Abstract
Background
Human epidermal growth factor receptor 2 (HER2) is the most crucial ErbB receptor tyrosine kinase (RTK) family member in HER2-positive (refered to HER2-overexpressing) breast cancer which are dependent on or "addictive" to the Phosphatidylinositol-3-kinase (PI3K) pathway. HER2-related target drugs trastuzumab and lapatinib have been the foundation of treatment of HER2--positive breast cancer. This study was designed to explore the relationship between PI3K pathway activation and the sensitivity to lapatinib in HER2--positive metastatic breast cancer patients pretreated with anthracyclins, taxanes and trastuzumab.
Methods
Sixty-seven HER2-positive metastatic breast cancer patients were recruited into a global lapatinib Expanded Access Program and 57 patients have primary tumor specimens available for determination of PI3K pathway status. PTEN status was determined by immunohistochemical staining and PIK3CA mutations were detected via PCR sequencing. All patients were treated with lapatinib 1250 mg/day continuously and capecitabine 1000 mg/m2 twice daily on a 2-week-on and 1-week-off schedule until disease progression, death, withdrawal of informed consent, or intolerable toxicity.
Results
PIK3CA mutations and PTEN loss were detected in 12.3% (7/57) and 31.6% (18/57) of the patients, respectively. Twenty-two patients with PI3K pathway activation (defined as PIK3CA mutation and/or PTEN expression loss) had a lower clinical benefit rate (36.4% versus 68.6%, P = 0.017) and a lower overall response rate (9.1% versus 31.4%, P = 0.05), when compared with the 35 patients with no activation. A retrospective analysis of first trastuzumab-containing regimen treatment data showed that PI3K pathway activation correlated with a shorter median progression-free survival (4.5 versus 9.0 months, P = 0.013).
Conclusions
PIK3CA mutations occur more frequently in elder patients for HER2-positive breast cancer. PIK3CA mutations and PTEN loss are not mutually exclusive. PI3K pathway activation resulting from PTEN loss or PIK3CA mutations may lead to drug resistance to lapatinib and trastuzumab (http://ClinicalTrials.gov number, NCT00338247).
Similar content being viewed by others
Background
Human epidermal growth factor receptor 2 (HER2) is the most crucial ErbB receptor tyrosine kinase (RTK) family member in breast cancer with overexpression in about one fourth of patients [1]. Since HER2 plays a key role in HER2-positive breast cancer, these patients usually have bad prognosis, and HER2-related target drugs have been the foundation of treatment. Trastuzumab, a HER2 monoclonal antibody against the extracellular domain of the molecule, has been a new standard in neo-adjuvant, adjuvant and palliative treatment of HER2-positive breast cancer [1–3]. However, trastuzumab mono-therapy shows a response rate of no more than 30% in palliative setting [4], and there is still a problem of primary or acquired resistance even with combination regimens. HER2-overexpressing breast cancer cells are dependent on or "addictive" to the Phosphatidylinositol-3-kinase (PI3K) pathway [5]. Published literatures showed that PI3K pathway activation is associated with primary resistance to trastuzumab, and trastuzumab exerts its antitumor effects only in the presence of a normal PI3K pathway [6–11].
PI3K pathway is one of the most important signaling pathways in cell, which is involved in many fundamental cellular processes, including proliferation, cell survival, motility and cell growth [12, 13]. Class IA PI3K, the most important member of the PI3K complex, is composed of a heterodimer with a p85 regulatory subunit and a p110 catalytic subunit (PIK3CA), residing downstream of multiple receptor kinase families including ErbB RTK family (EGFR, HER2, HER3, HER4) and transducing signals originating from them [12, 13]. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a phosphotase that converts membrane-associated phosphatidylinositol 3,4,5-triphosphate (PIP3) back to phosphatidylinositol 4,5-bisphosphate (PIP2) and negatively regulates signaling transduction of PI3K pathway [14, 15]. It is well known that dysregulation of PI3K pathway plays an important role in the development of malignancy, and the most common genetic alterations in this pathway are PIK3CA mutation and PTEN loss [16, 17], both of which can lead to constitutive activation of PI3K pathway and resistance to trastuzumab [7]. PTEN-related resistance to trastuzumab can be reversed by combined treatment with trastuzumab and the PI3K inhibitor LY294002 [18]. Therefore, PI3K pathway activation resulting from PIK3CA mutation and/or PTEN loss warrants further studies.
Up to now, little knowledge is available about the correlation between PI3K pathway status and efficacy and resistance of the other FDA-approved anti-HER2 agent, lapatinib. Laptinib, a dual tyrosine kinase inhibitor of EGFR and HER2, binds to the intracellular kinase domain [19]. It has no cross-resistance with trastuzumab since it is effective against breast cancer expressing p95HER2 [20], an active truncated form of HER2 and with HER2 epitope masked by mucin 4 [21]. Clinical data have shown the safety and efficacy of lapatinib alone and in combination with capecitabine, paclitaxel and letrozole and it is still effective in patients who have progressed on trastuzumab [22–24]. Therefore, the HER2 pathway is still an "addictive" oncogenic pathway in breast cancer pretreated with trastuzumab. However, several recent papers touching on PI3K pathway activation and lapatinib resistance conflicted with each other [18, 25–29], so we conducted this study to explore their correlation and the protocol was approved by the Fudan University Shanghai Cancer Center Institutional review board on June 30, 2008.
Methods
Patient Eligibility and Study Design
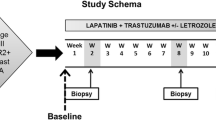
A global lapatinib Expanded Access Program was started to offer preapproval drug in order to provide clinical benefit to patients with HER2-positive metastatic breast cancer who had progressive diseases on treatment with regimens including anthracyclines, taxanes, and trastuzumab. Trastuzumab had to be used in metastatic setting. Tumors with either 3+ immunohistochemical staining for HER2 protein or HER2 gene amplification by fluorescence in situ hybridization were defined as HER2 positive in our institution. Women previously treated with capecitabine were eligible. Patients were required to have evaluable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST); an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; a left ventricular ejection fraction (LVEF) within the institution's normal range; a life expectancy of at least 12 weeks; and adequate renal, hepatic, and hematologic function. patients with central nervous system (CNS) metastases were eligible if they were clinically stable for at least 3 months after discontinuation of radiation therapy. patients with preexisting heart disease or conditions that could affect gastrointestinal absorption were ineligible. All patients gave written informed consent on recruitment into the global lapatinib Expanded Access Program and provision of the primary tumor sample for this translational study(http://ClinicalTrials.gov number, NCT00338247).
In this one-arm study, all patients receive the combination regimen consisting of lapatinib at a dose of 1250 mg daily on a continuous basis and capecitabine at a dose of 2000 mg per square meter of body-surface area in two divided doses on days 1 through 14 of a 21-day cycle. Standard recommendations for capecitabine dosage modifications were followed in the management of adverse events. Lapatinib was withheld for up to 14 days for grade 2 or more nonhematologic toxicity or any grade 3 or 4 hematologic toxicity. Patients were assessed every 6 weeks for the first 24 weeks, and then every 12 weeks while they were still receiving the study treatment. Patients who had no progressive disease but whose study treatment was withdrawn were assessed every 12 weeks until the commencement of alternative anticancer treatment, disease progression, or death. Efficacy was determined according to the RECIST criteria. Adverse events were assessed according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE, version 3.0). The clinical benefit was defined as a complete response, partial response, or stable disease for at least 6 months. Progression-free survival was calculated as the interval between the date of signing informed consent and the date of disease progression, or death from any cause.
PCR Sequencing and PIK3CA Mutation
DNA was extracted from formaldehyde-fixed, paraffin-embedded tumor tissue. PCR were performed with 10 to 100 ng of genomic DNA as template following a standard protocol. PIK3CA gene PCR primers were E9F CAAAGCAATTTCTACACGAGATCC; E9R GTAAAAACATGCTGAGATCAGCCACAT; E20F TGGAATGCCAGAACTACAATCTTT; E20R GGTCTTTGCCTGCTGAGAGTT. The PCR products were sequenced using the ABI3130XL automated capillary sequencer by Shanghai Tianhao Biotechnology Company.
Immunohistochemistry and PTEN Scoring
The antibody for PTEN IHC staining was a rabbit monoclonal anti-PTEN (D4.3) XPTM (Cell Signaling Beverly, MA, USA) diluted 1:200. The antibody was applied overnight at 4ºC. Then the tissues were incubated with the second antibody (1:500, Jackson lab) for 30 min. The color was developed with DAB solution about 1 min at room temperature and then stained in Harris hematoxylin solution for 3 min. Each set of slides included positive and negative control slides and normal cells in a tumor were used as an internal control.
PTEN immunoreactivity was examined by two independent observers who were blinded to the clinical data. A third pathologist was invited when the discordance was present between them. The staining was mainly visible in the cytoplasm of tumor cells. PTEN expression levels were semiquantified using immunoreactive scores (IRS) calculated by multiplying the percentage of PTEN-positive tumor cells (scored 0 to 4) with the PTEN staining intensity [1–3]. The tumor was graded as PTEN-negative (IRS 0-3), weak positive (IRS 4-6), positive (IRS 7-9), and strong positive (IRS 10-12)[6].
Statistical Analysis
The relationships between different variables were assessed by Chi-square tests and the trends were also examined by Chi-square tests when required. Differences in progression-free survival (PFS) and overall survival (OS) between groups were determined using the log-rank test. After a univariate analysis, the variables with significant correlation with PFS and OS, continuous variables and PI3K pathway status were put in a Cox proportional hazard regression model to determine which was an independent prognostic factor for PFS and OS, respectively. The statistical difference was considered significant if the P value was less than 0.05.
Results
Patient Characteristics
Sixty-seven Chinese patients were enrolled from Fudan University Cancer Center from Aug. 2008 to Mar. 2010. The median age was 49.0 years old (range 26-75). Fifty-seven patients had their tumor tissues available for detection of PI3K pathway activation (PIK3CA mutation and PTEN expression loss). Clinical and pathological data for the patients are showed in Table 1.
PIK3CA mutation and PTEN expression loss
The overall incidence of PIK3CA mutations was 12.3% (7 in 57 samples). The majority of mutations occurred at two hotspots, H1047R (7%, 4 samples) at exon 20 encoding the kinase domain (Figure 1a), and E542K (1.8%, 1 sample) at exon 9 encoding the helical domain (Figure 1b). L540F and T1052A mutations are rare and each was found in one tumor sample (Figure 1c-d).
PTEN expression loss was found in 18 patients (31.6%, Figure 2a). Thirty-nine patients were positive for PTEN expression [6], in which 17 (29.8%), 14 (24.6%), and 8 (14%) specimens were weak positive, positive and strong positive respectively (Figure 2b-d). In this study, PTEN loss was not mutually exclusive with PIK3CA mutations, since 3 of the 4 patients with H1047R mutation were also found to have no PTEN expression (Table 2).
Compared with the wild type, PI3K pathway activation (PIK3CA mutation and/or PTEN expression loss) was identified in a significantly older patient population (P = 0.016). The median age of patients with the PI3K pathway activation was 53.6 ± 7.9 years, while the median age of those with no PI3K pathway activation was 47.0 ± 10.9 years. The PI3K pathway activation status was not associated with all other clinicopathological parameters, such as hormone receptor status, HER2 protein expression status and disease free interval after radical mastectomy (all P > 0.05).
Patient outcome and PI3K pathway activation
On September 30, 2010, preliminary analysis was made on the basis of 50 disease progression events and 28 deaths. Median follow-up time was 15.3 ± 6.3 months. The median PFS of all 67 patients was 6.5 months (95%CI, 5.7-7.3 months), and the median PFS of the 57 patients who provided their tumor tissues for detection of PI3K pathway activation was also 6.5 months (95%CI, 5.4-7.6 months). The overall response rate (complete response plus partial response) was 22.4% for all 67 patients and 22.8% for the 57 patients whose tumor sample were available. The corresponding clinical benefit rates were 58.2% and 56.1%. The median overall survivals for both cohorts were 17.0 months.
An analysis of our data showed that PIK3CA mutation didn't correlate with overall response rate, clinical benefit rate or progression-free survival. For PTEN expression status, patients with wild type gene enjoyed a clinical benefit rate of 66.7%, which was statistically higher than 33.3% in those with no PTEN expression (P = 0.018). The overall response rate of 28.2% and median PFS of 8 months in the patients with PTEN expression were substantially higher, although the differences were not statistically significant (Table 3). When analyzing PIK3CA mutation together with PTEN expression loss since both can activate PI3K pathway, the clinical benefit was still observed for patients with no activation of PI3K pathway (68.6% versus 36.4%, P = 0.017; Figure 3). The overall response rate was also higher (31.4% versus 9.1%, P = 0.05; Figure 3). Both overall response and clinical benefit significantly correlated with PFS (Figure 4a-b), however, there was no significant association of PI3K pathway activation status with PFS or OS (Figure 4c).
Progression-free survival with objective response, clinical benefit, and PI3K pathway activation. The difference in PFS between patients with response (CR+PR) and those without response (a); the difference in PFS between patients with clinical benefit (CR+PR+SD > 6 months) and those without benefit (b); the difference in PFS between patients with pathway activation and those with no activation (c), and pathway activation status with efficacy of the first Trastuzumab-containing regimen (d).
A retrospective analysis was done to explore the relationship between PI3K pathway activation status and the efficacy of the other anti-HER2 drug, trastuzumab. We chose the progression-free survival of the first trastuzumab-containing regimen as an indicator for trastuzumab efficacy. The regimens were trastuzumab combined with one or two chemotherapy drugs, including docetaxel, vinorelbine, paclitaxel, gemcitabine, capecitabine and cisplatin. As previously reported, PI3K pathway activation shortened the median progression-free survival significantly (4.5 versus 9.0 months, P = 0.013; Figure 4d). PTEN expression status had a similar differentiating effect (6.0 versus 9.0 months, P = 0.024). However, the difference of PFS (4.5 versus 8.0 months) resulting from PIK3CA mutation wasn't significant.
Patient outcome and other factors
Response and survival of breast cancer might be affected by many other factors, such as age, ECOG performance status, hormone receptor status, HER2 expression, metastatic sites, number of metastatic sites and previous treatments. A univariate analysis of our data showed that only number of metastatic sites had a negative impact on overall response rate of the lapatinib and capecitbine doublet, while none of the above-mentioned factors was statistically associated with clinical benefit rate. Concerning survival, only previous capecitabine treatment was statistically associated with a shorter PFS (6.5 vs. 11.0 months, P = 0.011, Table 4) and OS (14.0 months vs. not reached, P = 0.003).
We put all the above-mentioned variants of PI3K pathway status, age and clinical benefit status into Cox's proportional hazards model to investigate how much PI3K pathway activation affected patient outcome. Clinical benefit rate was the only independent factor for PFS in HER2-positive breast cancer.
Discussion
PI3K pathway activation is the most common signal transduction pathway alteration in malignancies, including breast cancer [30]. It mostly results from PIK3CA mutation or amplification, PTEN loss [16]. Since the patients enrolled in this study were HER2-positive and high-level PIK3CA amplification without mutation is rare in this subpopulation [31, 32], so only PIK3CA mutation and PTEN loss were determined for the samples in our study.
Our data showed that PIK3CA mutation occurred in 12.3% of the patients, lower than 8-40% reported in other studies [33–38]. There are several reasons. First, only two exons accounting for approximately 85% of all mutations were determined in our study [33]. Second, mutation correlates with an old age and this phenomenon was confirmed by our study [39]. However, the median age of our patients was 49.0 years, about 10 years younger than Caucasian counterparts [39]. Third, the mutation was reported to occur more frequently in HER2-negative patients [39, 40], however, all patients in our study were HER2-positive.
Regarding mutations in hot spots, two common mutation points, H1047R and E542K were also present in our patients with no mutation of E545K observed [37, 39]. As to mutations in non-hot spots, two new points, L540F and T1052A mutations were first reported based on our knowledge. An analysis of our data showed that the ratio of hot spots to non-hot spots was 2.5 to 1, which is consistent with other reports [39]. Since there were only a few patients with the new mutation, our result needed further confirmation by other studies. Therefore, it remains a question whether the new mutation in non-hot spots results in an activation of PI3K pathway. As in other studies, these patients were considered to have a mutated gene in the analysis [39].
PTEN is a tumor suppressor gene, and can be down-regulated or lost of expression via deletion (common in breast cancer), mutation (rare in breast cancer), or promoter DNA methylation [14, 41, 42]. Loss of PTEN expression results in activation of PI3K pathway leading to development of cancer [15, 41]. PTEN loss is present in about one third of breast cancer patients, ranging from 15% to 48% [43–46]. Our study showed that the incidence of PTEN loss was 31.6%, which is consistent with other reported results.
Previous reports suggested that PIK3CA mutation and PTEN loss were mutually exclusive [37]. However, in 4 patients with H1047R mutations in our study, 3 patients were also found to have no PTEN expression. This fact was previously reported by Perez-Tenorio et al in 2009 [40]. PI3K mutation was indicated to be associated with ER positivity, HER2 negativity and primary tumor size, which were not observed in our study [37, 39, 40].
An analysis of our data showed that patients with PI3K pathway activation had a statistically significant shorter median PFS than those with no PI3K pathway activation (4.5 versus 9.0 months, P = 0.013), confirming the reported conclusion that PI3K pathway activation can result in resistance to trastuzumab [6–10]. Based on the published preclinical studies, these patients should be sensitive to lapatinib, a drug with a different mechanism of action [47]. There were some clinical data demonstrating that lapatinib induced objective responses in patients who had failed trastuzumab [22]. However, all patients were treated with lapatinib and capecitabine in our study, and PI3K pathway activation was still correlated with a lower clinical benefit rate (36.4% versus 68.6%, P = 0.017) and a lower overall response rate (9.1% versus 31.4%, P = 0.05), which is consistent with results of a smaller study reported by Cizkova et al [48]. Campone [49] et al pointed out that acquisition of resistance is frequently linked to an uncoupling between upstream signals emanating from HER2 itself and downstream signals related to PI3K, AKT and/or MAPK. Two studies showed that both knockdown of PTEN and transfection of mutant PIK3CA can result in lapatinib resistance and the mTOR/PI3K inhibitor, NVP-BEZ235 can reverse the resistance [26, 50].
However, there are also a few converse opinions. Based on the experimental results, O'Brien et al showed that lapatinib could overcome trastuzumab resistance via continued deactivation of PI3K/AKT/mTOR signaling [51]. A Japanese clinical study recruiting 122 patients attempted to illustrate the relationship between PI3K pathway activation and efficacy of lapatinib, but PIK3CA mutation was only found in 3 tissue samples among all 29 analyzed samples [52]. Recently, Toi et al indicated that low PTEN could predict response to lapatinib in a small phase 2 neoadjuvant trial [53]. Therefore, a definite conclusion regarding the PI3K pathway status and anti-HER2 therapy cannot be drawn up to now, and our study justifies further research.
It remains controversial whether the two gene alterations have any prognostic value. Li et al suggested that PIK3CA mutation was a negative prognostic factor [35]. On the contrary, a larger sample size study and a Japanese study indicated that it was a positive prognostic factor [36, 39]. Barbareschi [54] et al reported that mutation in exon 20 usually indicated good prognosis, while the mutation in exon 9 often meant bad prognosis. Perez-Tenorio [40] et al suggested that the two gene alterations should be combined with S phase fraction to give an accurate prediction of prognosis. Recently, Dupont Jensen [55] et al showed that there is a discrepancy of PIK3CA mutation between primary and metastatic tumors, urging on a simultaneous detection of the two matched samples. For the prognostic value of PTEN, it is relatively uniform and most investigators thought that PTEN loss is a negative prognostic factor [56, 57]. Our data showed that it was statistically associated with clinical benefit rate (P = 0.021). Due to a relatively smaller sample size of our study, no significant correlations between PI3K pathway status and clinicopathological parameters were found.
Conclusions
In conclusion, PIK3CA mutation occurs more frequently in elder patients and the ratio of mutations in hot spots to non-hot spots is about 2.5 to 1 in HER2-positive breast cancer patients. PTEN loss is present in about one third of patients. PIK3CA mutation and PTEN loss were not mutually exclusive. PI3K pathway activation may lead to drug resistance to lapatinib as well as trastuzumab.
Abbreviations
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome ten
- PI3K:
-
Phosphatidylinositol-3-kinase
- PIK3CA:
-
Phosphatidylinositol-3-kinase catalytic subunit
- EGFR:
-
Epidermal Growth Factor Receptor
- HER2:
-
Human Epidermal Growth Factor Receptor 2
- PFS:
-
Progression Free Survival
- OS:
-
Overall Survival
- ORR:
-
Overall Response Rate
- CBR:
-
Clinical Benefit Rate.
References
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001, 344 (11): 783-792. 10.1056/NEJM200103153441101.
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA: Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005, 353 (16): 1673-1684. 10.1056/NEJMoa052122.
Van Pelt AE, Mohsin S, Elledge RM, Hilsenbeck SG, Gutierrez MC, Lucci A, Kalidas M, Granchi T, Scott BG, Allred DC: Neoadjuvant trastuzumab and docetaxel in breast cancer: preliminary results. Clin Breast Cancer. 2003, 4 (5): 348-353. 10.3816/CBC.2003.n.040.
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M: Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002, 20 (3): 719-726. 10.1200/JCO.20.3.719.
She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N: Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS One. 2008, 3 (8): e3065.-
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT: PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004, 6 (2): 117-127. 10.1016/j.ccr.2004.06.022.
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M: A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007, 12 (4): 395-402. 10.1016/j.ccr.2007.08.030.
Fujita T, Doihara H, Kawasaki K, Takabatake D, Takahashi H, Washio K, Tsukuda K, Ogasawara Y, Shimizu N: PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer. 2006, 94 (2): 247-252. 10.1038/sj.bjc.6602926.
Fabi A, Metro G, Di Benedetto A, Nisticò C, Vici P, Melucci E, Antoniani B, Perracchio L, Sperduti I, Milella M: Clinical significance of PTEN and p-Akt co-expression in HER2-positive metastatic breast cancer patients treated with trastuzumab-based therapies. Oncology. 2010, 78 (2): 141-149. 10.1159/000312656.
Pandolfi PP: Breast cancer--loss of PTEN predicts resistance to treatment. N Engl J Med. 2004, 351 (22): 2337-2338. 10.1056/NEJMcibr043143.
Kataoka Y, Mukohara T, Shimada H, Saijo N, Hirai M, Minami H: Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010, 21 (2): 255-62. 10.1093/annonc/mdp304.
Bader AG, Kang S, Zhao L, Vogt PK: Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005, 5 (12): 921-929. 10.1038/nrc1753.
Engelman JA, Luo J, Cantley LC: The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006, 7 (8): 606-619. 10.1038/nrg1879.
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R: PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997, 275 (5308): 1943-1947. 10.1126/science.275.5308.1943.
Di Cristofano A, Pandolfi PP: The multiple roles of PTEN in tumor suppression. Cell. 2000, 100 (4): 387-390. 10.1016/S0092-8674(00)80674-1.
Brugge J, Hung MC, Mills GB: A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007, 12 (2): 104-107. 10.1016/j.ccr.2007.07.014.
Coughlin CM, Johnston DS, Strahs A, Burczynski ME, Bacus S, Hill J, Feingold JM, Zacharchuk C, Berkenblit A: Approaches and limitations of phosphatidylinositol-3-kinase pathway activation status as a predictive biomarker in the clinical development of targeted therapy. Breast Cancer Res Treat. 2010, 124 (1): 1-11. 10.1007/s10549-010-1108-4.
Kataoka Y, Mukohara T, Shimada H, Saijo N, Hirai M, Minami H: Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010, 21 (2): 255-262. 10.1093/annonc/mdp304.
Moy B, Kirkpatrick P, Kar S, Goss P: Lapatinib. Nat Rev Drug Discov. 2007, 6 (6): 431-432. 10.1038/nrd2332.
Scaltriti M, Chandarlapaty S, Prudkin L, Aura C, Jimenez J, Angelini PD, Sánchez G, Guzman M, Parra JL, Ellis C: Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010, 16 (9): 2688-2695. 10.1158/1078-0432.CCR-09-3407.
Mukohara T: Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci.
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B: Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006, 355 (26): 2733-2743. 10.1056/NEJMoa064320.
Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, Guerrera SF, Koehler M, Oliva C, Stein SH: Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008, 26 (34): 5544-5552. 10.1200/JCO.2008.16.2578.
Johnston S, Pippen J, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ: Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009, 27 (33): 5538-5546. 10.1200/JCO.2009.23.3734.
Xia W, Husain I, Liu L, Bacus S, Saini S, Spohn J, Pry K, Westlund R, Stein SH, Spector NL: Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007, 67 (3): 1170-1175. 10.1158/0008-5472.CAN-06-2101.
Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R: Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008, 68 (22): 9221-9230. 10.1158/0008-5472.CAN-08-1740.
Johnston S, Trudeau M, Kaufman B, Boussen H, Blackwell K, LoRusso P, Lombardi DP, Ben Ahmed S, Citrin DL, DeSilvio ML: Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008, 26 (7): 1066-1072. 10.1200/JCO.2007.13.9949.
Koninki K, Barok M, Tanner M, Staff S, Pitkanen J, Hemmila P, Ilvesaro J, Isola J: Multiple molecular mechanisms underlying trastuzumab and lapatinib resistance in JIMT-1 breast cancer cells. Cancer Lett. 2010, 294 (2): 211-219. 10.1016/j.canlet.2010.02.002.
O'Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, Duffy MJ, Crown J, O'Donovan N, Slamon DJ: Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010, 9 (6): 1489-1502. 10.1158/1535-7163.MCT-09-1171.
Engelman JA: Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009, 9 (8): 550-562. 10.1038/nrc2664.
Wu G, Xing M, Mambo E, Huang X, Liu J, Guo Z, Chatterjee A, Goldenberg D, Gollin SM, Sukumar S: Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res. 2005, 7 (5): R609-616. 10.1186/bcr1262.
Adélaïde J, Finetti P, Bekhouche I, Repellini L, Geneix J, Sircoulomb F, Charafe-Jauffret E, Cervera N, Desplans J, Parzy D: Integrated profiling of basal and luminal breast cancers. Cancer Res. 2007, 67 (24): 11565-11575. 10.1158/0008-5472.CAN-07-2536.
Buttitta F, Felicioni L, Barassi F, Martella C, Paolizzi D, Fresu G, Salvatore S, Cuccurullo F, Mezzetti A, Campani D: PIK3CA mutation and histological type in breast carcinoma: high frequency of mutations in lobular carcinoma. J Pathol. 2006, 208 (3): 350-355. 10.1002/path.1908.
Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA: Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004, 64 (21): 7678-7681. 10.1158/0008-5472.CAN-04-2933.
Li SY, Rong M, Grieu F, Iacopetta B: PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006, 96 (1): 91-95. 10.1007/s10549-005-9048-0.
Maruyama N, Miyoshi Y, Taguchi T, Tamaki Y, Monden M, Noguchi S: Clinicopathologic analysis of breast cancers with PIK3CA mutations in Japanese women. Clin Cancer Res. 2007, 13 (2 Pt 1): 408-414.
Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmström PO, Mansukhani M, Enoksson J: PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005, 65 (7): 2554-2559. 10.1158/0008-5472-CAN-04-3913.
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ: High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004, 304 (5670): 554.-10.1126/science.1096502.
Kalinsky K, Jacks LM, Heguy A, Patil S, Drobnjak M, Bhanot UK, Hedvat CV, Traina TA, Solit D, Gerald W: PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009, 15 (16): 5049-5059. 10.1158/1078-0432.CCR-09-0632.
Perez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjold B, Rutqvist LE, Skoog L, Stal O: PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007, 13 (12): 3577-3584. 10.1158/1078-0432.CCR-06-1609.
Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T: Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997, 15 (4): 356-362. 10.1038/ng0497-356.
Khan S, Kumagai T, Vora J, Bose N, Sehgal I, Koeffler PH, Bose S: PTEN promoter is methylated in a proportion of invasive breast cancers. Int J Cancer. 2004, 112 (3): 407-410. 10.1002/ijc.20447.
Bose S, Crane A, Hibshoosh H, Mansukhani M, Sandweis L, Parsons R: Reduced expression of PTEN correlates with breast cancer progression. Hum Pathol. 2002, 33 (4): 405-409. 10.1053/hupa.2002.124721.
Tsutsui S, Inoue H, Yasuda K, Suzuki K, Higashi H, Era S, Mori M: Reduced expression of PTEN protein and its prognostic implications in invasive ductal carcinoma of the breast. Oncology. 2005, 68 (4-6): 398-404. 10.1159/000086981.
Lee JS, Kim HS, Kim YB, Lee MC, Park CS, Min KW: Reduced PTEN expression is associated with poor outcome and angiogenesis in invasive ductal carcinoma of the breast. Appl Immunohistochem Mol Morphol. 2004, 12 (3): 205-210. 10.1097/00129039-200409000-00004.
Torres J, Navarro S, Rogla I, Ripoll F, Lluch A, Garcia-Conde J, Llombart-Bosch A, Cervera J, Pulido R: Heterogeneous lack of expression of the tumour suppressor PTEN protein in human neoplastic tissues. Eur J Cancer. 2001, 37 (1): 114-121. 10.1016/S0959-8049(00)00366-X.
Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ: Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006, 66 (3): 1630-1639. 10.1158/0008-5472.CAN-05-1182.
Cizkova M, Bouchalova K, Trojanec R, Dziechciarkova M, Vacher S, Radova L: Impact of PIK3CA, PTEN and RPS6 status on lapatinib treatment outcome in advanced-stage breast cancer patients. 2010, SABCS conference, abs. P2-09-30
Campone M, Juin P, Andre F, Bachelot T: Resistance to HER2 inhibitors: Is addition better than substitution? Rationale for the hypothetical concept of drug sedimentation. Crit Rev Oncol Hematol.
Brünner-Kubath C, Shabbir W, Saferding V, Wagner R, Singer CF, Valent P, Berger W, Marian B, Zielinski CC, Grusch M: The PI3 kinase/mTOR blocker NVP-BEZ235 overrides resistance against irreversible ErbB inhibitors in breast cancer cells. Breast Cancer Res Treat.
O'Brien NA, Wilcox K, McDermott M, Crown J, O'Donovan N, Slamon DJ: The role of the PI3K/AKT, RAS/MAPK and novel signaling pathways in resistance to HER2-targeted therapy. 2010, SABCS conference, abs. P4-01-06
Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H, Narasanna A, Chakrabarty A, Hilsenbeck SG, Huang J, Rimawi M, Schiff R, Arteaga C, Osborne CK, Chang JC: Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011, 29 (2): 166-73. 10.1200/JCO.2009.27.7814.
Toi M, Iwata H, Fujiwara Y, Ito Y, Nakamura S, Tokuda Y, Taguchi T, Rai Y, Aogi K, Arai T, Watanabe J, Wakamatsu T, Katsura K, Ellis CE, Gagnon RC, Allen KE, Sasaki Y, Takashima S: Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer. 2009, 101 (10): 1676-82. 10.1038/sj.bjc.6605343.
Barbareschi M, Buttitta F, Felicioni L, Cotrupi S, Barassi F, Del Grammastro M, Ferro A, Dalla Palma P, Galligioni E, Marchetti A: Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin Cancer Res. 2007, 13 (20): 6064-6069. 10.1158/1078-0432.CCR-07-0266.
Dupont Jensen J, Laenkholm AV, Knoop A, Ewertz M, Bandaru R, Weihua L, Hackl W, Barrett JC, Gardner H: PIK3CA mutations may be discordant between primary and corresponding metastatic disease in Breast Cancer. Clin Cancer Res.
Depowski PL, Rosenthal SI, Ross JS: Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001, 14 (7): 672-676. 10.1038/modpathol.3880371.
Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, Koujak S, Ferrando AA, Malmström P, Memeo L: Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA. 2007, 104 (18): 7564-7569. 10.1073/pnas.0702507104.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/11/248/prepub
Acknowledgements
This expanded drug access program was supported in part by GlaxoSmithKline. We thank all the patients who participated in the study. We also thank all the personnel in the hospital who help the study accomplished successfully.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LW designed the study, carried out the experiments, statistical analysis and drafted the manuscript. QZ contributed equally. JZ, SS, HG, ZJ, BW, ZS and ZW participated in the clinical work. XH proposed this study, organized the research team, interpreted all the data, and writing the manuscript. All authors read and approved the final manuscript.
Leiping Wang, Qunling Zhang contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wang, L., Zhang, Q., Zhang, J. et al. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer 11, 248 (2011). https://doi.org/10.1186/1471-2407-11-248
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-11-248