Abstract
Background
The epidermal growth factor receptor (EGFR)/RAS/RAF/MEK/MAPK pathway is an important pathway in the carcinogenesis, invasion and metastasis of colorectal cancers (CRCs). We conducted a retrospective study to determine the prognostic values of EGFR expression and KRAS mutation in patients with metastatic CRC (mCRC) based on synchronous or metachronous status.
Methods
From October 2002 to March 2012, 205 patients with mCRC were retrospectively analyzed; 98 were found to have metachronous mCRC while 107 were found to have synchronous mCRC. The EGFR expressions were determinate by IHC (immunohistochemistry) analysis and categorized 1+ (weak intensity), 2+ (moderate intensity), and 3+ (strong intensity). Genomic DNA was isolated from frozen primary CRC tissues and direct sequencing of KRAS was performed. The clinicopathological features of these mCRC patients were retrospectively investigated according to EGFR expression and KRAS mutation status. Moreover, we analyzed the prognostic values of EGFR expression and KRAS mutation among these patients.
Results
Of the 205 patients with mCRC, EGFR expression was analyzed in 167 patients, and positive EGFR expression was noted in 140 of those patients (83.8%). KRAS mutation was investigated in 205 patients and mutations were noted in 88 of those patients (42.9%). In patients with metachronous mCRC, positive EGFR expression was significantly correlated with well-and moderately-differentiated tumors (P = 0.028), poorer disease-free survival (DFS) (P < 0.001), and overall survival (OS) (P < 0.001). Furthermore, positive EGFR expression was a significant independent prognostic factor of DFS (P = 0.006, HR: 4.012, 95% CI: 1.130–8.445) and OS (P = 0.028, HR: 3.090, 95% CI: 1.477–10.900) in metachronous mCRC patients. KRAS mutation status was not significantly related to DFS and OS of patients with metachronous mCRC; likewise, KRAS mutation status was not significantly different in the progression-free survival (PFS) and OS of patients with synchronous mCRC (all P > 0.05).
Conclusions
The present study demonstrated that EGFR expression has prognostic value only for patients with metachronous mCRC. However, KRAS mutation did not have prognostic value in patients with metachronous or synchronous mCRC.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death in the United States where an estimated 142,820 newly diagnosed cases of CRC and an estimated 50,830 cancer deaths from CRC were reported in 2013 [1]. In Taiwan, CRC is the most common cancer type, having increased rapidly in prevalence, and the third leading cause of cancer-related death as of 2012. The incidence of CRC was 32.38 per 100,000 (7,213 new diagnoses of CRC) in 2000 and 60.72 per 100,000 (14,040 new diagnoses of CRC) in 2010 [2]. In Taiwan, 5131 people died from CRC in 2012 and the death rate was 22.0 per 100,000 [2]. The prognoses of metastasis colorectal cancer (mCRC) have improved in the past decade, with the median overall survival (OS) rate increasing from 12 months to more than 24 months [3, 4]. These improvements are considered to be a result of the development of combinations of standard chemotherapy, including fluoropyrimidine/folinic acid, irinotecan (FOLFIRI), and oxaliplatin (FOLFOX), and the introduction of new targeted biological agents such as cetuximab, panitumumab, and bevacizumab.
EGFR is a 170-KDa transmembrane receptor with an intracellular tyrosine kinase domain. EGFR is a member of the ErbB receptor family. After EFGR is bounded by EGF, EFGR forms a functionally active dimer (homodimer or heterodimer) that causes phosphorylation of tyrosine kinases in the intracellular domain of EGFR. Subsequently, complex intracellular signals to the cytoplasm and then to the nucleus are triggered by this phosphorylation [5]. Two major downstream signaling pathways are mediated by EGFR: the RAS/RAF/MEK/MAPK pathway and the PI3K–Akt pathway. The functions of the EGFR/RAS/RAF/MEK/MAPK pathway are associated with gene transcription, cell-cycle progression from the G1 phase to the S phase, and cell proliferation. Moreover, the EGFR/RAS/RAF/MEK/MAPK pathway has also been reported to play a critical role in the carcinogenesis, migration, invasion, and metastasis of CRC [5]. EGFR overexpression was previously thought to be associated with more advanced disease and worse prognoses. The prognostic value of EGFR in CRC has been investigated extensively, but it remains controversial [6–10]. Although KRAS mutation has been studied for the predictive value of tumor response to anti-EGFR treatment and also has been confirmed to be the highly predictive of resistance to anti-EGFR treatment [11–18], the prognostic value of KRAS mutation in synchronous and metachronous mCRC remains controversial [18–28]. Therefore, we conducted a retrospective study to evaluate the prognostic value of EGFR expression and KRAS mutation in patients with synchronous or metachronous mCRC. Synchronous metastasis was defined as metastatic disease at the time of the primary CRC diagnosis. Metachronous metastasis was defined as the absence of metastatic disease at the time of initial CRC diagnosis with metastatic disease developing more than 3 months after resection of the primary CRC.
Methods
Patients
This retrospective study included 205 patients with histologically proven synchronous or metachronous mCRC who received surgical treatment from a single-institution between October 2002 and July 2012. The present study was approved by the Institutional Review Board of the Kaohsiung Medical University Hospital. Patients’ clinical outcomes and survival statuses were regularly followed up. Available variables included: age of diagnosis, sex, tumor location, histological type, TNM classification, vascular invasion, perineural invasion, and preoperative and postoperative serum level of CEA. The TNM classification was defined according to the criteria of the American Joint Commission on Cancer/International Union Against Cancer (AJCC/UICC) [29]. All patients were followed up until their deaths, their last follow-up, or December 31, 2012. Overall survival (OS) was defined as the time from the date of primary treatment to the date of death from any cause or until the date of the last follow-up. Disease-free survival (DFS) for patients with metachronous mCRC was defined as the time from the date of primary treatment to the date of diagnosis for recurrence or metastatic disease or to the date of the last follow-up. Progress-free survival (PFS) for patients with synchronous mCRC was defined as the time from the date of primary treatment to the date of tumor progression or to the date of death from any cause, or to the date of the last follow-up.
Immunohistochemical analysis for EGFR expression
Formalin-fixed and paraffin-embedded tissue blocks were cut into 3 μm sections and deparaffinized, rehydrated, and autoclaved at 121°C for 5 min in Target Retrieval solution (Dako, Glostrup, Denmark), pH 6.0, to retrieve antigens. Endogenous peroxidase was blocked by 3% hydrogen peroxide for 5 min at room temperature. After washing with a Tris buffer solution, the sections were incubated with EGFR for 1 hour at room temperature. Then, DAKO REAL EnVision Detection System-HRP (DAKO, Glostrup, Denmark) was applied for 30 minutes at room temperature. Finally, sections were incubated in 3′, 3-diaminobenzidine for 5 minutes, followed by Mayer’s hematoxylin counterstaining. Dehydration was performed through two changes of 95% ethanol and two changes of 100% ethanol, and the samples were cleared in three changes of xylene and then mounted. Negative controls were obtained by replacing the primary antibody with non-immune serum. Immunoreactivity of EGFR was evaluated by two independent researchers who were blinded to patient outcome.
Expression patterns of EGFR were determined in a semi-quantitative manner by light microscopy. Immunoreactivity for EGFR (membrane staining) was categorized in accordance with the presence of tumor cell staining and staining intensity. The intensity of EGFR immunoreactivity was scored with a 3-tier system as follow [7, 30]: 1+ (weak intensity); 2+ (moderate intensity); and 3+ (strong intensity) (Figure 1). Negative EGFR expression means absence of membrane staining above background in all tumor cells. Positive EGFR expression is defined as any IHC (immunohistochemistry) complete or incomplete membrane staining of tumor cells, including intensity 1+, 2+ or 3 +.
DNA extraction and direct sequencing of KRAS
Genomic DNA was isolated from frozen primary CRC tissues, using proteinase-K (Stratagene, La Jolla, CA, USA) digestion and the phenol/chloroform extraction procedure according to the method outlined by Sambrook et al. [31]. The designed sequences of oligonucleotide primers for exons 2 and 3 of the KRAS and the operational procedure of direct sequencing were based on those of our previously study [18, 32].
Statistical analysis
All data were statistically analyzed using the Statistical Package for the Social Sciences, version 19.0 (SPSS Inc., Chicago, IL, USA). The correlation between clinicopathological features and EGFR expression or KRAS mutation was compared using a Chi-square test (for categorical variables) and Student t-test (for continuous variables). The Cox proportional-hazards model was used for univariate and multivariate analyses to identify the independent prognostic factors for OS, DFS and PFS. OS, DFS, and PFS were calculated by the Kaplan-Meier method, and the differences in survival rates were analyzed by the log-rank test. A P value less than 0.05 was considered to be statistically significant.
Results
Characteristics of patients with mCRC
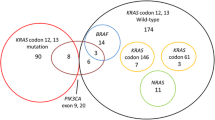
Of the 205 patients with mCRC, 98 patients (47.8%) were metachronous and 107 patients (52.2%) were synchronous. The mean age of the 205 patients was 61.0 ± 12.8 (range, 29–86) years of age. There were 120 males and 85 females. The median follow-up time for the 205 patients was 30.2 ± 20.9 (range, 1–137.3) months. Immunohistochemical analyses for EGFR expression were performed in 174 patients and positive expression was noted in 140 of those patients (80.5%). KRAS mutation status was evaluated in 205 patients, and mutation was noted in 88 of those patients (42.9%).
Characteristics of patients with metachronous mCRC
The clinical and pathological data regarding the 98 patients with metachronous mCRC are summarized in Table 1. Immunohistochemical analyses for EGFR expression were performed in 84 patients, and positive EGFR expression was noted in 67 of those patients (79.8%). There were no significant differences in mean ages, gender, tumor location, AJCC/UICC cancer stage, retrieved lymph node number, vascular invasion, perineural invasion, pre-operative serum CEA level, and post-operative serum CEA level between patients with positive EGFR expression and those with negative EGFR expression. However, OS (36.72 vs. 62.51 months, P < 0.001) and DFS (14.48 vs. 34.27 months, P < 0.001) rates of patients with positive EGFR expression were significantly poorer than those of patients with negative EGFR expression.
KRAS mutation status was evaluated in 98 patients and mutation was noted in 44 of those patients (44.9%). There were no significant differences in mean ages, tumor location, histological type, AJCC/UICC cancer stage, retrieved lymph node number, vascular invasion, perineural invasion, pre-operative serum CEA level, and post-operative serum CEA level between patients with wild-type KRAS and those with mutated KRAS. OS (42.54 vs. 37.41 months, P = 0.293) and DFS (18.38 vs. 16.43 months, P = 0.487) were not significantly different between patients with wild-type KRAS and those with mutated KRAS.
Characteristics of patients with synchronous mCRC
The clinical and pathological data regarding the 107 patients with synchronous mCRC are summarized in Table 2. Immunohistochemical analyses for EGFR expression were performed in 90 patients, and a positive EGFR expression was noted in 73 patients (88.0%). There were no significant differences in mean ages, gender, tumor location, histological type, tumor depth, lymph node metastasis, retrieved lymph node number, vascular invasion, perineural invasion, pre-operative serum CEA level, and post-operative serum CEA level between patients with positive EGFR expression and those with negative EGFR expression. Moreover, OS (22.08 vs. 24.70 months, P = 0.523) and PFS (9.65 vs. 7.44 months, P = 0.417) were not significantly different between patients with positive EGFR expression and those negative EGFR expression. The differences of clinical and pathological data regarding the patients with metachronous mCRC and the patients with synchronous mCRC are summarized in Additional file 1: Table S1.
KRAS mutation status was evaluated in 107 patients, and mutation was noted in 44 of those patients (41.1%). There were no significant differences in mean ages, gender, tumor location, histological type, tumor depth, lymph node metastasis, retrieved lymph node number, vascular invasion, perineural invasion, pre-operative serum CEA level, and post-operative serum CEA level between patients with wild-type KRAS and those with mutated KRAS. Moreover, OS (23.04 vs. 18.74 months, P = 0.074) and PFS (10.22 vs. 7.95 months, P = 0.101) were also not significantly different.
Univariate and multivariable analyses of survival impact of EGFR expression and KRASmutation in patients with metachronous mCRC
The univariate and multivariate analyses were performed to investigate independent prognostic factors for OS and DFS in patients with metachronous mCRC using the Cox proportional-hazards model (Table 3). Positive EGFR expression was demonstrated to be independent negative prognostic factors for OS (P = 0.028; HR, 3.090; 95% CI, 1.130–8.445) and DFS (P = 0.006; HR, 4.012; 95% CI, 1.477–10.900). However, KRAS mutation was not a significant prognostic factor for OS (P = 0.140; HR, 1.815; 95% CI, 0.823–4.004) and DFS (P = 0.260; HR, 1.440; 95% CI, 0.656–0.081). The Kaplan-Meier survival analysis also demonstrated that patients with positive EGFR expressions had worse OS (P = 0.003) and DFS (P < 0.001) (Figure 2A and 2B). The median OS times of patients with positive EGFR expression and those with negative EGFR expression were 49.50 and 76.20 months (P = 0.003; 95% CI, 41.223–57.777 and 52.175–99.920), respectively. The 5-year OS rates of patients with positive EGFR expression and those with negative EGFR expression were 23% and 79%, respectively. The median DFS times of patients with positive EGFR expression and those with negative EGFR expression were 20.96 and 50.17 months (P < 0.001; 95% CI, 17.216–24.708 and 38.822–61.510), respectively. The 3-year DFS rates of patients with positive EGFR expression and those with negative EGFR expression were 16% and 51%, respectively.
The Kaplan-Meier survival curve for patients with metachronous mCRC. A. Overall survival stratified by EGFR expression. B. Disease-free survival stratified by EGFR expression. C. Overall survival stratified by KRAS mutation status. D. Disease-free survival stratified by KRAS mutation status. E. Overall survival stratified by wild-type KRAS, codon 12, and codon 13. F. Disease-free survival stratified by wild-type KRAS, codon 12, and codon 13.
The Kaplan-Meier survival analysis demonstrated no significant difference between patients with wild-type KRAS and those with mutated KRAS in terms of OS (P = 0.461) and DFS (P = 0.783) (Figure 2C and 2D). The median OS times of patients with wild-type KRAS and those with mutated KRAS were 66.10 and 50.30 months (P = 0.461; 95% CI, 39.430–92.770 and 40.770–59.830), respectively. The median DFS times of patients with wild-type KRAS and those with mutated KRAS were 37.90 and 22.80 months (P = 0.783; 95% CI, 11.120–44.680 and 14.470–31.130), respectively. Furthermore, we analyzed the OS and DFS of patients with wild-type KRAS (N = 54), mutated KRAS codon 12 (N = 32), and mutated KRAS codon 13 (N = 12). No significant difference was noted in terms of OS (P = 0.656) and DFS (P = 0.977) (Figures 2E and 2F).
Univariate and multivariable analyses of survival impact of EGFR expression and KRASmutation in patients with synchronous mCRC
Univariate and multivariate analyses were performed to investigate the independent prognostic factors for OS and PFS in patients with synchronous mCRC using the Cox proportional-hazards model (Table 4). No variable was demonstrated to be an independent prognostic factor for OS and PFS in patients with synchronous mCRC. The Kaplan-Meier survival analysis demonstrated no significant difference between patients with positive EGFR expression and those with negative EGFR expression in terms of OS (P = 0.883) and PFS (P = 0.945) (Figure 3A and 3B). The median OS times of patients with positive EGFR expression and those with negative EGFR expression were 22.30 and 21.70 months (P = 0.883; 95% CI, 18.836–25.764 and 6.972–36.428), respectively. The median PFS times of patients with positive EGFR expression and those with negative EGFR expression were 8.20 and 11.70 months (P = 0.945; 95% CI, 6.356–10.044 and 8.425–14.975), respectively. In addition, the Kaplan-Meier survival analysis demonstrated no significant difference between patients with wild-type KRAS and those with mutated KRAS in terms of OS (P = 0.544) and PFS (P = 0.555) (Figure 3C and 3D). The median OS times of patients with wild-type KRAS and those with mutated KRAS were 22.50 and 21.30 months (P = 0.544; 95% CI, 21.036–23.964 and 17.967–24.633), respectively. The median PFS times of patients with wild-type KRAS and those with mutated KRAS were 9.30 and 11.70 months (P = 0.555; 95% CI, 7.395–11.205 and 4.696–18.704), respectively. Furthermore, we analyzed the OS and DFS of patients with wild-type KRAS (N = 63), mutated KRAS codon 12 (N = 37), and mutated KRAS codon 13 (N = 7). No significant difference was noted in terms of OS (P = 0.656) and PFS (P = 0.977) (Figure 3E and 3F).
The Kaplan-Meier survival curve for patients with synchronous mCRC. A. Overall survival stratified by EGFR expression. B. Disease-free survival stratified by EGFR expression. C. Overall survival stratified by KRAS mutation status. D. Disease-free survival stratified by KRAS mutation status. E. Overall survival stratified by wild-type KRAS, codon 12, and codon 13. F. Disease-free survival stratified by wild-type KRAS, codon 12, and codon 13.
Discussion
Of the 205 patients analyzed in this study, 98 patients had metachronous and 107 had synchronous mCRC. Positive EGFR expression was found in 80.5% patients through immunohistochemical analyses. The positive rate of EGFR expression in CRC was reported to be 25% to 82% [7]. KRAS mutation status was evaluated in 205 patients and mutation was noted in 88 of those patients (42.9%), in concordance with the mutation rate of KRAS in CRC (35% to 42%) [19]. In our patients with metachronous mCRC, EGFR expression was associated with differentiation grade of the tumor, with more moderate differentiation in patients with positive EGFR expression (P = 0.028), in accordance with the report of Andreyev et al. [25]. However, the association was not noted in our synchronous mCRC patients. The association between histological grade and EGFR expression is still controversial [6–8, 33, 34].
For the prognostic value of EGFR for patients with metachronous mCRC, we have demonstrated EGFR as an independent negative prognostic factor for OS and DFS by multivariate Cox proportional-hazards model. The Kaplan-Meier survival analysis also showed that patients with positive EGFR expression had worse OS and DFS. Galizia et al. [7] have shown that there is strong association between disease-specific survival and EGFR expression status, and a more than 10-fold risk of cancer related death in patients with positive EGFR expression compared with patients with negative EGFR expression. The difference was even stronger in patients with Duke’s C and D colon cancer than in those with Duke’s A and B colon cancer [7]. Ljuslinder et al. [6] have shown an association between worse outcomes and higher EGFR expression at invasive margin. Giralt et al. [9] evaluated the relationship between prognosis and EGFR expression in patients with locally advanced rectal cancer (LARC) receiving preoperative radiotherapy, and they found that the pathological response rate was lower in patients with positive EGFR expression than in those with negative EGFR expression. Azria et al. [10] conducted a similar study to evaluate the prognostic impact of EGFR expression on locoregional recurrence in patients with LARC receiving preoperative radiotherapy. The locoregional recurrence rate was higher in patients with EFGR extent ≧25% than in patients with EFGR ≦25% (20% vs. 7%). The locoregional recurrence-free survival rate at 2 years was 94% and 84%, respectively (P = 0.06). EFGR extent ≧25% was a significant factor for locoregional recurrence (P = 0.037; HR, 7.18; 95% CI, 1.17–46). Theodoropoulos et al. [34] reported a significant association between high EGFR expression and advanced T3 and T4 stages (P = 0.001), which implied that EGFR overexpression was associated with tumor invasion. Furthermore, they also demonstrated a trend between positive EGFR expression and poorer OS. Deng et al. [35] reported a significant association between high EGFR expression in primary tumor and poorer OS (P = 0.046); however, the association was not noted in stage IV patients, which is in agreement with our present study. The association between EFGR expression and worse survival has also been noted in other malignancies, such as gastric cancer [36, 37], esophageal cancer [38], and breast cancer [39]. In contrast, Spano et al. [8] and McKay et al. [33] reported no significant association between EGFR expression and survival.
Through the multivariate Cox proportional-hazards analyses and Kaplan-Meier survival analysis used in this study, KRAS mutation status was found not to be a significant independent prognostic factor of OS, DFS, and PFS for patients with metachronous mCRC and synchronous mCRC. Roth et al. [19] reported a mutated rate of 37% of KRAS mutation from 1299 patients with stages II and III colon cancer. No significant association between survival (OS and relapse-free survival) and KRAS mutation status was demonstrated. Moreover, no difference was noted between survival (OS and relapse-free survival) and type of KRAS mutation stratified by condon 12 and 13 in patients with stages II and III colon cancer, which is in agreement with our analyses of patients with metachronous mCRC. Rose et al. [20] assessed the survival impact of KRAS mutation status in 110 patients with metachronous and synchronous mCRC. The OS of patients with metachronous and those with synchronous mCRC was not influenced by KRAS mutation status (P = 0.55 and 0.37, respectively), which is also consistent with our present study. Three studies [21–23] from Asia evaluating the survival impact of KRAS mutation status in CRC patients also showed that there was no prognostic value of KRAS mutation status for OS and PFS. Recently, a systemic review and meta-analysis regarding the prognostic value of KRAS mutation status demonstrated that there was no association between KRAS mutation status and the prognosis of patients with CRC [24]. In contrast, 2 large collaborative Kirsten Ras in Colorectal Cancer Collaborative Group (RASCAL) studies [25, 26] evaluated the prognostic role in CRC. It was concluded in these studies that mutated KRAS were significantly associated with an increased risk of relapse and death and a significant association between failure-free survival and G12V of mutated KRAS in Duke’s C patients. Richman et al. [27] reported similar PFS and worse OS in patients with mutated KRAS compared to patients with wild-type KRAS. Roth et al. indicated that the KRAS mutations were assessed by each referring center according to three types of methodologies, and meta-analysis results could be affected by variations between trials [19]. In a meta-analysis assessing the predictive and prognostic value of KRAS mutations in CRC patients treated with cetuximab, it was concluded that mCRC patients with mutated KRAS could have worse PFS and OS when treated with cetuximab [28].
To our best knowledge, this study is the first to evaluate the prognostic values of EGFR expression and KRAS mutation simultaneously in patients with metachronous and synchronous mCRC. However, there are some limitations to the present study. First, the present study is a single-institution retrospective study. The study’s relatively small sample size is another limitation. Third, the KRAS mutation status was evaluated only as wild-type and mutant (codon 12 and codon 13) and we did not analyze other rare mutation types.
Conclusion
In conclusion, we have demonstrated that EGFR expression has prognostic value only for patients with metachronous mCRC, while having no such value for patients with synchronous mCRC. Our data indicate EGFR as an independent negative prognostic factor for OS and DFS in patients with metachronous mCRC. Analyzing EGFR expression may help identify high-risk patients requiring more aggressive therapeutic modalities in the setting of metachronous mCRC. However, KRAS mutation did not have prognostic value for patients with metachronous or synchronous mCRC.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- CRC:
-
Colorectal cancer
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- CEA:
-
Carcinoembryonic antigen
- AJCC:
-
American joint commission on cancer
- IHC:
-
Immunohistochemistry
- PD:
-
Poorly differentiated
- MD:
-
Moderately differentiated
- WD:
-
Well differentiated.
References
Jemal A, Siegel R, Xu J, Ward E: Cancer statistics, 2013. CA Cancer J Clin. 2013, 63: 11-30. 10.3322/caac.21166.
Department of health, the Executive Yuan, Republic of China: Health and vital statistics. http://www.mohw.gov.tw/cht/DOS/Index.aspx,
Teufel A, Steinmann S, Siebler J, Zanke C, Hohl H, Adami B, Schroeder M, Klein O, Höhler T, Galle PR, Heike M, Moehler M: Irinotecan plus folinic acid/continuous 5-fluorouracil as simplified bimonthly FOLFIRI regimen for first-line therapy of metastatic colorectal cancer. BMC Cancer. 2004, 4: 38-10.1186/1471-2407-4-38.
Folprecht G, Lutz MP, Schöffski P, Seufferlein T, Nolting A, Pollert P, Köhne CH: Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006, 17: 450-456. 10.1093/annonc/mdj084.
Ciardiello F, Tortora G: EGFR antagonists in cancer treatment. N Engl J Med. 2008, 358: 1160-1174. 10.1056/NEJMra0707704.
Ljuslinder I, Melin B, Henriksson ML, Öberg Å, Palmqvist R: Increased epidermal growth factor receptor expression at the invasive margin is a negative prognostic factor in colorectal cancer. Int J Cancer. 2011, 128: 2031-2037. 10.1002/ijc.25559.
Galizia G, Lieto E, Ferraraccio F, De Vita F, Castellano P, Orditura M, Imperatore V, La Mura A, La Manna G, Pinto M, Catalano G, Pignatelli C, Ciardiello F: Prognostic significance of epidermal growth factor receptor expression in colon cancer patients undergoing curative surgery. Ann Surg Oncol. 2006, 13: 823-835. 10.1245/ASO.2006.05.052.
Spano JP, Lagorce C, Atlan D, Milano G, Domont J, Benamouzig R, Attar A, Benichou J, Martin A, Morere JF, Raphael M, Penault-Llorca F, Breau JL, Fagard R, Khayat D, Wind P: Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol. 2005, 16: 102-108. 10.1093/annonc/mdi006.
Giralt J, de las Heras M, Cerezo L, Eraso A, Hermosilla E, Velez D, Lujan J, Espin E, Rosello J, Majó J, Benavente S, Armengol M, de Torres I, Grupo Español de Investigacion Clinica en Oncologia Radioterápica (GICOR): The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy: a multicenter, retrospective analysis. Radiother Oncol. 2005, 74: 101-108. 10.1016/j.radonc.2004.12.021.
Azria D, Bibeau F, Barbier N, Zouhair A, Lemanski C, Rouanet P, Ychou M, Senesse P, Ozsahin M, Pèlegrin A, Dubois JB, Thèzenas S: Prognostic impact of epidermal growth factor receptor (EGFR) expression on loco-regional recurrence after preoperative radiotherapy in rectal cancer. BMC Cancer. 2005, 20: 5-62.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004, 351: 337-345. 10.1056/NEJMoa033025.
Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB: Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005, 23: 1803-1810. 10.1200/JCO.2005.08.037.
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG: Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007, 25: 1658-1664. 10.1200/JCO.2006.08.1620.
Wierzbicki R, Jonker DJ, Moore MJ, Berry SR, Loehrer PJ, Youssoufian H, Rowinsky EK: A phase II, multicenter study of cetuximab monotherapy in patients with refractory, metastatic colorectal carcinoma with absent epidermal growth factor receptor immunostaining. Invest New Drugs. 2011, 29: 167-174. 10.1007/s10637-009-9341-6.
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD: Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008, 26: 1626-1634. 10.1200/JCO.2007.14.7116.
Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR: K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008, 359: 1757-1765. 10.1056/NEJMoa0804385.
Yen LC, Yeh YS, Chen CW, Wang HM, Tsai HL, Lu CY, Chang YT, Chu KS, Lin SR, Wang JY: Detection of KRAS oncogene in peripheral blood as a predictor of the response to cetuximab plus chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res. 2009, 15: 4508-4513. 10.1158/1078-0432.CCR-08-3179.
Yen LC, Uen YH, Wu DC, Lu CY, Yu FJ, Wu IC, Lin SR, Wang JY: Activating KRAS mutations and overexpression of epidermal growth factor receptor as independent predictors in metastatic colorectal cancer patients treated with cetuximab. Ann Surg. 2010, 251: 254-260. 10.1097/SLA.0b013e3181bc9d96.
Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, Aranda E, Nordlinger B, Cisar L, Labianca R, Cunningham D, Van Cutsem E, Bosman F: Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol. 2010, 28: 466-474. 10.1200/JCO.2009.23.3452.
Rose JS, Serna DS, Martin LK, Li X, Weatherby LM, Abdel-Misih S, Zhao W, Bekaii-Saab T: Influence of KRAS mutation status in metachronous and synchronous metastatic colorectal adenocarcinoma. Cancer. 2012, 118: 6243-6252. 10.1002/cncr.27666.
Liou JM, Wu MS, Shun CT, Chiu HM, Chen MJ, Chen CC, Wang HP, Lin JT, Liang JT: Mutations in BRAF correlate with poor survival of colorectal cancers in Chinese population. Int J Colorectal Dis. 2011, 26: 1387-1395. 10.1007/s00384-011-1229-1.
Zlobec I, Bihl MP, Schwarb H, Terracciano L, Lugli A: Clinicopathological and protein characterization of BRAF- and K-RAS-mutated colorectal cancer and implications for prognosis. Int J Cancer. 2010, 127: 367-380.
Kim ST, Park KH, Kim JS, Shin SW, Kim YH: Impact of KRAS mutation status on outcomes in metastatic colon cancer patients without anti-epidermal growth factor receptor therapy. Cancer Res Treat. 2013, 45: 55-62. 10.4143/crt.2013.45.1.55.
Ren J, Li G, Ge J, Li X, Zhao Y: Is K-ras gene mutation a prognostic factor for colorectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. 2012, 55: 913-923. 10.1097/DCR.0b013e318251d8d9.
Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA: Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst. 1998, 90: 675-684. 10.1093/jnci/90.9.675.
Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N, Beranek M, Jandik P, Benamouzig R, Jullian E, Laurent-Puig P, Olschwang S, Muller O, Hoffmann I, Rabes HM, Zietz C, Troungos C, Valavanis C, Yuen ST, Ho JW, Croke CT, O'Donoghue DP, Giaretti W, Rapallo A, Russo A, Bazan V, et al: Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer. 2001, 85: 692-696. 10.1054/bjoc.2001.1964.
Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, Taylor G, Barrett JH, Quirke P: KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009, 27: 5931-5937. 10.1200/JCO.2009.22.4295.
Qiu LX, Mao C, Zhang J, Zhu XD, Liao RY, Xue K, Li J, Chen Q: Predictive and prognostic value of KRAS mutations in metastatic colorectal cancer patients treated with cetuximab: a meta-analysis of 22 studies. Eur J Cancer. 2010, 46: 2781-2787. 10.1016/j.ejca.2010.05.022.
Edge SB, Byrd DR, Compton CC, et al: AJCC cancer staging manual. 2010, New York: Springer, 2010-7
Scartozzi M, Bearzi I, Berardi R, Mandolesi A, Fabris G, Cascinu S: Epidermal growth factor receptor (EGFR) status in primary colorectal tumors does not correlate with EGFR expression in related metastatic sites: implications for treatment with EGFR-targeted monoclonal antibodies. J Clin Oncol. 2004, 22: 4772-4778. 10.1200/JCO.2004.00.117.
Sambrook J, Fitsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. 1989, NY: Cold Spring Harbor Press
Wang JY, Hsieh JS, Chen FM, Yeh CS, Alexandersen K, Huang TJ, Chen D, Lin SR: High frequency of activated K-ras codon 15 mutant in colorectal carcinomas from Taiwanese patients. Int J Cancer. 2003, 107: 387-393. 10.1002/ijc.11417.
McKay JA, Murray LJ, Curran S, Ross VG, Clark C, Murray GI, Cassidy J, McLeod HL: Evaluation of the epidermal growth factor receptor (EGFR) in colorectal tumours and lymph node metastases. Eur J Cancer. 2002, 38: 2258-2264. 10.1016/S0959-8049(02)00234-4.
Theodoropoulos GE, Karafoka E, Papailiou JG, Stamopoulos P, Zambirinis CP, Bramis K, Panoussopoulos SG, Leandros E, Bramis J: P53 and EGFR expression in colorectal cancer: a reappraisal of ‘old’ tissue markers in patients with long follow-up. Anticancer Res. 2009, 29: 785-791.
Deng Y, Kurland BF, Wang J, Bi J, Li W, Rao S, Lan P, Lin T, Lin E: High epidermal growth factor receptor expression in metastatic colorectal cancer lymph nodes may be more prognostic of poor survival than in primary tumor. Am J Clin Oncol. 2009, 32: 245-252. 10.1097/COC.0b013e3181891326.
Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F, Galizia G: Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008, 15: 69-79. 10.1245/s10434-007-9596-0.
Galizia G, Lieto E, Orditura M, Castellano P, Mura AL, Imperatore V, Pinto M, Zamboli A, De Vita F, Ferraraccio F: Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J Surg. 2007, 31: 1458-1468. 10.1007/s00268-007-9016-4.
Wang KL, Wu TT, Choi IS, Wang H, Resetkova E, Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Albarracin CT: Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer. 2007, 109: 658-667. 10.1002/cncr.22445.
Buchholz TA, Tu X, Ang KK, Esteva FJ, Kuerer HM, Pusztai L, Cristofanilli M, Singletary SE, Hortobagyi GN, Sahin AA: Epidermal growth factor receptor expression correlates with poor survival in patients who have breast carcinoma treated with doxorubicin-based neoadjuvant chemotherapy. Cancer. 2005, 104: 676-681. 10.1002/cncr.21217.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/13/599/prepub
Acknowledgements
This work was supported by the foundation Biosignature in Colorectal Cancers, Academia Sinica, Taiwan, as well as an Excellence for Cancer Research Center Grant funded by the Department of Health, Executive Yuan, Taiwan, Republic of China (DOH102-TD-C-111-002) and the Kaohsiung Medical University Hospital (KMUH100-0 M13).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
CWH analyzed the data and wrote the manuscript. HLT, CMH, CJM, CYL, CHK and DCW made substantial contributions in data acquisition, statistical analyses, and data interpretation, and helped in manuscript preparation. YTC and CYC participated in making formalin-fixed and paraffin-embedded tissue blocks, immunohistochemical staining of EGFR, and interpretation of EGFR expression. JYW participated in study design and coordination. All authors have read and approved the final manuscript.
Electronic supplementary material
12885_2013_4212_MOESM1_ESM.docx
Additional file 1: Table S1: Baseline characteristics of metachronous and synchronous metastatic colorectal cancer patients. (DOCX 17 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Huang, CW., Tsai, HL., Chen, YT. et al. The prognostic values of EGFR expression and KRAS mutation in patients with synchronous or metachronous metastatic colorectal cancer. BMC Cancer 13, 599 (2013). https://doi.org/10.1186/1471-2407-13-599
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-13-599