological diagnosis of prostate adenocarcinoma and progressive disease (PD) defined as at least two consecutive increases in PSA, new metastatic lesion on radionuclide bone scan, or progressive tumor lesions on cross-sectional imaging, despite adequate androgen ablative therapy. Patients showed positive IgG responses to at least two of the 31 different candidate peptides (Table 1). Any number of previous hormonal therapies was allowed. Patients were required to wait at least four weeks for entry into the study after the completion of prior radiation therapy, chemotherapy, or a change in hormonal therapy. Other inclusion criteria included age ≥ 20 years; Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; life expectancy of at least 12 weeks; positive status for HLA-A2, -A24, -A3 supertype (−A3, -A11, -A31, and -A33), or -A26; adequate hematologic, hepatic, and renal function; and negative status for hepatitis virus B and C. Exclusion criteria included an acute infection; a history of severe allergic reactions; pulmonary, cardiac, or other systemic diseases; and other inappropriate conditions for enrollment as judged by clinicians.
Study design and treatment
This was a single institution, single arm, open-label, phase II study. The endpoints of this study were primarily safety and feasibility of PPV in patients with CRPC. Secondary endpoints were to assess the PSA kinetics and immune responses. In addition, we identified potential factors for predicting OS and selecting suitable patients for this treatment. This study protocol was approved by Kurume University Ethical Committee. Written informed consent was obtained from all patients before any study procedures.
In this study, 31 peptides, whose safety and immunological effects had been confirmed in previously conducted clinical studies [10–18], were employed for vaccination [12 peptides for HLA-A2, 14 peptides for HLA-A24, 9 peptides for the HLA-A3 supertype (A3, A11, A31, or A33), and 4 peptides for HLA-A26] (Table 1). All peptides were prepared under conditions of Good Manufacturing Practice using a Multiple Peptide System (San Diego, CA). The selection of 2 to 4 peptides for vaccination to each patient was based on HLA typing and high titer level of peptide-specific IgG to candidate peptides. Each of the selected peptides was mixed with incomplete Freund’s adjuvant (Montanide ISA-51VG; Seppic, Paris, France) and emulsified in the 5 ml plastic syringe, and a maximum of four peptides of 1.5 ml emulsion (3 mg/peptide) were injected subcutaneously into the lateral thigh area once a week for 6 weeks. The peptides were re-selected according to peptide-specific IgG levels at every cycle of 6 vaccinations and administered at 2-, 3-, or 4-week intervals until withdrawal of consent or unacceptable toxicity.
Assessment of clinical activity
Patients were monitored at each visit by history and physical examinations. Serum PSA test and routine laboratory studies were performed every 6 vaccinations for any adverse effects. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI-CTCAE Ver3).
All patients underwent relevant radiologic studies and bone scans every 6 months or at the progression of symptoms. PD was defined as radiographic progression evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) criteria [19] or clinical progression.
To assess the PSA response for each patient, percent PSA change from baseline was calculated for each phase of the study (pre- and during vaccination). In addition, PSA doubling time (PSADT) was calculated using all serum PSA values for a specified period, and using a minimum of three PSA values by the formula log2/b, where b denotes the least square estimate of the linear regression model of the log-transformed PSA values on time. For analytical purposes, negative PSADT estimates and high positive PSADT estimates (>36 months) were censored at 36 months.
To investigate biomarkers for OS that may allow patient selection and prediction of a response to PPV, serum amyloid A (SAA), C-reactive protein (CRP), and interleukin (IL)-6 in plasma at baseline were additionally examined by enzyme-linked immunosorbent assay (ELISA), respectively.
Measurement of humoral and T-cell responses specific to the vaccinated peptides
To study the humoral responses specific to the vaccinated peptides, peptide-specific IgG levels were measured by a Luminex system (Luminex, Austin, TX), as reported previously [20]. If the total titers of selected peptide-specific IgG in any cycles of post-vaccination plasma were more than 2-fold higher than those in the pre-vaccination plasma, the changes were considered to be a positive response.
Although T-cell subsets using flowcytometry was not analyzed in this study, T-cell responses specific to the vaccinated peptides were evaluated by IFN-γ ELISPOT assay using peripheral blood mononuclear cells (PBMCs), as reported previously [18]. Peptide-specific T-cell responses were evaluated by the differences between the numbers of spots per 105 x PBMCs in response to the vaccine peptides and those to the control peptide at pre- and 6th vaccination; at least 2-fold more spots at the 6th vaccination than at pre-vaccination was considered positive.
Statistical analysis
All patients who received more than 6 vaccinations were considered evaluable for tumor response, and all patients entered were included in the survival analysis. Data were analyzed at the end of November, 2012 using commercially available computer software. The Student’s t-test and the chi-square test were used to compare quantitative and categorical variables, respectively. Survival was calculated from the date of first treatment until the date of any cause of death. Patients lost to follow-up were censored at the last known date of survival. The Kaplan-Meier method was used to estimate actuarial survival curves, and groups were compared using a log-rank test. Cox proportional hazards regression model was used for univariate and multivariate analyses to identify factors that had a significant impact on survival. All baseline parameters in the survival and proportional hazards regression analysis were analyzed as dichotomous variables using median or cut-off values. A two-sided significance level of 5% was considered statistically significant.
Results
Characteristics of the patients
Between April 2009 and August 2011, 100 patients with CRPC were enrolled in this trial at Kurume University Hospital. All 100 patients received at least one vaccination with a median of 16 vaccinations (range, 1 to 40) and were included in the safety assessment and survival analysis. Three patients did not complete 6 vaccinations (1 cycle) and were excluded from the assessment of PSA response and immune responses. The reason for these failures to complete 6 vaccinations was withdrawal of consent. The median age of participants was 69 years (range, 51 to 92 years), and the ECOG performance status was 0 in 91of the patients and 1 in the remaining 9. The median PSA and pre-vaccination PSADT at the entry to the study was 29.8 ng/ml (range, 0.2 to 2481 ng/ml) and 2 months (range, 0.3 to 36+ months), respectively. Fifty-seven patients had a Gleason score of ≥ 8 and 86 patients had metastasis. All patients had experienced progression after androgen deprivation therapy as an initial or secondary therapy. Forty patients had received docetaxel based chemotherapy with a median cycle of 6.5 as a third line treatment. Baseline patient characteristics are shown in Table 2.
Adverse events
The overall toxicities are shown in Table 3. The most frequent adverse events were local redness and swelling at injection sites, bone pain, hypoalbuminemia, lymphocytopenia, appetite loss, fatigue, increased ALP, and anemia, which were grade 1 or 2 in most cases. There were no grade 4 toxicities and no treatment-related deaths. A total of 51 grade 3 toxicities including anemia, bone pain, increased ALP, lymphocytopenia, decreased white blood cells, increased creatinine, injection site reaction, and increased AST and ALT were observed during the study. All of these severe adverse events were concluded to be not directly associated with the vaccinations, but with cancer progression or other causes by the independent safety evaluation committee in this trial.
Clinical outcome
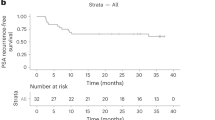
Forty-eight (49%) patients exhibited some decrease in PSA from baseline, ranging from 1.9% to 99.6% (Figure 1A). Confirmed ≥50% PSA decline at any point during PPV was observed in 21 patients (22%), with a median time of 4 months to ≥50% PSA decline and a median duration of ≥50% PSA decline of 3 months. Delayed PSA response was observed. Patients with ≥50% PSA decline during PPV showed longer survival than remaining patients ( p = 0.035) (Figure 1B). The median estimated PSADT pre- and during PPV were 2 and 3.89 months, respectively. Fifty-four (56%) patients displayed at least 2-fold increase over the pre-treatment PSADT (range, 2.1- to 75-fold), and these patients with a prolongation of PSADT showed longer survival than patients without a prolongation of PSADT (p = 0.013) (Figure 1C and D). To compare the difference in PSA responses with clinical outcomes, patients were divided into three groups: responder group with survival longer than 20 months after PPV, non-responder group with death within 12 months after PPV, and another group with the remaining patients. Average% PSA changes in the responder group were significantly lower than those in the non-responder group at 2 to 5 months (p < 0.005) and those in the other group at 5 to 10 months (p < 0.005) during the PPV. In addition, average% PSA changes in the responder group showed a trend of PSA plateau. Average% PSA changes from baseline among three groups before and during PPV are shown in Figure 1E.
PSA kinetics and overall survival. (A) Waterfall plot showing the maximal PSA changes (%) from baseline during personalized peptide vaccination (PPV) at any time point. (B) Overall survival by >50% PSA decline. (C) The ratio of PSADT changes for each patient pre- and during PPV is plotted. The ratio of PSADT changes was calculated by dividing PSADT during treatment by pre-treatment PSADT. A ratio greater than 2 indicates prolongation of PSADT. (D) Overall survival by prolongation of PSDT. (E) Longitudinal average PSA changes (%) before and during PPV. Green histograms: Responder group (alive for more than 20 months). Red histograms: Non-responder group (death within 12 months). Gray histograms: Other group.
There was no complete response or partial response in terms of measurable disease. The median time to disease progression, as defined by clinical and/or radiologic criteria, was 10.9 months (95% CI, 6 to 19 months). At the time of analysis with a median follow-up of 18 months (95% CI, 14.1 to 24 months), 64 deaths had occurred. Median survival time was 18.8 months (95% CI, 14.9 to 28.6 months) in all patients. Median survival time in chemotherapy naive patients and in patients after docetaxel chemotherapy were 21.6 months and 11.6 months, respectively.
Immunological response
The number of selected peptides were 4 peptides in 62 patients, 3 peptides in 17 patients and 2 peptides in 21 patients at the first screening. Same peptide at the first screening were only selected in 29 of 97 (30%) patients at second screening and in 10 of 66 (15%) patients at the third screening, remaining patients received at least 1 different peptide during the study. The most frequently selected peptides were Lck486 (40 patients), CypB129 (31 patients), PAP213 (24 patients), SART2-93 (21 patients), PSA248 (20 patients), Lck488 (17 patients) and WHSC2-123 (16 patients) at the first screening. All 31 peptides were selected at any screening in the study.
Total IgG responses specific to the vaccinated peptide were augmented in 42 of 97 (43%) patients, 62 of 66 (94%) patients, 36 of 36 (100%) patients, 16 of 16 (100%) patients, and 7 of 7 (100%) patients at the 6th, 12th, 18th, 24th, and 30th vaccinations, respectively. Finally, positive IgG responses during PPV were observed in 76/97 (79%) patients. PBMCs from 97 patients were available for IFN-γ Elispot assay at the pre- and 6th vaccination. Peptide-specific T-cell responses were detectable in 42 patients (43%) at the 6th vaccination. There was no obvious correlation between IgG and CTL responses. Positive immune responses of both IgG and CTL based on baseline characteristics including age, PS, HLA typing, PSA, Gleason score, presence of metastasis and prior chemotherapy are shown in Figure 2. There was no difference in positive immune responses among baseline characteristics. In comparing immune responses with PSA kinetics, although average PSA changes did not correlate with immune responses, average ratio of PSADT was significantly higher in patients with positive IgG (8 vs. 4, p < 0.0001) and CTL (8.8 vs. 6.1, p = 0.0007) responses (Figure 3).
Survival analysis
Cox proportional hazards regression analysis was performed to determine factors that would predict disease death (Table 4). Univariate Cox analysis showed that good performance status (p < 0.0001), positive IgG response (p < 0.0001), low CRP (p = 0.012), prolongation of PSADT (p = 0.018), low PSA (p = 0.004), prior chemotherapy status (p = 0.037), positive T-cell response (p = 0.039), and presentation of ≥50% PSA decline (p = 0.046) were significantly associated with survival.
The factors showing p less than 0.05 in the univariate analysis were included in multivariate analysis of the model. Finally, positive IgG response (p = 0.001) and prolongation of PSADT (p = 0.004) during PPV, as well as baseline good performance status (p = 0.004), low CRP levels (p = 0.006), and low PSA levels (p = 0.008), were significantly favorable factors for OS (Table 4).
Discussion
As observed in several clinical trials, immunotherapy can induce novel patterns of antitumor responses distinct from those of chemotherapy, which are consequently not captured by the WHO or RECIST criteria [5]. On the other hand, there is debate regarding the utility of PSA changes, especially with immunotherapy, and the PSA Working Group 2 has advocated using radiographic progression-free survival as a preferred endpoint for phase II trials [21]. Others have argued that changes in PSADT may be a marker of drug effect, understanding that shorter PSADT corresponds to worse prognosis and, thus, a favorable change in PSADT suggests drug activity [22, 23]. However, clinical trials of recently developed drugs, such as sipuleucel-T [6], cabazitaxel [24], and abiraterone acetate [25], for the treatment of progressive CRPC patients did not analyze the usefulness of PSADT as a surrogate marker of response in CRPC patients. In the current study, we attempted careful and stringent collection of multiple PSA values in order to calculate PSADT changes before and during PPV accurately. While delayed PSA responses were observed, we did see a statistically significant increase in PSADT. Importantly, patients with prolongation of PSADT showed statistically longer survival (p = 0.018). These results suggest that the development of late immune responses is associated with changes in PSADT.
The evaluation of T-cell immune responses to target self antigens after vaccine clinical trials presents several challenges. Antigen-specific T-cells can be evaluated by their peptide target specificity, proliferative capacity, cytokine secretion, cytolytic activity, and membrane markers of activation. At present, the best measure of antigen-specific T-cells is unknown, as is the optimal time to evaluate immune responses. In our current analysis, we evaluated both humoral responses determined by peptide-specific IgG levels using a Luminex system and antigen-specific CD8+ T-cell responses by using IFN-γ ELISPOT assays, to provide a more direct quantitative assessment after immunization. Delayed 50% PSA decline and prolongation of PSADT were observed in patients with positive IgG and T-cell respkonses, and these immune responses were associated with OS. These results suggest that further immunological analysis at multiple time points might be needed to determine whether T-cell response or the development of late immune responses is associated with clinical responses.
Cancer vaccinations do not always extract good immune and/or clinical responses in vaccinated patients. This study showed that IgG responses and prolongation of PSADT during PPV, along with baseline performance status, CRP, and PSA levels, were well correlated with OS in patients with CRPC treated by PPV. These results suggest that risk stratification based on these factors could be helpful for estimating the OS in patients with CRPC treated by immunotherapy.
Despite these encouraging observations, the current study must be interpreted as hypothesis-generating due to several limitations. This single-arm phase II study without a concurrent control arm did not allow estimation of the potential clinical or immune effects of this treatment. Another potential limitation of this study regarding OS is the lack of treatment data after the treatment phase of the trial. Imbalances due to chance may have occurred in treatments after progression. However, only docetaxel has been shown to affect survival in this population of patients, and only by a few months. The median survival of 18.8 months (95% CI, 14.1 to 24 months) observed in this study surpassed the survival that was observed from docetaxel-based clinical trials in a similar population by TAX-327 (median survival, 19.2 months) and South West Oncology Group 9906 (median survival, 17.5 months) [7, 8]. Thus, we think it unlikely that a potential imbalance in post-study treatments could explain the survival results.
Conclusions
This study showed that PPV in patients with CRPC was active and well tolerated, improving survival with immune responses, delayed PSA responses, and prolongation of PSADT. Further randomized trials are needed to confirm these preliminary results.
Abbreviations
- CR:
-
Complete response
- CT:
-
Computed tomography
- CRPC:
-
Castration-resistant prostate cancer
- CTL:
-
Cytotoxic T lymphocytes
- EOCG:
-
Eastern cooperative oncology group
- HLA:
-
Human leukocyte antigen
- IFN- γ:
-
Interferon-γ
- IgG:
-
Immunoglobulin G
- OS:
-
Overall survival
- PBMC:
-
Peripheral blood mononuclear cells
- PPV:
-
Personalized peptide vaccination
- PSA:
-
Prostate specific antigen
- PSADT:
-
Prostate specific antigen doubling time.
References
Vollmer RT, Dawson NA, Vogelzang NJ: The dynamics of prostate specific antigen in hormone refractory prostate carcinoma: an analysis of cancer and leukemia group B study 9181 of megestrol acetate. Cancer. 1998, 83: 1989-1994. 10.1002/(SICI)1097-0142(19981101)83:9<1989::AID-CNCR15>3.0.CO;2-V.
Armstrong AJ, Garrett-Mayer E, Ou Yang YC, Carducci MA, Tannock I, de Wit R, Eisenberger M: Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007, 25: 3965-3970. 10.1200/JCO.2007.11.4769.
Scher HI, Kelly WM, Zhang ZF, Ouyang P, Sun M, Schwartz M, Ding C, Wang W, Horak ID, Kremer AB: Post-therapy serum prostate-specific antigen level and survival in patients with androgen-independent prostate cancer. J Natl Cancer Inst. 1999, 91: 244-251. 10.1093/jnci/91.3.244.
Petrylak DP, Ankerst DP, Jiang CS, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME, Burch PA, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED: Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99–16. Natl Cancer Inst. 2006, 98: 516-521. 10.1093/jnci/djj129.
Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J: Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010, 102: 1388-1397. 10.1093/jnci/djq310.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF: IMPACT Study Investigators: Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010, 363: 411-422. 10.1056/NEJMoa1001294.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA: TAX 327 Investigators: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004, 351: 1488-1490. 10.1056/NEJMp048178.
Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED: Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004, 351: 1513-1520. 10.1056/NEJMoa041318.
Stein WD, Gulley JL, Schlom J, Madan RA, Dahut W, Figg WD, Ning YM, Arlen PM, Price D, Bates SE, Fojo T: Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011, 17: 907-917. 10.1158/1078-0432.CCR-10-1762.
Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, Itoh K, Noda S: Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003, 57: 80-92. 10.1002/pros.10276.
Noguchi M, Itoh K, Suekane S, Yao A, Suetsugu N, Katagiri K, Yamada A, Yamana H, Noda S: Phase I trial of patient-oriented vaccination in HLA-A2-positive patients with metastatic hormone-refractory prostate cancer. Cancer Sci. 2004, 95: 77-84. 10.1111/j.1349-7006.2004.tb03174.x.
Noguchi M, Itoh K, Suekane S, Morinaga A, Sukehiro A, Suetsugu N, Katagiri K, Yamada A, Noda S: Immunological monitoring during combination of patient-oriented peptide vaccination and estramustine phosphate in patients with metastatic hormone refractory prostate cancer. Prostate. 2004, 60: 32-45. 10.1002/pros.20011.
Noguchi M, Itoh K, Yao A, Mine T, Yamada A, Obata Y, Furuta M, Harada M, Suekane S, Matsuoka K: Immunological evaluation of individualized peptide vaccination with a low dose of estramustine for HLA-A24+ HRPC patients. Prostate. 2005, 63: 1-12. 10.1002/pros.20157.
Noguchi M, Mine T, Yamada A, Obata Y, Yoshida K, Mizoguchi J, Harada M, Suekane S, Itoh K, Matsuoka K: Combination therapy of personalized peptide vaccination and low-dose estramustine phosphate for metastatic hormone refractory prostate cancer patients: an analysis of prognostic factors in the treatment. Oncol Res. 2007, 16: 341-349.
Noguchi M, Kakuma T, Uemura H, Nasu Y, Kumon H, Hirao Y, Moriya F, Suekane S, Matsuoka K, Komatsu N, Shichijo S, Yamada A, Itoh K: A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother. 2010, 59: 1001-1009. 10.1007/s00262-010-0822-4.
Noguchi M, Uemura H, Naito S, Akaza H, Yamada A, Itoh K: A phase I study of personalized peptide vaccination using 14 kinds of vaccine in combination with low-dose estramustine in HLA-A24-positive patients with castration-resistant prostate cancer. Prostate. 2011, 71: 470-479. 10.1002/pros.21261.
Matsumoto K, Noguchi M, Satoh T, Tabata K, Fujita T, Iwamura M, Yamada A, Komatsu N, Baba S, Itoh K: A phase I study of personalized peptide vaccination for advanced urothelial carcinoma patients who failed treatment with methotrexate, vinblastine, adriamycin and cisplatin. BJU Int. 2011, 108: 831-838.
Yoshiyama K, Terazaki Y, Matsueda S, Shichijo S, Noguchi M, Yamada A, Mine T, Ioji T, Itoh K, Shirouzu K, Sasada T, Takamori S: Personalized peptide vaccination in patients with refractory non-small cell lung cancer. Int J Oncol. 2012, 24: 795-801.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG: New guidelines to evaluate the response to treatment in solid tumor: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000, 92: 205-216. 10.1093/jnci/92.3.205.
Komatsu N, Shichijo S, Nakagawa M, Itoh K: New multiplexed flow cytometric assay to measure anti-peptide antibody: a novel tool for monitoring immune responses to peptides used for immunization. Scand J Cin Lab Invest. 2004, 64: 1-11. 10.1080/00365510310003391.
Scher HI, Halabi S, Tannoch I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M, Prostate Cancer Clinical Trials Working Group: Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008, 226: 1148-1159.
McNeel DG, Dunphy E, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, Liu G, Eickhoff JC, Wilding G: Safety and immunological efficacy of a DNA vaccine encoding prostatic and phosphatase in patients with D0 prostate cancer. J Clin Oncol. 2009, 27: 4047-4054. 10.1200/JCO.2008.19.9968.
Sweeney C, Liu G, Yiannoutsos C, Kolesar J, Horvath D, Staab MJ, Fife K, Armstrong V, Treston A, Sidor C, Wilding G: A phase II, multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin Cancer Res. 2005, 11: 6625-6633. 10.1158/1078-0432.CCR-05-0440.
De Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO: TROPIC Investigators: Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomized open-label trial. Lancet. 2010, 376: 1147-1154. 10.1016/S0140-6736(10)61389-X.
De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI, COU-AA-301 Investigators: Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011, 364: 1995-2005. 10.1056/NEJMoa1014618.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/13/613/prepub
Details of all funding sources
This study was supported in part by Grants-in-Aid (KAKENHI) (no.22591782 to M.Noguchi), and by the grants from the Regional Innovation Cluster Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
K. Itoh is a consultant/advisory board member in Green Peptide Co. A.Yamada is a part-time executive of Green Peptide Co. No potential conflicts of interest were disclosed by other authors.
Authors' contributions
NM conceived of the study, and participated in its design and coordination and drafted the manuscript. KI and AY participated in its design and helped to draft the manuscipt. FM, SS, RO performed the clinical trial and collected the data. SM and TS carried out the immunoassays. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Noguchi, M., Moriya, F., Suekane, S. et al. A phase II trial of personalized peptide vaccination in castration-resistant prostate cancer patients: prolongation of prostate-specific antigen doubling time. BMC Cancer 13, 613 (2013). https://doi.org/10.1186/1471-2407-13-613
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-13-613