Abstract
Background
The activation of signal transducer and activator of transcription 3 (Stat3) pathway correlates with tumor growth, survival, drug resistance and poor prognosis in osteosarcoma. To explore the potential therapeutic values of this pathway, we assessed both the expression and the activation of Stat3 pathway in several pairs of multidrug resistant (MDR) osteosarcoma cell lines, and tissues. To explore the potential therapeutic values of this pathway, we analyzed the ability of the synthetic oleanane triterpenoid, C-28 methyl ester of 2-cyano-3,12-dioxoolen-1,9-dien-28-oic acid (CDDO-Me), to inhibit Stat3 expression and activation as well as its effects on doxorubicin sensitivity in osteosarcoma cells.
Methods
Expression of Stat3, phosphorylated Stat3 (pStat3) and Stat3 targeted proteins, including Bcl-XL, Survivin and MCL-1 were determined in drug sensitive and MDR osteosarcoma cell lines and tissues by Western blot analysis. The effect of CDDO-Me on osteosarcoma cell growth was evaluated by MTT and apoptosis by PARP cleavage assay and caspase-3/7 activity.
Results
Stat3 pathway was activated in osteosarcoma tissues and in MDR cell lines. CDDO-Me inhibited growth and induced apoptosis in osteosarcoma cell lines. Treatment with CDDO-Me significantly decreased the level of nuclear translocation and phosphorylation of Stat3. The inhibition of Stat3 pathway correlated with the suppression of the anti-apoptotic Stat3 targeted genes Bcl-XL, survivin, and MCL-1. Furthermore, CDDO-Me increased the cytotoxic effects of doxorubicin in the MDR osteosarcoma cell lines.
Conclusions
Stat3 pathway is overexpressed in MDR osteosarcoma cells. CDDO-Me significantly inhibited Stat3 phosphorylation, Stat3 nuclear translocation and induced apoptosis in osteosarcoma. This study provides the framework for the clinical evaluation of CDDO-Me, either as monotherapy or perhaps even more effectively in combination with doxorubicin to treat osteosarcoma and overcome drug resistance.
Similar content being viewed by others
Background
Osteosarcoma is the most common malignant tumor of bone, which mainly affects children and adolescents [1, 2]. Current treatment of osteosarcoma consists of multi agent chemotherapy and surgical resection[3]. The advancement in intensive chemotherapy has significantly improved the 5-year survival rate from 10% with surgery alone to approximately 60-70% when combined with chemotherapy [1–4]. Even so, for 20 years, survival rate has not changed and nearly 30-40% of the patients still experience local recurrence or metastasis, possibly because of development of multidrug resistance (MDR). Overcoming drug resistance is one approach to improve the survival rate of osteosarcoma patients. The development of drug resistance is associated with many events, such as activation of transcription factors, overexpression of antiapoptotic proteins, and overexpression of multidrug resistance gene 1 (MDR1) [5–8]. Successful management of osteosarcoma might be greatly aided by the use of novel agents that could overcome drug resistance.
Signal Transducer and Activator of Transcription 3 (Stat3) is one of the transcription factors that play an important role in tumor cell growth, survival, proliferation, differentiation, apoptosis, metastasis, angiogenesis and drug resistance [9–17]. Stat3 is activated (phosphorylated) by Janus-activated kinase (JAK)-1 or JAK-2 in response to interleukin-6 (IL-6) family of cytokines and growth factors [18]. Stat3 then forms homodimers that translocate to the cell nucleus and binds to promoters of target genes, activating oncogenes such as c-myc and cyclin D, and antiapoptotic proteins [19]. Constitutive activation of Stat3 pathway has been found in many cancer cells including osteosarcoma [9–11, 13, 14, 16, 17, 20]. Furthermore, constitutively activated Stat3 pathway correlates with malignant tumor phenotype, resistance to chemotherapeutic drugs, and poor prognosis in some cancers [8, 15, 17, 21–27].
Several reports have shown inhibition of Stat3 pathways in cancer cells, resulting in a dramatic increase of apoptosis [13, 17, 20, 28–32]. The novel synthetic oleanane triterpenoid, C-28 methyl ester of 2-cyano-3,12-dioxoolen-1,9-dien-28-oic acid (CDDO-Me), is a promising new class of agents for the prevention and treatment of cancer [33–35]. When CDDO-Me is applied at low concentrations, it demonstrated a variety of anti-inflammatory effects. At higher concentrations, the compound inhibits cancer cell growth and proliferation in a wide variety of cell lines including ovarian, cervical, breast, liver, leukemia, and lung cancer [17, 32–38]. CDDO-Me is currently in phase I/II clinical trials for cancer treatment [36–39]. CDDO-Me-induced apoptosis is associated with the activation of caspase 3 and 8, cytochrome c, SOCS-1, and SHP-1, and inhibition of NF-kB, Cox2 and VEGF [32, 33, 35, 40]. To date, the effect of CDDO-Me on MDR osteosarcoma cells is unclear.
In this study, we investigated the molecular mechanism of CDDO-Me induced apoptosis and the effects of combinations of CDDO-Me with doxorubicin which is known to have established activity in the clinical therapy for osteosarcoma.
Methods
Cell lines, tissues, antibodies and drugs
Human osteosarcoma cells KHOS, U-2OS, SaOS were obtained from the American Type Tissue Collection (Rockville, MD) and a normal human hipbone osteoblast cell line HOB-c was obtained from the Promo Cell GmbH (Heidelberg, Germany). The MDR cell line U-2OSTR was established as previously reported [1, 2]. Briefly, the U-2OS cell lines were selected over a period of 8 months by continuous culturing in medium containing step-wise increases in drugs. Dr. Efstathios Gonos (Institute of Biological Research and Biotechnology, Athens, Greece) kindly provided the MDR KHOSR2 cell line [41]. In addition, osteosarcoma tissue samples were obtained from the Massachusetts General Hospital Sarcoma Tissue Bank. Surgically treated patients diagnosed with osteosarcoma were identified and utilized for the study and were used in accordance with the policies of the Institutional Review Board of the institution. Doxorubicin was obtained through unused residual clinical material at the Massachusetts General Hospital. CDDO-Me was kindly provided by Dr. Jeff Supko (Massachusetts General Hospital). The stock solution of drugs were prepared according to the drug specifications and stored at -20°C. The rabbit polyclonal antibodies to Stat3, Bcl-XL, MCL-1, PARP, and the mouse monoclonal antibodies to phosphorylated Stat3 (pStat3), survivin were purchased from Cell Signaling Technologies (Cambridge, MA). The mouse monoclonal antibody to β-actin and MTT reagent were purchased from Sigma-Aldrich (St. Louis, MO). The Pgp1 monoclonal antibody C219 was purchased from Signet (Dedham, MA). Goat antimouse-HRP and goat antirabbit-HRP were purchased from BIO-Rad (Hercules, CA). SuperSignal® West Pico Chemiluminescent Substrate was purchased from PIERCE (Rockford, IL).
Cell culture
Osteoblast cells HOB-c were cultured in Osteoblast Growth Medium (Promo Cell), and all other cell lines were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (all obtained from Invitrogen, Carlsbad, CA). All cells were incubated at 37°C in 5% CO2-95% air atmosphere and passaged when near confluent monolayers were achieved using trypsin-EDTA solution. Resistant cell lines were continuously cultured in 0.1 μM doxorubicin. Cells were free of mycoplasma contamination as tested by MycoAlert® Mycoplasma Detection Kit from Cambrex (Rockland, ME).
Western blotting
Protein lysates from osteosarcoma cells and tissues were generated through lysis with 1× radioimmunoprecipitation assay lysis buffer (Upstate Biotechnology, Charlottesville, VA). The concentration of the protein was analyzed by DC Protein assay (Bio-Rad Laboratories, Hercules, CA) and spectrophotometer (Beckman DU-640, Beckman Instruments, Inc., Columbia, MD). Thirty micrograms of total protein was processed on Nu-Page 4-12% Bis-Tris Gel (Invitrogen) and transferred to a pure nitrocellulose membrane (Bio-Rad) then immunoblotted with specific antibodies. Primary antibodies were incubated at 1:1000 dilution in Tris-buffered saline, pH 7.4, with 0.1% Tween-20 and 5% nonfat milk (Bio-Rad) overnight at 4°C. Horseradish peroxidase-conjugated secondary antibodies (Bio-Rad) were incubated in Tris-buffered saline, pH 7.4, with 0.1% Tween-20 and 5% nonfat milk, at 1:2,000 dilutions for 1 hour at room temperature. Positive immunoreactions were detected by using SuperSingal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL).
Cell viability analysis
The In vitro cell viability analysis was performed by MTT assay as described previously [42]. For analyzing effects of CDDO-Me on KHOS, KHOSR2, U-2OS, U-2OSTR and HOB-c, 2 × 103 cells per well were plated in 96-well plates in culture medium containing increasing concentrations of CDDO-Me. For the effect of varying concentrations of CDDO-Me on U-2OSTR and KHOSR2, 2 × 103 cells per well were plated in 96-well plates in culture medium containing increasing concentrations of doxorubicin and CDDO-Me at final concentrations of 0.1 and 0.3 μmol/L, respectively. After 7 days of culture at 37°C, 10 μl MTT (5 mg/ml in PBS, obtained from Sigma) was added to each well and the plates were incubated for 3 h. The resulting formazan product was dissolved with acid-iso-propanol and the absorbance at a wavelength of 490 nm (A490) was read on a SPECTRAmax Microplate Spectrophotometer (Molecular Devices, Sunnyvale, CA). The absorbance values were normalized by assigning the value of the control line in the medium without drug to 1.0 and the value of the no cell control to 0. Experiments were performed in triplicate. The half inhibitory concentration (IC50) was defined as the compound or chemo drug concentration required decreasing the A490 to 50% of the control value.
Apoptosis assay
Lysates from whole-cell were immunoblotted with specific antibodies to PARP (Cell Signaling Technology) and its cleavage products. Positive immunoreactions were detected by using Super Signal West Pico Chemiluminescent Substrate. As a second parameter of apoptotic cell death, we measured caspase-3/7 activities in HOB-c, KHOS and KHOSR2 after treatment with CDDO-Me by using Apo-ONE Homogenous caspase-3/7 system according to the manufacturer's instructions (Promega, Madison, WI). The intensity of the emitted fluorescence was determined at a wavelength of 521 nm with the use of a SPECTRAmax® Microplate Spectrofluorometer (Molecular Devices).
Real-time analysis of the effect of CDDO-Me on Stat3 nucleocytoplasmic translocation
To study the effects of CDDO-Me on Stat3 nuclear translocation in live cells, a real-time cell based assay was used as described below. Stable transfectants expressing EGFP-Stat3 were generated with the osteosarcoma cell line U-2OS using standard lipofectmine transfection techniques with G418 selection. EGFP-Stat3 expressing cells were seeded at a density of 4 × 103 cells per well in 96 well plates and incubated overnight at 37°C. The cells were then treated with either 1 μM CDDO-Me with 30 ng/ml human recombinant IL-6 (R&D Systems, Minneapolis, MN) or IL-6 alone. To counterstain the nuclei, the cells were incubated with 1 μg/ml Hoechst 33342 (Invitrogen, Carlsbad, CA) for 1 minute. IL-6 dependent nuclear translocation of EFGP-Stat3 was analyzed using an Olympus 1X71 fluorescence microscope and the pictures were captured as digital images using IPLab Software from Scanalytics (Rockville, MD).
Data analysis
Values shown are representative of triplicate determinations in two or more experiments. The IC50 was calculated and the effects of treatment were evaluated using a two-sided Student's t test (GraphPad PRISM® 4 software, GraphPad Software, San Diego, CA). Errors are a SD (standard deviation) of averaged results and with value p < 0.05 was considered as a significant difference between means.
The combination index (CI) for experimental treatment combinations was calculated to determine the synergistic, additive, or antagonistic effects using the Chou-Talalay method and GraphPad PRISM® 4 software[43]. When CI = 1, the equation represents the conservation isobologram and indicates additive effects. CI values of <1.0 indicates synergistic effect, > 1.0 indicates antagonistic effect.
Results
Stat3 pathway is activated in osteosarcoma cell lines and tissues
To evaluate the expression and activation of Stat3 pathway, we analyzed the protein expression in several pairs of both drug sensitive and MDR osteosarcoma cell lines and 8 samples of osteosarcoma tissues. Western blot analysis demonstrated that Stat3 and pStat3 were constitutively overexpressed in drug sensitive cell lines KHOS, U-2OS, SaOS and MDR cell lines KHOSR2, U-2OSTR. Normal osteoblast cell line HOB-c expressed Stat3 as well as low levels of pStat3 (Fig. 1A). In osteosarcoma tissues, Stat3 was overexpressed in all of the samples and pStat3 was overexpressed in 7 out of 8 (88%) samples (Fig. 1B). Following this preliminary study, we analyzed the expression of Stat3-mediated antiapoptotic proteins Bcl-XL, survivin, and MCL-1. Western blot analysis demonstrated that Bcl-XL, survivin, and MCL-1 were constitutively overexpressed in drug sensitive cell lines and MDR osteosarcoma cell lines. Osteoblast cell lines HOB-c controls showed no expression of Stat3-mediated anti-apoptotic proteins. Pgp1 is also over expressed in MDR cell lines (Fig. 1A). Osteosarcoma tissues showed heterogeneous expression of Stat3 mediated antiapoptotic proteins and Pgp1 (Fig. 1B).
Activation of Stat3, pStat3, Stat3-mediated antiapoptotic proteins, and Pgp1 in osteosarcoma cell lines and tissues. A) Osteoblast cell lines, drug sensitive and MDR osteosarcoma cell lines. B) Osteosarcoma tissues. Expression was assessed with total cellular protein isolated from the indicated cell lines and immunoblotted with specific antibodies as described in Materials and Methods. The blots were also probed with an anti-actin monoclonal antibody to assess relative protein levels in the sample lanes.
CDDO-Me inhibits growth and induces apoptosis in osteosarcoma cells
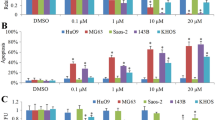
To confirm that CDDO-Me inhibits cell growth, KHOS, U-2OS, KHOSR2, U-2OSTR, and HOB-c were evaluated using MTT assay. The results showed that the growth of all cell lines was inhibited after treatment with CDDO-Me (Fig. 2A). CDDO-Me showed significantly higher antiproliferative activity in osteosarcoma cells than in osteoblast cells (p < 0.0001). The IC50 of each cells were HOB-c: 0.8 μM, KHOS: 0.15 μM, KHOSR2: 0.33 μM, U-2OS: 0.17, U-2OSTR: 0.39 μM. The effect of CDDO-Me on the induction of apoptosis was assessed by evaluating PARP cleavage and caspase assay for both drug sensitive and MDR osteosarcoma cell lines. PARP cleavage was detected in all cells after 24 hours of treatment with CDDO-Me. A dose-response analysis revealed the appearance of PARP cleavage products in the presence of 0.5 μmol/L of CDDO-Me for KHOS, KHOSR2 and 1.0 μmol/L of CDDO-Me for U-2OS, U-2OSTR (Fig. 2B). In addition, caspase-3/7 activity was significantly increased when KHOS and KHOSR2 were treated with increasing concentration of CDDO-Me (Fig. 3).
CDDO-Me inhibits growth and induces apoptosis in osteosarcoma cell lines. A) To analyze the effect of CDDO-Me, KHOS, U-2OS, KHOSR2, U-2OSTR, and HOB-c were exposed to varying concentrations of CDDO-Me for 7 days. Growth inhibition was assessed by MTT. B) Parental cells KHOS, U-2OS and MDR cells KHOSR2 and U-2OSTR were treated with CDDO-Me in a dose-dependent manner. Total cellular proteins were subjected to immunoblotting with specific antibodies to PARP and β-actin. PARP cleavage was detected in all four osteosarcoma cell lines after treatment with CDDO-Me. The anti-PARP antibodies demonstrate 118 kd full length PARP and 85 kd cleaved PARP fragment.
CDDO-Me inhibits IL-6 induced nuclear translocation of Stat3
To identify the interruption of IL-6 dependent Stat3 nuclear translocation by CDDO-Me, a novel real-time cell-based method was developed to image the EGFP-Stat3 chimera in the nucleus and cytoplasm in human osteosarcoma cell line U-2OS. Resting cells demonstrated that the majority of EGFP-Stat3 was cytoplasmic (Fig. 4A, A') until the addition of IL-6, which then promptly induced translocation of fluorescent Stat3 to the nucleus in U-2OS cells (Fig. 4B). Pretreatment of the cells with CDDO-Me (1 μM) blocked IL-6 dependent EGFP-Stat3 nuclear translocation (Fig. 4B').
CDDO-Me inhibits EGFP-Stat3 nuclear translocation in osteosarcoma cell line U-2OS. U-2OS cells which stably express the EGFP-Stat3 fusion protein were incubated for 4 h with either IL-6 alone (A-D) or CDDO-Me (1 μM) followed immediately thereafter with the addition of IL-6 to a final concentration of 30 ng/ml (A'-D'). To counterstain the nuclei, the cells were incubated with 1 μg/ml Hoechst 33342 (Invitrogen, Carlsbad, CA) for 1 minute. Cells were photographed 1 h later. Subcellular localization of the fusion protein was assessed by fluorescence microscopy.
CDDO-Me inhibits Stat3 pathway in a dose-dependent manner
After identifying CDDO-Me as an inhibitor of Stat3 nuclear translocation in osteosarcoma cells, the effect of CDDO-Me on Stat3 phosphorylation was examined in osteosarcoma drug sensitive and MDR cell lines. To evaluate the dose-dependent inhibition of Stat3 activation, the cell lines were treated with CDDO-Me alternatively with varying doses for 24 h. In a dose-dependent manner, concentrations as low as 0.5 μM CDDO-Me inhibited Stat3 phosphorylation (Fig. 5). Stat3 phosphorylation and nuclear translocation are required for Stat3 transcriptional activity. We hypothesized that inhibition of nuclear transport should suppress transcription and subsequent translation of Stat3-mediated proteins. Therefore, we next examined whether exposure of cell lines to CDDO-Me resulted in decreased expression of the antiapoptotic proteins Bcl-XL, survivin and MCL-1 by dose-dependent inhibition (Fig. 5). Results showed incubation of CDDO-Me for 24 h caused significant down-regulated Bcl-XL, survivin and MCL-1 expression in both drug sensitive (Fig. 5A) and MDR (Fig. 5B) cell lines in a dose-dependent manner.
Dose-dependent inhibition of Stat3 phosphorylation and down-regulated Stat3-mediated antiapoptotic proteins by CDDO-Me. Parental cells KHOS, U-2OS (A) and MDR cells KHOSR2, U-2OSTR (B) were treated with CDDDO-Me for 24 hours in and then harvested and processed for Western blotting. For Western blot analysis, 30 μg of total cellular proteins were subjected to immunoblotting with specific antibodies.
CDDO-Me inhibits Stat3 pathway and induces apoptosis in a time-dependent manner
To evaluate the time-dependent inhibition of Stat3 activation, the cell lines were treated with 1 μM of CDDO-Me for varying time periods for 24 h. In a time-dependent manner, the pStat3 level decreased as early as 4 hours after the addition of 1 μM of CDDO-Me treatment (Fig. 6). We next examined whether exposure of cell lines to CDDO-Me resulted in decreased expression of the antiapoptotic proteins Bcl-XL, survivin and MCL-1 by time-dependent inhibition (Fig. 6). Results showed incubation of CDDO-Me for 24 h caused significant down-regulation of Bcl-XL, survivin and MCL-1 expression in both drug sensitive (Fig. 6A) and MDR (Fig. 6B) cell lines in a time dependent manner. The effect of CDDO-Me on the induction of apoptosis was assessed by evaluating PARP cleavage in a time-dependent manner. PARP cleavage was detected in all cells after 12 hours of treatment with 1 μM of CDDO-Me (Fig. 6).
Time-dependent inhibition of Stat3 phosphorylation and down-regulated Stat3-mediated antiapoptotic proteins by CDDO-Me. Parental cells KHOS, U-2OS (A) and MDR cells KHOSR2, U-2OSTR (B) were treated for 24 h with CDDO-Me (1 μM), and then harvested and processed for Western blotting. For Western blot analysis, 30 μg of total cellular proteins were subjected to immunoblotting with specific antibodies.
CDDO-Me has no effect on Pgp-1 expression
While CDDO-Me significantly inhibits Stat3 phosphorylation in KHOSR2, U-2OSTRcells, there was no significant change in Pgp1 expression as observed in the Pgp1-over expressing cell lines (Fig. 5B and 6B).
CDDO-Me shows synergy with doxorubicin and reduces resistance in MDR osteosarcoma cell lines
To examine whether interruption of the Stat3 pathway shows synergistic effect when combined with chemotherapy, we analyzed the effect of CDDO-Me in the presence or absence of doxorubicin. Treatment of MDR cell lines with doxorubicin, CDDO-Me or combination of both drugs were analyzed using MTT. The combination of the two agents doxorubicin and CDDO-Me resulted in significantly greater cell death than either drug alone (Fig 7A). The combination index (CI) values were <1 in all cases and thereby identified synergistic interaction at all combinations between CDDO-Me and doxorubicin at various combination. The CI of each combinations were KHOSR2/doxorubicin+CDDO-Me(0.1 μM): 0.98, KHOSR2/doxorubicin+CDDO-Me(0.3 μM): 0.64, U-2OSTR/doxorubicin+CDDO-Me(0.1 μM): 0.83, U-2OSTR/doxorubicin+CDDO-Me(0.3 μM): 0.51.
CDDO-Me shows synergy with doxorubicin and overcomes drug resistance in MDR osteosarcoma cell lines. A) CDDO-Me inhibits cell growth in KHOSR2 and U-2OSTR. Cells were seeded at a density of 8,000 cells per well in a 96-well plate for 24 hours. The cells were then treated with 0.6 μM doxorubicin, 0.1 μM CDDO-Me, 0.3 μM CDDO-Me or combination of different two drugs for additional 24 hours. The cells were analyzed by MTT assay as described in Materials and Methods (B and C). CDDO-Me together with doxorubicin overcome drug resistance in both MDR KHOSR2 and U-2OSTR cells.
To investigate the inhibition of Stat3 pathway's effect on drug sensitive osteosarcoma cell lines KHOS, U-2OS and MDR osteosarcoma cell lines KHOSR2, U-2OSTR, they were exposed to varying concentrations of doxorubicin and CDDO-Me for 7 days. MTT demonstrated that CDDO-Me has a synergistic effect on doxorubicin-induced antiproliferative effect in KHOSR2 and U-2OSTR (Fig 7B and 7C). In addition, MTT assay demonstrated that CDDO-Me increases doxorubicin's effect and partially overcomes doxorubicin-resistance. The IC50 of each cells were KHOS: 0.07 μM, KHOSR2/CDDO-Me(0.3 μM): 0.11 μM, KHOSR2/CDDO-Me(0.1 μM): 0.29 μM, KHOSR2: 0.86 μM (Fig 6B), and U-2OS: 0.06 μM, U-2OSTR/CDDO-Me(0.3 μM) 0.12 μM, U-2OSTR/CDDO-Me(0.1 μM): 0.24 μM, U-2OSTR: 0.93 μM (Fig 7C).
Discussion
Stat3 pathway plays an important role in tumor cell growth and proliferation, and is constantly activated in many human cancer cell lines and tumor tissues [9–14, 17, 20]. Constitutive activation of Stat3 pathway could be an early indicator of drug resistance[15]. Activation of Stat3 pathway was also reported to be present in osteosarcoma cells and tissues [14, 20]. In this study, we observed that elevated levels of Stat3 and pStat3 are detected in osteosarcoma drug sensitive cell lines KHOS, U-2OS, SaOS and osteosarcoma MDR cell lines KHOSR2, U-2OSTR. The data is consistent with previous studies that Stat3 pathways play an important role in not only drug sensitive but also drug resistant osteosarcoma cells [14, 20]. The downstream effect of Stat3 activation is the Stat3-dependent regulation of several antiapoptotic genes including Bcl-XL, survivin, and MCL-1. Overexpressions of these survival-promoting genes have been shown to be highly expressed and prevent apoptosis in human cancer cells, especially in high-grade tumors [13, 44–46]. In our study, these antiapoptotic genes were highly expressed in both drug sensitive and resistant osteosarcoma cell lines, but not in normal human osteoblast cells.
Nuclear translocation of pStat3 is a crucial event for its transcriptional function. Blocking the phosphorylation and translocation of Stat3 is a rational approach for the inhibition of Stat3 activation. Recently, Stat3 has been implicated as a promising target for cancer therapy [12, 13, 20, 28–31]. Stat3 inhibitors, SD-1029 and SD-1008, greatly induce apoptosis in drug resistant ovarian cancer cells by blocking Stat3 nuclear translocation [29, 30]. Stat3, pStat3 and Stat3 targeted antiapoptotic proteins are over expressed in drug resistance osteosarcoma cells. Inhibition of the Stat3 pathway and interruption of antiapoptotic response may also play an important role for treatment of these cells. Our result is consistent with recent reports showing that the novel Stat3 inhibitor, Indirubin, significantly induces apoptosis in human breast cancer cells [13]. In addition, LLL3, Stat3 DNA binding and transcription activities inhibitor, and Stat3 siRNA significantly decrease cell proliferation and viability, ultimately inducing apoptosis in osteosarcoma cells[20].
CDDO is widely used in Asian herbal medicine and was originally identified as an active compound for anti-inflammatory and anti-carcinogenic treatments [34, 39]. The novel compound CDDO-Me has been shown to be highly effective in vivo models for the prevention and treatment of cancer [33–35]. CDDO-Me is able to induce the differentiation of tumor cells, suppress the growth of tumor cells, and induce apoptosis in cancer cells that are resistant to conventional chemotherapeutic agents [32]. Recent report has shown that CDDO-Me inhibits activation of the JAK/Stat3 pathway by forming adducts with both JAK1 and Stat3 in human cervical and breast cancer cells [34]. Previously, we also demonstrated that CDDO-Me inhibits Stat3 pathway in ovarian cancer cells by down-regulation of antiapoptotic genetic expression, and resulted in a dramatic induction of apoptosis [17]. Furthermore, recent study showed that the novel Stat3 target gene Bcl-XL inhibitor, ABT-737, greatly enhanced the activities of paclitaxel in lung cancer cells [47]. In this study, CDDO-Me inhibits Stat3 phosphorylation and down-regulates Stat3-mediated antiapoptotic proteins Bcl-XL, survivin and, MCL-1, in both dose and time dependent manner. This was through apoptotic cell death in both drug sensitive and resistant osteosarcoma cells. Furthermore, the apoptotic threshold also increases the sensitivity of these cells to the cytotoxic effects of doxorubicin. The results are consistent with a series of studies showing that CDDO-related compounds are not monofunctional drugs that strictly target single steps in signal transduction pathways [32, 40, 48]. Biochemical and cellular assays support the hypothesis that the molecule CDDO-Me inhibits Stat3 activity with resultant inhibition of Stat3 phosphorylation, nuclear translocation, and decrease in Stat3-dependent transcription, leading to apoptosis and enhanced chemosensitivity.
Conclusion
Our results demonstrated that Stat3 pathways were constantly activated in osteosarcoma and MDR osteosarcoma cells. CDDO-Me significantly inhibits Stat3 phosphorylation and consequently Stat3 nuclear translocation, and eventually resulted in inducing apoptosis in these cells. In addition, this compound is able to overcome drug resistance in these cells.
References
Arndt CA, Crist WM: Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999, 341 (5): 342-352. 10.1056/NEJM199907293410507.
Mirabello L, Troisi RJ, Savage SA: Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009, 115 (7): 1531-43. 10.1002/cncr.24121.
Ferrari S, Palmerini E: Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007, 19 (4): 341-346. 10.1097/CCO.0b013e328122d73f.
Bacci G, Ferrari S, Bertoni F, Ruggieri P, Picci P, Longhi A, Casadei R, Fabbri N, Forni C, Versari M, et al: Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000, 18 (24): 4016-4027.
Yang C, Yang S, Wood KB, Hornicek FJ, Schwab JH, Fondren G, Mankin H, Duan Z: Multidrug resistant osteosarcoma cell lines exhibit deficiency of GADD45alpha expression. Apoptosis. 2009, 14 (1): 124-133. 10.1007/s10495-008-0282-x.
Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB: Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003, 22 (47): 7280-7295. 10.1038/sj.onc.1206934.
Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV: Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets. 2003, 3 (1): 1-19. 10.2174/1568009033333754.
Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, Hampel C, Lee H, Seiden MV: Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. 2006, 12 (17): 5055-5063. 10.1158/1078-0432.CCR-06-0861.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE: Stat3 as an oncogene. Cell. 1999, 98 (3): 295-303. 10.1016/S0092-8674(00)81959-5.
Bromberg J, Darnell JE: The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000, 19 (21): 2468-2473. 10.1038/sj.onc.1203476.
Buettner R, Mora LB, Jove R: Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002, 8 (4): 945-954.
Yu H, Jove R: The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004, 4 (2): 97-105. 10.1038/nrc1275.
Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, Hippe F, Vatter S, Merz KH, Eisenbrand G, et al: Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci USA. 2005, 102 (17): 5998-6003. 10.1073/pnas.0409467102.
Chen CL, Loy A, Cen L, Chan C, Hsieh FC, Cheng G, Wu B, Qualman SJ, Kunisada K, Yamauchi-Takihara K, et al: Signal transducer and activator of transcription 3 is involved in cell growth and survival of human rhabdomyosarcoma and osteosarcoma cells. BMC Cancer. 2007, 7: 111-10.1186/1471-2407-7-111.
Barre B, Vigneron A, Perkins N, Roninson IB, Gamelin E, Coqueret O: The STAT3 oncogene as a predictive marker of drug resistance. Trends Mol Med. 2007, 13 (1): 4-11. 10.1016/j.molmed.2006.11.001.
Lai R, Navid F, Rodriguez-Galindo C, Liu T, Fuller CE, Ganti R, Dien J, Dalton J, Billups C, Khoury JD: STAT3 is activated in a subset of the Ewing sarcoma family of tumours. J Pathol. 2006, 208 (5): 624-632. 10.1002/path.1941.
Duan Z, Ames RY, Ryan M, Hornicek FJ, Mankin H, Seiden MV: CDDO-Me, a synthetic triterpenoid, inhibits expression of IL-6 and Stat3 phosphorylation in multi-drug resistant ovarian cancer cells. Cancer Chemother Pharmacol. 2008
Murray PJ: The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007, 178 (5): 2623-2629.
Alvarez JV, Febbo PG, Ramaswamy S, Loda M, Richardson A, Frank DA: Identification of a genetic signature of activated signal transducer and activator of transcription 3 in human tumors. Cancer Res. 2005, 65 (12): 5054-5062. 10.1158/0008-5472.CAN-04-4281.
Fossey SL, Liao AT, McCleese JK, Bear MD, Lin J, Li PK, Kisseberth WC, London CA: Characterization of STAT3 activation and expression in canine and human osteosarcoma. BMC Cancer. 2009, 9: 81-10.1186/1471-2407-9-81.
Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB: Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002, 62 (12): 3351-3355.
Khoury JD, Medeiros LJ, Rassidakis GZ, Yared MA, Tsioli P, Leventaki V, Schmitt-Graeff A, Herling M, Amin HM, Lai R: Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK- anaplastic large cell lymphoma. Clin Cancer Res. 2003, 9 (10 Pt 1): 3692-3699.
Horiguchi A, Oya M, Shimada T, Uchida A, Marumo K, Murai M: Activation of signal transducer and activator of transcription 3 in renal cell carcinoma: a study of incidence and its association with pathological features and clinical outcome. J Urol. 2002, 168 (2): 762-765. 10.1016/S0022-5347(05)64741-6.
Benekli M, Xia Z, Donohue KA, Ford LA, Pixley LA, Baer MR, Baumann H, Wetzler M: Constitutive activity of signal transducer and activator of transcription 3 protein in acute myeloid leukemia blasts is associated with short disease-free survival. Blood. 2002, 99 (1): 252-257. 10.1182/blood.V99.1.252.
Dolled-Filhart M, Camp RL, Kowalski DP, Smith BL, Rimm DL: Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin Cancer Res. 2003, 9 (2): 594-600.
Hsiao JR, Jin YT, Tsai ST, Shiau AL, Wu CL, Su WC: Constitutive activation of STAT3 and STAT5 is present in the majority of nasopharyngeal carcinoma and correlates with better prognosis. Br J Cancer. 2003, 89 (2): 344-349. 10.1038/sj.bjc.6601003.
Sheen-Chen SM, Huang CC, Tang RP, Chou FF, Eng HL: Prognostic value of signal transducers and activators of transcription 3 in breast cancer. Cancer Epidemiol Biomarkers Prev. 2008, 17 (9): 2286-2290. 10.1158/1055-9965.EPI-08-0089.
Turkson J, Jove R: STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000, 19 (56): 6613-6626. 10.1038/sj.onc.1204086.
Duan Z, Bradner JE, Greenberg E, Levine R, Foster R, Mahoney J, Seiden MV: SD-1029 inhibits signal transducer and activator of transcription 3 nuclear translocation. Clin Cancer Res. 2006, 12 (22): 6844-6852. 10.1158/1078-0432.CCR-06-1330.
Duan Z, Bradner J, Greenberg E, Mazitschek R, Foster R, Mahoney J, Seiden MV: 8-benzyl-4-oxo-8-azabicyclo[3.2.1]oct-2-ene-6,7-dicarboxylic acid (SD-1008), a novel janus kinase 2 inhibitor, increases chemotherapy sensitivity in human ovarian cancer cells. Mol Pharmacol. 2007, 72 (5): 1137-1145. 10.1124/mol.107.038117.
Darnell JE: Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002, 2 (10): 740-749. 10.1038/nrc906.
Liby KT, Yore MM, Sporn MB: Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007, 7 (5): 357-369. 10.1038/nrc2129.
Deeb D, Gao X, Dulchavsky SA, Gautam SC: CDDO-Me inhibits proliferation, induces apoptosis, down-regulates Akt, mTOR, NF-kappaB and NF-kappaB-regulated antiapoptotic and proangiogenic proteins in TRAMP prostate cancer cells. J Exp Ther Oncol. 2008, 7 (1): 31-39.
Ahmad R, Raina D, Meyer C, Kufe D: Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)-->signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 2008, 68 (8): 2920-2926. 10.1158/0008-5472.CAN-07-3036.
Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB: A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006, 12 (6): 1828-1838. 10.1158/1078-0432.CCR-05-2044.
Hyer ML, Croxton R, Krajewska M, Krajewski S, Kress CL, Lu M, Suh N, Sporn MB, Cryns VL, Zapata JM, et al: Synthetic triterpenoids cooperate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res. 2005, 65 (11): 4799-4808. 10.1158/0008-5472.CAN-04-3319.
Lapillonne H, Konopleva M, Tsao T, Gold D, McQueen T, Sutherland RL, Madden T, Andreeff M: Activation of peroxisome proliferator-activated receptor gamma by a novel synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res. 2003, 63 (18): 5926-5939.
Konopleva M, Zhang W, Shi YX, McQueen T, Tsao T, Abdelrahim M, Munsell MF, Johansen M, Yu D, Madden T, et al: Synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest in HER2-overexpressing breast cancer cells. Mol Cancer Ther. 2006, 5 (2): 317-328. 10.1158/1535-7163.MCT-05-0350.
Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Maue RA, Place AE, Porter DM, Spinella MJ, et al: A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res. 1999, 59 (2): 336-341.
Yu H, Kortylewski M, Pardoll D: Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007, 7 (1): 41-51. 10.1038/nri1995.
Lourda M, Trougakos IP, Gonos ES: Development of resistance to chemotherapeutic drugs in human osteosarcoma cell lines largely depends on up-regulation of Clusterin/Apolipoprotein J. Int J Cancer. 2007, 120 (3): 611-622. 10.1002/ijc.22327.
Duan Z, Duan Y, Lamendola DE, Yusuf RZ, Naeem R, Penson RT, Seiden MV: Overexpression of MAGE/GAGE genes in paclitaxel/doxorubicin-resistant human cancer cell lines. Clin Cancer Res. 2003, 9 (7): 2778-2785.
Chou TC, Talalay P: Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984, 22: 27-55. 10.1016/0065-2571(84)90007-4.
Silver DL, Naora H, Liu J, Cheng W, Montell DJ: Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004, 64 (10): 3550-3558. 10.1158/0008-5472.CAN-03-3959.
Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al: Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006, 12 (1): 11-19. 10.1158/1078-0432.CCR-04-1752.
Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, et al: Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res. 2006, 12 (1): 20-28. 10.1158/1078-0432.CCR-04-1749.
Wesarg E, Hoffarth S, Wiewrodt R, Kroll M, Biesterfeld S, Huber C, Schuler M: Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int J Cancer. 2007, 121 (11): 2387-2394. 10.1002/ijc.22977.
Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB: The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006, 5 (12): 3232-3239. 10.1158/1535-7163.MCT-06-0444.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/10/187/prepub
Acknowledgements
This project was supported, in part, by a grant from the Gaetagno and Wechsler funds. KR is supported by Department of Orthopaedic Surgery, Nihon University School of Medicine. ZD is supported, in part, by a grant from Sarcoma Foundation of America, and a grant from the National Cancer Institute, NIH (Nanotechnology Platform Partnership), R01-CA119617. EC is supported by the Jennifer Hunter Yates Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KR designed and carried out all the studies, analyzed the data, and drafted the manuscript. MS helped in carrying out the experiments and assisted with the manuscript draft. EC provided guidance with the study and assisted with the manuscript draft. CY helped in carrying out the experiments. FJH and HJM provided experimental reagents, osteosarcoma tissues and guided the team in the analysis of results and discussions. ZD is the principal investigator who led the research effort, provided guidance with the studies, assisted in data analysis and interpretation, and edited the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ryu, K., Susa, M., Choy, E. et al. Oleanane triterpenoid CDDO-Me induces apoptosis in multidrug resistant osteosarcoma cells through inhibition of Stat3 pathway. BMC Cancer 10, 187 (2010). https://doi.org/10.1186/1471-2407-10-187
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-10-187