Abstract
Background
This study aimed to systematically review the evidence from randomized controlled trials (RCTs) and to conduct a meta-analysis of the effects of yoga on physical and psychosocial outcomes in cancer patients and survivors.
Methods
A systematic literature search in ten databases was conducted in November 2011. Studies were included if they had an RCT design, focused on cancer patients or survivors, included physical postures in the yoga program, compared yoga with a non-exercise or waitlist control group, and evaluated physical and/or psychosocial outcomes. Two researchers independently rated the quality of the included RCTs, and high quality was defined as >50% of the total possible score. Effect sizes (Cohen’s d) were calculated for outcomes studied in more than three studies among patients with breast cancer using means and standard deviations of post-test scores of the intervention and control groups.
Results
Sixteen publications of 13 RCTs met the inclusion criteria, of which one included patients with lymphomas and the others focused on patients with breast cancer. The median quality score was 67% (range: 22–89%). The included studies evaluated 23 physical and 20 psychosocial outcomes. Of the outcomes studied in more than three studies among patients with breast cancer, we found large reductions in distress, anxiety, and depression (d = −0.69 to −0.75), moderate reductions in fatigue (d = −0.51), moderate increases in general quality of life, emotional function and social function (d = 0.33 to 0.49), and a small increase in functional well-being (d = 0.31). Effects on physical function and sleep were small and not significant.
Conclusion
Yoga appeared to be a feasible intervention and beneficial effects on several physical and psychosocial symptoms were reported. In patients with breast cancer, effect size on functional well-being was small, and they were moderate to large for psychosocial outcomes.
Similar content being viewed by others
Background
Cancer represents a major public health concern. In Western countries, approximately one in three persons will be directly affected by cancer before the age of 75 years, with breast cancer, melanoma, colorectal cancer and prostate cancer comprising the most common types [1, 2]. Due to medical advances, survival rates have improved over the past decade. For example, currently, the 5-year survival rates across all cancers are approximately 56% for male and 62% for female patients in Australia [1] and 58% and 64%, respectively, in the Netherlands [2]. However, cancer and its treatment are often associated with prolonged adverse physical and psychosocial symptoms, including reduced physical function and fitness and increased risk of anxiety, depression, and fatigue [3, 4]. This greatly impacts the patient’s quality of life (QoL) [5, 6]. Therefore, there is a need for effective methods to manage physical and psychosocial symptoms and to improve QoL of cancer patients and survivors.
Psychosocial interventions such as counselling, support groups and cognitive behavioural therapies may help patients cope with cancer and the psychosocial problems associated with cancer and cancer treatment, but are less likely to help with common physical issues such as loss of strength and flexibility, weight gain, and reduced physical function [7]. Findings from previous reviews and meta-analyses suggest that aerobic and resistance exercise attenuate a range of the physical problems associated with cancer and cancer treatment [3, 4, 6, 8–16]. The benefits of these types of exercise include not only improved physical function, but also reduced fatigue and improved QoL. Unfortunately, many cancer patients perceive various barriers to exercise [17–21]. The most common physical barriers are physical discomfort and feeling sick. Psychosocial barriers include having low mood, feelings of self-consciousness relating to appearance and body image, fatigue and fear for overdoing it [20, 22, 23]. Because of these barriers, approximately one out of three adult cancer patients turns to complementary and alternative medicine techniques, mindfulness, or yoga, to help manage their symptoms [24–26].
Yoga is a ‘mind-body’ exercise, a combination of physical poses with breathing and meditation [27]. Several studies in the non-cancer population reported positive effects of yoga on physical outcomes including perceptual and motor skills [28], cardiopulmonary function [29], fitness [30], muscle strength, flexibility, stiffness, and joint pain [31–33]. Furthermore, a recent review of 10 studies comparing the effects of yoga asanas (postures) with those of ‘regular’ exercise, indicated that yoga may be as effective as exercise for improving health outcomes such as blood glucose and lipids, fatigue, pain, and sleep in healthy people and in people with conditions such as diabetes and multiple sclerosis [34].
Previous reviews [35, 36] and a meta-analysis [37] of intervention studies have reported that yoga is feasible for patients with cancer, with improved sleep, QoL, mood and levels of stress. The current study extends previous work by our exclusive focus on 1) randomised controlled trials (RCTs), the most rigorous intervention study design; 2) yoga interventions that included physical postures and were not part of a larger program such as Mindfulness-Based Stress Reduction; and 3) a focus on both physical and psychosocial outcomes.
The aim of the present study is to conduct a systematic review and meta-analysis of the effects of yoga in cancer patients and survivors, focusing on both physical and psychosocial outcomes.
Methods
Literature search
IR, medical librarian, conducted the literature search in ten databases: AgeLine and AMED (Allied and Complementary Medicine Database), British Nursing Index, CINAHL, CENTRAL (The Cochrane Central Register of Controlled Trials), EMBASE, PEDro, PsycINFO, PubMed and SPORTDiscus (earliest to November 2011). In order to identify all relevant papers, a search was conducted with both thesaurus terms and free terms for ‘yoga’ in combination with an extensive list of search terms to identify intervention studies. RCTs were identified using search terms for certain publication types (e.g. randomized controlled trial and controlled clinical trial in PubMed) in combination with a list of free text terms in title and abstracts that could be used to describe RCTs (e.g. randomi*ed, randomly, trial, groups). Detailed search profiles are available on request from IR. Additional articles were identified by manually checking the reference list of included papers.
Study inclusion criteria
Study inclusion criteria were: (i) design: RCT; (ii) population: adults with any cancer diagnosis either during or post treatment; (iii) intervention: yoga including physical postures (asanas); (iv) control group: non-exercise or wait-list; (v) outcome: physical and psychosocial outcomes. Only full-text articles written in English were included. Studies that included yoga as part of a larger intervention program (e.g., Mindfulness-Based Stress Reduction, meditation, or pranayama (breathing control) only) were excluded.
Selection process and quality assessment
Titles and abstracts of the references were reviewed to exclude articles out of scope (JvU). Full-text articles of potentially relevant records were assessed for eligibility by two independent reviewers (LB and JvU).
LB and JvU independently assessed the quality of the included papers using a Delphi list developed by Verhagen et al. [38], which consists of nine equally weighted quality criteria to assess different methodological aspects (see below). This list has previously been used for the evaluation of methodological quality in systematic reviews of exercise programs [39–41]. Criteria have a ‘yes’ (=1), ‘no’ (=0) or ‘don’t know’ (=0) answer format. Disagreements between the reviewers were discussed and resolved, and in case of doubt, a third reviewer (MC) was consulted. Authors were contacted for additional information if it was not possible to score an item based on the information provided in the paper. Items scoring a “yes” contribute to the quality scores, ranging from 0 to 9 points. Where outcomes were assessed by self-report only, criterion 5 (blinding of the outcome assessor) was not applicable, and studies could obtain a maximum quality score of 8 points. A study was classified as a low quality study if the quality score was lower than 50% of the maximum possible score [41].
Criteria considered for quality assessment according to Verhagen et al. [38]
-
1.
Was a method of randomization performed?
-
2.
Was the treatment allocation concealed?
-
3.
Were the groups similar at baseline?
-
4.
Were the eligibility criteria specified?
-
5.
Was the outcome assessor blinded?
-
6.
Was the yoga instructor blinded (i.e. unaware of the study aim)?
-
7.
Was the participant blinded?
-
8.
Were point estimates and measures of variability (between groups comparison) presented for the primary outcomes?
-
9.
Did the analysis include an intention-to-treat analysis?
Data extraction
The following data were extracted by LB: (i) study population; (ii) type, intensity, frequency and duration of intervention, (iii) control group; (iv) outcome measures; and (v) effects on physical and/or psychosocial outcomes.
Meta-analysis
Effect sizes were calculated (standardized mean difference d) for all individual studies by subtracting the average post-test score of the control group (Mc) from that of the yoga intervention group (My) and dividing the result by the pooled standard deviations of the yoga intervention group and the control groups (SDyc) [42]. An effect size of 0.5 thus indicates that the mean of the experimental group is half a standard deviation larger than the mean of the control group. Effect sizes of 0.56 to 1.2 are large, while effect sizes of 0.33 to 0.55 are moderate and effect sizes of 0 to 0.32 are small [43].
For outcomes that were investigated in >3 studies, individual effect sizes were pooled in Comprehensive Meta-Analysis (CMA; version 2.2.046). Because only one study did not include patients with breast cancer [44], the meta-analyses was conducted on data from studies including patients with breast cancer only. As we expected considerable heterogeneity, we calculated pooled effect sizes with the random effects model. This model assumes that the included studies are drawn from ‘populations’ of studies that differ from each other systematically (heterogeneity). In this model, the prevalence resulting from the included studies not only differs because of the random error within studies (fixed effects model), but also because of true variation in prevalence from one study to the next. We first tested the heterogeneity under the fixed model using the statistics I2 and Q. I2 describes the variance between studies as a proportion of the total variance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity, with 25% as low, 50% as moderate, and 75% as high heterogeneity [45]. When P values of the Q are above 0.05, the total variance is due to variance within studies and not to variance between studies. We ran the analyses on all studies and with outliers excluded. Studies with extreme values of which the 95% confidence interval had no overlap with the 95% confidence interval of the pooled estimate were considered as outliers.
Results
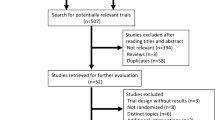
After removing duplicates, the literature searches yielded a total of 1909 unique records. For 171 potentially relevant records, we checked full text (Figure 1). The majority of the studies (n = 79) were excluded because they were not designed as a RCT. Of the records identified in the database search, 15 records met the inclusion criteria. We found one additional RCT [31] from the reference list of the review by Smith and Pukall [35]. Both Vadiraja et al. [46–48] and Raghavendra et al. [49, 50] published more than one paper on the same RCT, each describing different outcome measures and/or subpopulations. Thus 16 papers [31, 32, 44, 46–58] of 13 RCTs were included in this systematic review. Details of the populations, yoga interventions, and outcomes of the included studies are presented in Tables 1, 2 and 3.
Quality assessment
Results of the methodological quality assessment are presented in Table 4. Median quality score was 67% (range 22–89%). All but one study [31] were of high quality. All included studies used randomization. In all but one [31] study treatment allocation was concealed, and groups were comparable at baseline, or dissimilarities at baseline were adequately adjusted for in the analyses. All studies adequately specified the eligibility criteria of the study population. The outcome assessor was blinded in five papers [32, 51, 52, 57, 58], but this criterion was not applicable in the seven papers using self-reported outcomes only [44, 47, 49, 50, 55]. In five papers [51, 52, 55–57], the yoga instructor was blinded as he or she was unaware of the study aim. Participants were blinded in two papers [51, 58]; Banerjee [51] informed us that their study was double blinded. In four papers, point estimates and 95% confidence intervals (CI) for between group differences were reported [47, 50, 54, 58]. One paper [44] reported 95% CI only, and three papers [46, 48, 55] only presented effect sizes, without 95% CI. In nine papers [32, 47, 48, 50, 52–55, 58], data were analyzed on an intention-to-treat basis.
Study population
Details of the study populations are reported in Table 1. Twelve studies included patients with breast cancer and one study focused on patients with lymphomas [44]. Five studies in patients with breast cancer studies took place during cancer treatment: three studies (five papers [46–48, 51, 55]) during radiotherapy, one study [31] during hormone therapy, and one study (two papers, [49, 50]) during chemotherapy with or without additional radiotherapy. Five studies [32, 52, 56–58] focused on breast cancer survivors who had completed treatment, and two studies [53, 54] included patients and survivors both during and after treatment. The study in patients with lymphomas included patients during and after active treatment [44]. Sample sizes ranged from 18 to 128 patients, with seven studies including less than 50 patients, and only one study with more than 100 patients. Average age of the participants ranged from 44 to 63 years. One study did not report the age of the patients [50]. Eleven studies in patient with breast cancer included women only, one study [52] in mainly breast cancer patients (85%) included 5% men, and the study in lymphoma patients [44] included 39% men.
Yoga program
The content of the yoga programs is summarized in Table 2. All included a supervised yoga program with physical poses (yoga asanas), combined with breathing techniques (pranayama) and relaxation or meditation (savasana or dhanya).
All yoga classes were led by experienced yoga instructors. Median program duration was seven weeks with a range of six weeks to six months. In the study by Rao et al. [50], the program duration depended on the number of chemotherapy cycles, which ranged from four to eight. In this latter study, supervised sessions were conducted for 30 min before chemotherapy once every ten days. Furthermore, patients were provided with audiotapes of the exercises for home practice and asked to practice 1 h daily for 6 days/week during intervals between chemotherapy cycles [49]. In general, the number of classes per week ranged from one to three, and home practice was encouraged in nine studies, supported by audio or videotapes. Session duration ranged from 30 to 120 min; three studies did not report the session duration [31, 44, 50].
In nine studies [31, 32, 44, 52–57] the yoga program was compared with a wait-list control group. In three studies [46–51], the control group received supportive therapy with education, counseling, or coping preparation. In one study, the control group received health education classes [58].
Effects
Tables 5 and 6 present an overview of the effects of yoga on physical and psychosocial outcomes, respectively (for details, see Table 3). Fourteen papers reported on both physical and psychosocial outcomes, and two papers reported on psychosocial outcomes only.
Physical outcomes
Twenty-three physical outcomes were examined in thirteen of the included papers (Table 5). In addition to self-reported physical function and functional well-being, outcomes included nine physical symptoms (e.g., pain, nausea, and dyspnoea), nine measures of physical activity and fitness, and three biological variables. However, except for physical function, functional well being, and pain, the outcomes were studied in only three studies or less, thus we considered this evidence insufficient to draw conclusions on the effectiveness of yoga on these outcomes. After excluding an outlier [55], the pooled effect size of yoga on physical function in patients with breast cancer was small and insignificant (d = 0.17; 95% CI = −0.06 to 0.40), see Table 7. Further, in patients with breast cancer, yoga resulted in a small but significant increase in functional well-being (d = 0.31; 95% CI = 0.04 to 0.58). Pain was evaluated in four studies, of which standard deviations to calculate effect sizes were not available in two studies [32, 44]. The average effect size of the other two studies among patients with breast cancer [48, 55] was large (d = −0.64; 95% CI = −0.98 to −0.31).
Psychosocial outcomes
Twenty psychosocial outcomes were examined in the fifteen included papers (Table 6). The effects of yoga on distress, anxiety, depression, fatigue, sleep, general QoL, emotional function and social function were evaluated in three or more studies. After excluding outliers, yoga resulted in significant large reductions in distress (d = −0.75; 95% CI = −1.09 to −0.42), anxiety (d = −0.77; 95% CI = −1.08 to −0.46), and depression (d = −0.69; 95% CI = −1.02 to −0.37), moderate reductions in fatigue (d = −0.51; 95% CI = −0.79 to −0.22), and moderate increases in general HRQoL (d = 0.37; 95% CI = 0.11 to 0.62), emotional function (d = 0.49; 95% CI = 0.16 to 0.81), and social function (d = 0.33; 95% CI = 0.12 to 0.54) in breast cancer patients, see Table 7. Effects on sleep disturbances were small and insignificant (d = −0.26; 95% CI = −0.53 to 0.02). In patients with lymphoma, however, Cohen et al. [44] found a significant reduction in sleep disturbances (d = −1.00; 95% CI = −3.8 to −0.8). Although some studies found beneficial effects on other psychosocial outcomes, including positive and negative effect, mood, spirituality and relaxation (Table 6), these were studied in less than three studies. Therefore, this evidence was considered to be insufficient.
Dropout and attendance
Dropout from the studies, defined as the number of randomized participants without post-intervention measurement ranged from 0 to 38%. Attendance at the yoga classes was reported in nine studies [32, 44, 47, 53–57], and varied between 58 and 88% (Table 2). Vadiraja et al. [47] reported that the level of adherence did not influence results on QoL, positive and negative affect [47]. Four other studies reported on the influence of intervention adherence on outcomes. Danhauer et al. [53] reported that better intervention adherence was associated with higher self-reported physical function and QoL. In contrast, Moadel et al. [54] found similar improvements in QoL among participants with low and high class attendance, but found a positive association between intervention attendance and improved mood. Carson et al. [32] also showed that greater mean yoga practice time was associated with less fatigue, less symptom bother, and more acceptance at post-treatment, and tended to be associated with less sleep disturbances. Littman et al. [57] reported that generally, the benefits were greater among women who attended more facility-based classes, but results were not entirely consistent.
Safety
Five studies evaluated adverse events and provided this information in the manuscripts [47, 50, 53, 57, 58]. Four studies reported that there were no adverse events and one study [58] reported one adverse event of a participant with a history of back problems, who experienced a back spasm in yoga class. After evaluation by her physician, she was able to return to class and complete the intervention.
Discussion
This review and meta-analysis described and evaluated sixteen papers examining yoga as an intervention to manage physical and psychosocial symptoms in cancer patients and survivors. In contrast to previous reviews [35, 36] and meta-analysis [37] we only included studies focusing on yoga interventions with physical postures, and evaluating the effectiveness on physical and/or psychosocial outcomes. Yoga appeared to be a feasible intervention, and beneficial effects on several physical and psychosocial symptoms were reported, with a small effect on functional well-being and moderate to large effects on various psychosocial outcomes.
Physical outcomes
Due to the limited number of studies per physical outcome, evidence for physical effects of yoga was generally insufficient to draw firm conclusions. The effects of yoga on physical function and functional well-being were small. This may be related to the short intervention duration; only two studies lasted 12 weeks or longer [54, 57], all others were shorter, ranging from 6 to 10 weeks (median = 7). To improve physical function and fitness, longer intervention duration may be required. The lack of significant improvements in physical function and fitness may also be related to the relatively low intensity of certain types of yoga [52, 59]. Nevertheless, in healthy older adults, a 6-month yoga intervention resulted in improved physical outcomes such as timed 1-leg stand, flexibility, and energy [60]. These beneficial effects may be related to the lower baseline cardiorespiratory fitness of older adults compared with younger patients, and the longer intervention duration in that specific study. Significant improvements in treadmill time and estimated peak oxygen uptake as a result of yoga have also been shown in a small group of patients with chronic heart failure [61]. A systematic review of studies comparing yoga with other forms of exercise concluded that in both healthy people and in patients with chronic diseases, yoga may be as effective or better than other forms of exercise at improving a variety of health-related outcome measures, including physical outcomes such as muscle strength and flexibility [34]. One study with healthy sedentary elderly people has reported that peak oxygen uptake increased by 11% after yoga, compared with 24% after aerobic training [62]. Although patients perceived that they had improved fitness after 12 weeks of yoga [63], future empirical evidence should indicate whether yoga is as beneficial as endurance or strength exercise in improving physical fitness in (physically inactive) cancer patients.
Psychosocial outcomes
This review found that yoga has large beneficial effects on distress, anxiety and depression, moderate beneficial effects on fatigue, general HRQoL, emotional function and social function, and a small and insignificant effect on sleep. There was insufficient evidence for effects on psychosocial outcomes that were studied less frequently including cognitive function, vigor, anger-hostility, spirituality, relaxation and mental health. More studies evaluating the effects of yoga on these outcomes are needed before we can draw firm conclusions.
The finding that yoga improves QoL, and reduces distress and depression concurs with findings from previous reviews and meta-analysis of yoga interventions for cancer patients and survivors [35, 37]. In contrast, the current meta-analysis could not confirm previous findings on reductions in sleep disturbances in patients with breast cancer. Fatigue is among the most frequently occurring and debilitating complaints associated with cancer and cancer treatments [5, 64]. Therefore, it is important to find effective strategies to reduce fatigue in cancer patients. In contrast to the meta-analysis of Lin et al. [37], we found a moderate significant effect size on fatigue. This is in line with a recent study of Bower et al. [58] who showed beneficial effects after 12 weeks of yoga classes on persistent fatigue in breast cancer survivors. In addition, patients themselves also perceived improvements in QoL, fatigue, stress, anxiety and depression [63]. The moderate-to-large effect sizes on these psychosocial outcomes seem larger than the small to moderate effect sizes of exercise [12, 16, 65–67] or psychosocial interventions [12, 66, 68]. However, our results have to be interpreted with caution due to small sample sizes in most studies. Furthermore effect sizes may also be influenced by patient selection, i.e. including also non-fatigued or non-depressed patients. Therefore, future studies should obtain insight in the most effective interventions to improve psychosocial outcomes. The current meta-analysis showed that yoga may be such an intervention.
Methodological quality of studies
This review included a quality rating, and only one paper was of low quality. A major concern regarding the methodological quality of most included studies was that not all participants completed the yoga program and data were not analysed on an intention-to-treat basis. This may have introduced bias, overestimating the benefits of yoga. Only half of the studies reported class attendance, of which four studies indicated that intervention adherence was positively associated with some outcomes [32, 53, 54, 57]. Whether adherence to the yoga sessions was affected by cancer-related symptoms or side-effects of cancer treatment was not reported.
Most studies separately reported the descriptive results of the outcomes for the yoga and control groups, and presented only p-values for the group differences. Many studies however, did not report effect sizes or other point estimates of the between-group differences, and their confidence intervals. Therefore we calculated standardized mean differences using means and standard deviations of the post-test values.
Furthermore, as with all exercise interventions, blinding was difficult. Because the control group usually consisted of either wait-list or usual care, participants were not blinded to the intervention, possibly introducing bias.
Strengths and limitations
The extensive search in ten databases, the inclusion of RCTs, the methodological quality assessment and conduction of a meta-analysis are strengths of the study. Further, by only including studies focusing on yoga interventions that contained physical postures (asanas), we attempted to reduce the variability between the yoga interventions, thereby increasing the comparability of studies. Nevertheless, there may still remain some variability between the different types of yoga interventions included in this review. This may be reflected by the high heterogeneity. Other sources of high heterogeneity may be differences in instruments used to define the outcome, differences in patient groups (i.e. different stage of cancer, or different timing of the intervention with respect to primary cancer treatment), or differences in control groups. Because of the small number of studies, we were unable to conduct subgroup analyses to further reduce heterogeneity. Although we used random effects modelling to take into account the large heterogeneity, overall effect sizes should be interpreted with caution as they may vary somewhat among subgroups.
In general, publication bias endangers the external validity of reviews and meta-analyses. Also in this study, publication bias cannot be ruled out. Another limitation is the small sample size of some studies. In addition, some studies were conducted by the same research group, and other studies had multiple outcomes, increasing the probability of type 1 errors. Furthermore, most studies offered yoga to people based on having cancer, not based on having physical or psychosocial problems, which may have resulted in an underestimation of the beneficial effects of yoga.
The yoga interventions were conducted during various forms of cancer treatment; some were conducted post-treatment, and some studies included a mixed sample of patients during and post-treatment. Due to the limited number of studies, it is difficult to draw conclusions on the optimal timing for yoga interventions. Future studies should consider this issue.
Further, although effect sizes for many psychosocial outcomes were generally moderate to large, effect sizes of yoga interventions on physical function and functional well-being were small. This may indicate that the effect is small, or that some patients may have physical benefits from yoga whereas others may not, which may be indicative of the heterogeneity of cancer patients. Future studies with large sample sizes should identify moderators of the effect of yoga on physical and psychosocial outcomes in order to identify subgroups of patients whom may specifically benefit from yoga.
Finally, this review only included papers published in the English language. Although our searches were not limited to language, we may have missed important findings from yoga in Asia, in which practicing yoga is much more common than in Western countries. Nevertheless, this review included six studies that were conducted in Asia.
Clinical implications
The emerging literature provides preliminary support for the feasibility and efficacy of yoga interventions for cancer patients. Only one adverse effect was reported in five studies that assessed adverse events, and the results indicate that yoga may improve physical well-being and psychosocial outcomes. Although the literature suggests yoga may be effective in improving physical outcomes in other patients and healthy elderly [29, 30, 34], evidence in cancer patients and survivors is generally insufficient to draw firm conclusions at this stage. In contrast, physical exercise has been shown to be effective in improving physical function and fitness in cancer patients and survivors [3, 4, 10, 16]. However, for cancer patients and survivors who are unable or unwilling to participate in traditional aerobic or resistance exercise programs yoga may be an appropriate form of exercise [63] as it is especially suitable for those who perceive barriers to other forms of exercise [69]. Breast cancer patients have been found to perceive more barriers to exercise than age-matched controls, and higher barriers were associated with less exercise [70]. In a recent pilot study, breast cancer survivors reported minimal barriers and high motivation for participating in a yoga program [63]. All participants reported that yoga was beneficial and enjoyable, that they were confident that they could do the exercises, and that they were motivated to attend all classes [63]. However, evidence for yoga as an effective intervention to improve physical function and fitness is lacking and should be established by future studies. Future studies should also systematically assess and report adverse events related to yoga.
Conclusion
This systematic review and meta-analysis of RCTs showed that yoga has strong beneficial effects on distress, anxiety and depression, moderate effects on fatigue, general HRQoL, emotional function and social function, small effects on functional well-being, and no significant effects on physical function and sleep disturbances. Results of the current review must be interpreted with caution due to the relative small sample sizes of most of the included studies. RCTs with larger sample sizes are needed to improve our understanding of the physical and psychosocial effects of yoga. Future studies should also address the optimal duration and frequency of yoga, the effects in patients with types of cancer other than breast cancer, and the optimal time point in the cancer and cancer treatment or rehabilitation trajectories for offering yoga interventions [63].
References
Australian Institute of Health and Welfare: Cancer survival and prevalence in Australia. 2008, CAT no CAN 38, Canberra
IKCnet.Dutch Cancer Registration: Survival. 2009, http://www.ikcnet.nl,
Courneya KS, Friedenreich CM: Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999, 21: 171-179. 10.1007/BF02908298.
Courneya KS: Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003, 35: 1846-1852. 10.1249/01.MSS.0000093622.41587.B6.
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al: Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000, 5: 353-360. 10.1634/theoncologist.5-5-353.
Dimeo FC: Effects of exercise on cancer-related fatigue. Cancer. 2001, 92: 1689-1693. 10.1002/1097-0142(20010915)92:6+<1689::AID-CNCR1498>3.0.CO;2-H.
Emslie C, Whyte F, Campbell A, Mutrie N, Lee L, Ritchie D, et al: 'I wouldn't have been interested in just sitting round a table talking about cancer'; exploring the experiences of women with breast cancer in a group exercise trial. Health Educ Res. 2007, 22: 827-838.
Cheema B, Gaul CA, Lane K, Fiatarone Singh MA: Progressive resistance training in breast cancer: a systematic review of clinical trials. Breast Cancer Res Treat. 2008, 109: 9-26. 10.1007/s10549-007-9638-0.
Conn CA, Kozak WE, Tooten PC, Gruys E, Borer KT, Kluger MJ: Effect of voluntary exercise and food restriction in response to lipopolysaccharide in hamsters. J Appl Physiol. 1995, 78: 466-477.
De Backer IC, Schep G, Backx FJ, Vreugdenhil G, Kuipers H: Resistance training in cancer survivors: a systematic review. Int J Sports Med. 2009, 30: 703-712. 10.1055/s-0029-1225330.
Galvao DA, Newton RU: Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005, 23: 899-909. 10.1200/JCO.2005.06.085.
Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ: Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007, 26: 660-667.
Kangas M, Bovbjerg DH, Montgomery GH: Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008, 134: 700-741.
Kirshbaum MN: A review of the benefits of whole body exercise during and after treatment for breast cancer. J Clin Nurs. 2007, 16: 104-121. 10.1111/j.1365-2702.2006.01638.x.
Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G: Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005, 23: 3830-3842. 10.1200/JCO.2005.02.148.
Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH: An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010, 4: 87-100. 10.1007/s11764-009-0110-5.
Courneya KS, McKenzie DC, Reid RD, Mackey JR, Gelmon K, Friedenreich CM, et al: Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Ann Behav Med. 2008, 35: 116-122.
Midtgaard J, Baadsgaard MT, Moller T, Rasmussen B, Quist M, Andersen C, et al: Self-reported physical activity behaviour; exercise motivation and information among Danish adult cancer patients undergoing chemotherapy. Eur J Oncol Nurs. 2009, 13: 116-121. 10.1016/j.ejon.2009.01.006.
Rogers LQ, Courneya KS, Verhulst S, Markwell SJ, McAuley E: Factors associated with exercise counseling and program preferences among breast cancer survivors. J Phys Act Health. 2008, 5: 688-705.
Whitehead S, Lavelle K: Older breast cancer survivors' views and preferences for physical activity. Qual Health Res. 2009, 19: 894-906. 10.1177/1049732309337523.
Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Vallance JK, et al: A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Ann Behav Med. 2005, 29: 147-153. 10.1207/s15324796abm2902_9.
Perna FM, Craft L, Carver CS, Antoni MH: Negative affect and barriers to exercise among early stage breast cancer patients. Health Psychol. 2008, 27: 275-279.
Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS: Predictors of adherence and contamination in a randomized trial of exercise in colorectal cancer survivors. Psychooncology. 2004, 13: 857-866. 10.1002/pon.802.
Ernst E, Cassileth BR: The prevalence of complementary/alternative medicine in cancer: a systematic review. Cancer. 1998, 83: 777-782. 10.1002/(SICI)1097-0142(19980815)83:4<777::AID-CNCR22>3.0.CO;2-O.
Bernstein BJ, Grasso T: Prevalence of complementary and alternative medicine use in cancer patients. Oncology (Williston Park). 2001, 15: 1267-1272.
Molassiotis A, Fernadez-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, et al: Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005, 16: 655-663. 10.1093/annonc/mdi110.
Lipton L: Using yoga to treat disease: an evidence-based review. JAAPA. 2008, 21: 34-36. 38, 41
Telles S, Ramaprabhu V, Reddy SK: Effect of yoga training on maze learning. Indian J Physiol Pharmacol. 2000, 44: 197-201.
Raub JA: Psychophysiologic effects of Hatha Yoga on musculoskeletal and cardiopulmonary function: a literature review. J Altern Complement Med. 2002, 8: 797-812. 10.1089/10755530260511810.
Tran MD, Holly RG, Lashbrook J, Amsterdam EA: Effects of Hatha Yoga Practice on the Health-Related Aspects of Physical Fitness. Prev Cardiol. 2001, 4: 165-170. 10.1111/j.1520-037X.2001.00542.x.
Blank SE, Kittel J, Haberman MR: Active practice of Iyengar Yoga as an intervention for breast cancer survivors. Int J Yoga Therapy. 2010, 15: 51-59.
Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL: Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009, 17: 1301-1309. 10.1007/s00520-009-0587-5.
Galantino M, Cannon N, Hoelker T, Iannaco J, Quinn L: Potential benefits of walking and yoga on perceived level of cognitive decline and persistent fatigue in women with breast cancer. Rehabil Oncol. 2007, 25: 3-16.
Ross A, Thomas S: The health benefits of yoga and exercise: a review of comparison studies. J Altern Complement Med. 2010, 16: 3-12. 10.1089/acm.2009.0044.
Smith KB, Pukall CF: An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009, 18: 465-475. 10.1002/pon.1411.
Bower JE, Woolery A, Sternlieb B, Garet D: Yoga for cancer patients and survivors. Cancer Control. 2005, 12: 165-171.
Lin KY, Hu YT, Chang KJ, Lin HF, Tsauo JY: Effects of yoga on psychological health, quality of life, and physical health of patients with cancer: a meta-analysis. Evid Based Complement Alternat Med. 2011, Epub
Verhagen AP, de Vet HC, de Bie RA, Boers M, van den Brandt PA: The art of quality assessment of RCTs included in systematic reviews. J Clin Epidemiol. 2001, 54: 651-654. 10.1016/S0895-4356(00)00360-7.
Chinapaw MJ, van Uffelen JG, Riphagen I, van Mechelen W: The functional effects of physical exercise training in frail older people: a systematic review. Sports Med. 2008, 38: 781-793. 10.2165/00007256-200838090-00006.
van Uffelen JG, Chinapaw MJ, Hopman-Rock M, van Mechelen W: The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clin J Sport Med. 2008, 18: 486-500. 10.1097/JSM.0b013e3181845f0b.
Verhagen AP, Karels C, Bierma-Zeinstra SM, Feleus A, Dahaghin S, Burdorf A, et al: Exercise proves effective in a systematic review of work-related complaints of the arm, neck, or shoulder. J Clin Epidemiol. 2007, 60: 110-117.
Cuijpers P, van Straten A, Bohlmeijer E, Hollon SD, Andersson G: The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychol Med. 2010, 40: 211-223. 10.1017/S0033291709006114.
Lipsey MW: Design sensitivity; statistical power for experimental research. 1990, Sage, Newbury Park
Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A: Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004, 100: 2253-2260. 10.1002/cncr.20236.
Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ. 2003, 327: 557-560. 10.1136/bmj.327.7414.557.
Vadiraja HS, Raghavendra RM, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, et al: Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009, 8: 37-46. 10.1177/1534735409331456.
Vadiraja HS, Rao MR, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, et al: Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complement Ther Med. 2009, 17: 274-280. 10.1016/j.ctim.2009.06.004.
Vadiraja HS, Rao RM, Hongasandra NR, Nagarathna R, Rekha M, Vanitha N, et al: Effects of yoga on symptom management in breast cancer patients: A randomized controlled trial. Int J Yoga. 2009, 2: 73-79. 10.4103/0973-6131.60048.
Raghavendra RM, Nagarathna R, Nagendra HR, Gopinath KS, Srinath BS, Ravi BD, et al: Effects of an integrated yoga programme on chemotherapy-induced nausea and emesis in breast cancer patients. Eur J Cancer Care (Engl). 2007, 16: 462-474. 10.1111/j.1365-2354.2006.00739.x.
Rao RM, Raghuram N, Nagendra HR, Gopinath KS, Srinath BS, Diwakar RB, et al: Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complement Ther Med. 2009, 17: 1-8. 10.1016/j.ctim.2008.05.005.
Banerjee B, Vadiraj HS, Ram A, Rao R, Jayapal M, Gopinath KS, et al: Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007, 6: 242-250. 10.1177/1534735407306214.
Culos-Reed SN, Carlson LE, Daroux LM, Hately-Aldous S: A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psychooncology. 2006, 15: 891-897. 10.1002/pon.1021.
Danhauer SC, Mihalko SL, Russell GB, Campbell CR, Felder L, Daley K, et al: Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009, 18: 360-368. 10.1002/pon.1503.
Moadel AB, Shah C, Wylie-Rosett J, Harris MS, Patel SR, Hall CB, et al: Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007, 25: 4387-4395. 10.1200/JCO.2006.06.6027.
Chandwani KD, Thornton B, Perkins GH, Arun B, Raghuram NV, Nagendra HR, et al: Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010, 8: 43-55.
Banasik J, Williams H, Haberman M, Blank SE, Bendel R: Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011, 3: 135-142.
Littman AJ, Bertram LC, Ceballos R, Ulrich CM, Ramaprasad J, McGregor B, et al: Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: effects on quality of life and anthropometric measures. Support Care Cancer. 2012, 20: 267-277. 10.1007/s00520-010-1066-8.
Bower JE, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, et al: Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2012, 118: 3766-3775. 10.1002/cncr.26702.
Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, et al: Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993, 25: 71-80. 10.1249/00005768-199301000-00011.
Oken BS, Zajdel D, Kishiyama S, Flegal K, Dehen C, Haas M, et al: Randomized, controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern Ther Health Med. 2006, 12: 40-47.
Pullen PR, Nagamia SH, Mehta PK, Thompson WR, Benardot D, Hammoud R, et al: Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail. 2008, 14: 407-413. 10.1016/j.cardfail.2007.12.007.
Bowman AJ, Clayton RH, Murray A, Reed JW, Subhan MM, Ford GA: Effects of aerobic exercise training and yoga on the baroreflex in healthy elderly persons. Eur J Clin Invest. 1997, 27: 443-449. 10.1046/j.1365-2362.1997.1340681.x.
Speed-Andrews AE, Stevinson C, Belanger LJ, Mirus JJ, Courneya KS: Pilot evaluation of an iyengar yoga program for breast cancer survivors. Cancer Nurs. 2010, 33: 369-381. 10.1097/NCC.0b013e3181cfb55a.
de Jong N: Courtens AM, bu-Saad HH, Schouten HC: Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nurs. 2002, 25: 283-297. 10.1097/00002820-200208000-00004.
Cramp F, Daniel J: Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008, CD006145-2
Duijts SF, Faber MM, van Oldenburg HS BM, Aaronson NK: Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors--a meta-analysis. Psychooncology. 2011, 20: 115-126. 10.1002/pon.1728.
Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM: The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol (R Coll Radiol). 2010, 22: 208-221. 10.1016/j.clon.2009.12.005.
Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G: Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev. 2009, CD006953-1
Brawley LR, Culos-Reed SN, Angove J, Hoffman-Goetz L: Understanding the barriers to phyiscal activity for cancer patients: review and recommendations. J Psychosoc Oncol. 2002, 20: 1-21.
Courneya KS, Friedenreich CM: Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997, 3: 215-226. 10.1089/acm.1997.3.215.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/12/559/prepub
Acknowledgments
The contribution of LM Buffart was supported by a fellowship granted by the EMGO Institute for Health and Care Research and a grant from the Alpe d’HuZes/KWF Fund, provided by the Dutch Cancer Society. JGZ van Uffelen was supported by a NHMRC program grant (Owen, Bauman and Brown; #569663) at The University of Queensland, School of Human Movement Studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LB, JvU and MC have made substantial contributions to conception and design of the manuscript. LB and JvU have screened papers and conducted the quality rating and meta-analysis. IR has conducted the literature search. LB and JvU have been involved in drafting the manuscript. MC, IR, WB, JB, WvM have been involved in critically revising the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Buffart, L.M., van Uffelen, J.G., Riphagen, I.I. et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 12, 559 (2012). https://doi.org/10.1186/1471-2407-12-559
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-12-559