Abstract
Background
Antenatal magnesium sulphate, widely used in obstetrics to improve maternal and infant outcomes, may be associated with adverse effects for the mother sufficient for treatment cessation. This systematic review aimed to quantify maternal adverse effects attributed to treatment, assess how adverse effects vary according to different regimens, and explore women’s experiences with this treatment.
Methods
Bibliographic databases were searched from their inceptions to July 2012 for studies of any design that reported on maternal adverse effects associated with antenatal magnesium sulphate given to improve maternal or infant outcomes. Primary outcomes were life-threatening adverse effects of treatment (death, cardiac arrest, respiratory arrest). For randomised controlled trials, data were meta-analysed, and risk ratios (RR) pooled using fixed-effects or random-effects models. For non-randomised studies, data were tabulated by design, and presented as RR, odds ratios or percentages, and summarised narratively.
Results
A total of 143 publications were included (21 randomised trials, 15 non-randomised comparative studies, 32 case series and 75 reports of individual cases), of mixed methodological quality. Compared with placebo or no treatment, magnesium sulphate was not associated with an increased risk of maternal death, cardiac arrest or respiratory arrest. Magnesium sulphate significantly increased the risk of 'any adverse effects’ overall (RR 4.62, 95% CI 2.42-8.83; 4 trials, 13,322 women), and treatment cessation due to adverse effects (RR 2.77; 95% CI 2.32-3.30; 5 trials, 13,666 women). Few subgroup differences were observed (between indications for use and treatment regimens). In one trial, a lower dose regimen (2 g/3 hours) compared with a higher dose regimen (5 g/4 hours) significantly reduced treatment cessation (RR 0.05; 95% CI 0.01-0.39, 126 women). Adverse effect estimates from studies of other designs largely supported data from randomised trials. Case reports supported an association between iatrogenic overdose of magnesium sulphate and life-threatening consequences.
Conclusions
Appropriate administration of antenatal magnesium sulphate was not shown to be associated with serious maternal adverse effects, though an increase in 'minor’ adverse effects and treatment cessation was shown. Larger trials are needed to determine optimal regimens, achieving maximal effectiveness with minimal adverse effects, for each antenatal indication for use. Vigilance in the use of magnesium sulphate is essential for women’s safety.
Similar content being viewed by others
Background
Magnesium sulphate has a long history of use in obstetrics. It is supported as the first line treatment for women with eclampsia [1–3], and is the drug of choice for women with severe pre-eclampsia [4]; it has been widely used as a tocolytic, however, benefit for this indication remains unproven [5, 6]. Most recently antenatal magnesium sulphate has been supported for neuroprotection of the fetus, and it is thus now recommended for women at risk of very preterm birth [7].
Although life-threatening maternal adverse effects of magnesium sulphate are considered extremely rare in obstetrics [8], severe consequences of magnesium toxicity including respiratory arrest, cardiac arrest and death have been detailed in case reports. The 'well recognised’ and more commonly reported maternal adverse effects of magnesium sulphate include flushing, increased warmth and sweating due to the peripheral vasodilatory effects of magnesium, and nausea, vomiting, headaches, muscle weakness, blurred vision, and intravenous (IV) or intramuscular (IM) site pain or discomfort [8]. Though such maternal adverse effects may be considered comparatively 'minor,’ they have been associated with the need for early cessation of this therapy, which has benefits when used for maternal and fetal neuroprotection [4, 7].
While maternal adverse effects following antenatal magnesium sulphate administration are well known [4–7, 9], the risk of individual events is unclear, and there has been a dearth of evidence regarding how such adverse effects vary by different regimens. Variation in aspects of the regimens such as the route of administration, dose, and duration, may help to explain differences in adverse effects of magnesium sulphate experienced among women receiving treatment. Although recent evidence suggests that on average, there are not large differences in the risk estimates of adverse effects from randomised controlled trials and observational studies [10], some uncertainty remains regarding the consistency of estimates provided by diverse study designs [11].
In view of the extremely widespread use of antenatal magnesium sulphate in obstetric practice, in this systematic review we aimed to quantify the extent of maternal adverse effects attributed to treatment, explore any variation in risk estimates between study designs, and to assess how such maternal adverse effects vary according to different regimens for administration and different indications for use. Implementation of this therapy may be strengthened, and the safety improved, if guidelines and recommendations for practice can be based on such knowledge. As it is known that maternal adverse effects may affect adherence and therapy cessation, we additionally aimed to explore and better understand women’s responses to their experiences with this therapy.
Methods
Search strategy
Additional file 1 provides the PRISMA checklist. A comprehensive search of the bibliographic databases, MEDLINE, Embase, CENTRAL (Cochrane Central Register of Controlled Trials) and TOXLINE, was undertaken from their respective inceptions to July 2012, using a combination of MeSH and free text terms [12]. The search strategies used are given in Additional file 2. No date or language restrictions were applied, however, because of logistical constraints, for non-English papers only those with an available partial/full translation were retrieved. Conference Proceeding Citation Index-Science, OpenSIGLE, ClinicalTrials.gov, Current Controlled Trials metaRegister of Controlled Trials, International Clinical Trials Registry, and Google were additionally searched using key word searches (including to identify any publically available incident reports from patient safety organisations). The reference lists of any eligible articles identified were checked for additional references. The blog search engine blogsearch.google.com and the search engine Google, limited to Discussions, were searched using key words ([“magnesium sulfate” OR “magnesium sulphate”] AND pregnancy) (sorted by relevance). Because of logistical constraints, it was pre-specified that sampling would cease once 20 relevant blogs and 10 relevant discussion forum threads were identified, with up to a total of five relevant threads from each original site sampled, if available.
Inclusion criteria
Studies
We included intervention (randomised, cluster-randomised, quasi-randomised and non-randomised comparative studies) and observational studies (cohort, case-control, cross-sectional, case series and case reports). We included studies available as abstracts only, along with full-text publications. Personal blogs and discussion forum threads from pregnancy-related internet sites were included, along with incident reports from patient safety organisations.
Participants, interventions and comparisons
We included women given antenatal magnesium sulphate: for pre-eclampsia/eclampsia (including when it was continued/initiated in the immediate postpartum period); for tocolysis to women in preterm labour or who had had at least one episode of threatened preterm labour; for neuroprotection of the fetus, to women considered at risk of preterm birth (less than 37 weeks’ gestation), or at term, regardless of the regimen for administration (including iatrogenic overdoses). We excluded studies where women were given oral magnesium sulphate, and where magnesium sulphate was given as an adjuvant during anaesthesia, or where magnesium sulphate was given in combination with another agent for tocolysis. We included instances where magnesium sulphate was compared to no placebo, placebo or to a different magnesium sulphate regimen, and/or, where the study’s exposure was magnesium sulphate. We excluded studies where magnesium sulphate was compared to an alternative therapy.
Outcomes
We included studies that reported data on maternal adverse effects associated with magnesium sulphate. Primary outcomes were life-threatening adverse effects of treatment (death, cardiac arrest, respiratory arrest). Secondary outcomes included other maternal-reported or clinical maternal adverse effects attributed to treatment (e.g. warmth and flushing, arm discomfort), outcomes associated with interventions to reduce potential/actual adverse effects (e.g. use of calcium gluconate, discontinuation of treatment), and other outcomes of interest (including caesarean section, pulmonary oedema, and postpartum haemorrhage). We used the definitions as used by the study authors.
Study selection
After screening all titles and abstracts, we obtained the full-text article for any study which appeared to meet the inclusion criteria based on the title and/or abstract, along with any reviews that may have provided relevant references. All full-text articles and abstracts were assessed for inclusion. Each stage was carried out by one reviewer (ESB) with the second reviewer (PFM) assessing a random sample (10% of the total). We resolved any discrepancies through discussion, or if required, we consulted the third reviewer (CAC).
Data extraction and management
Once a study was included, data were extracted using a standardised form. Data extracted included information regarding study design, participants, the magnesium sulphate regimen(s), the control/comparison if applicable, maternal adverse effects reported and results relevant to the review, the risk of bias, confounding and relevance. For personal blogs and discussion forum threads, information regarding perceived purpose and/or benefits of treatment, and women’s experiences with treatment, particularly considering adverse effects were extracted. Extraction was carried out by one reviewer (ESB), with the second reviewer (PFM) independently extracting a random sample (10% of the total, and all included randomised controlled trials). We resolved any discrepancies through discussion, or if required, we consulted the third reviewer (CAC).
Assessment of study quality/ risk of bias
Quality appraisal of intervention studies was undertaken utilising established guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions [13]. The quality assessment of observational studies was guided by recommendations from the Cochrane Handbook on assessing the quality of non-randomised studies [13] (which highlights that attention must be paid particularly to selection bias) and principles of the Newcastle-Ottawa Scale [14], where we judged the quality of each study on three main aspects: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure of outcome of interest for case-control or cohort studies respectively; for case series we primarily considered selection of the study group. As it has been suggested that the quality of adverse effect detection and reporting is not always adequately assessed, it was also important to consider the methods used to detect adverse effects and how rigorous these methods were, along with an assessment of incomplete reporting [12, 15].
Data synthesis and analysis
The analysis and presentation of results were categorised by study design. Statistical analyses were performed using Review Manager, version 5.1 (The Cochrane Collaboration, Copenhagen, Denmark).
For intervention studies we presented quantitative data from individual studies where possible as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes. For all outcomes, we carried out analyses as far as possible on an intention-to-treat basis. Pooled estimates (summary RR with 95% CI) were calculated using fixed-effect meta-analysis (Mantel-Haenszel method) where there was a sufficient quantity of data, with clinical homogeneity. Where we considered that there was clinical heterogeneity sufficient to expect the underlying effects differed between trials, or there was substantial statistical heterogeneity (where I2 was greater than 30% and either T2 was greater than zero, or there was a low P-value (less than 0.10) in the Chi2 test), summary estimates were calculated using random-effects meta-analysis.
Separate comparisons were performed for those studies assessing magnesium sulphate versus no treatment/placebo, and those comparing different magnesium sulphate regimens. For all review outcomes, we conducted subgroup analyses based on indication for use (i.e. given for pre-eclampsia/eclampsia; fetal neuroprotection; tocolysis), as this was considered likely to influence outcomes. Additional subgroup analyses were planned if sufficient data were available based on aspects of the magnesium sulphate regimen (i.e. route of administration; dose). We assessed subgroup differences by interaction tests available within Review Manager, and where applicable, we have quoted the Chi2 statistic and P-value, and the interaction test I2 value. We included only primary outcomes and the outcomes: discontinuation due to adverse effects, calcium gluconate use, and 'any adverse effects’ in subgroup analyses.
For observational studies (cohort, case-control, cross-sectional, case series) we presented effect estimates where possible as percentages, RR or odds ratios (OR) with 95% CIs, adjusted RR or OR if reported with 95% CIs, or P-values only, in tabular format based on study type; we used narrative synthesis to summarise the studies. Data from case reports were tabulated and subsequently grouped according to themes. For personal blogs and discussion forum threads, relevant text was tabulated (considering perceived purpose/benefits of magnesium sulphate and experience of magnesium sulphate therapy, before, during and after treatment), and thematic analysis techniques were used to identify and summarise emerging themes.
Results
Study selection
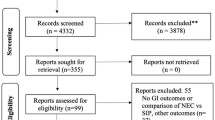
The results of the search strategy, including the sources of the studies, culling and final inclusion of studies are shown in Figure 1. The initial database searching identified 5,062 articles. Review of the abstracts/titles and exclusion of irrelevant/duplicate articles yielded 1,034 articles. Of these articles, we excluded 896 for the documented reasons. We therefore included 138 studies, along with an additional five studies identified through other searching; a total of 143 studies (see Additional file 3 for References to all included reports). In the case of multiple publications from the same study, we included the report with the most relevant data relating to adverse effects.
Flow diagram of included studies. *Numbers indicate level of evidence, according to Australian Government National Health and Medical Research Council (NHMRC) Evidence Hierarchy Available at: https://www.nhmrc.gov.au/_files_nhmrc/file/guidelines/developers/nhmrc_levels_grades_evidence_120423.pdf.
Evidence from randomised controlled trials
Twenty one randomised trials (16,812 women) were included, the characteristics of which are detailed in Additional file 4, and the risk of bias assessment presented in Figures 2, 3 and 4. The trials assessed a variety of different treatment regimens with varying comparators, and are therefore assessed under six different comparisons:
-
1.
Magnesium sulphate versus placebo or no treatment (11 trials).
-
2.
Lower dose versus higher dose magnesium sulphate IM maintenance (2 trials).
-
3.
Magnesium sulphate IV maintenance versus IM maintenance (3 trials).
-
4.
Short versus standard (24 hour) postpartum magnesium sulphate maintenance (2 trials).
-
5.
Lower dose versus higher dose magnesium sulphate IV maintenance (2 trials).
-
6.
'Ready-to-use’ magnesium sulphate solution versus a reference drug requiring dilution (1 trial).
Considering the risk of bias for trials in Comparison 1 (magnesium sulphate versus placebo or no treatment), sequence generation and allocation concealment were adequate in the majority of trials (6/11 and 8/11 trials respectively) (Figure 2). For other trials, it was unclear whether this was adequate, and for one trial, allocation concealment was not considered adequate. For six trials, blinding of personnel, women and outcome assessors was considered adequate, whilst for four trials this was not considered adequate, and for one trial this was unclear. The randomised trials in Comparisons 2-6 (different magnesium sulphate regimens) were considered at a comparatively higher risk of bias overall (Figure 3). For the majority of trials, it was unclear whether sequence generation and allocation concealment were adequate (5/10 and 8/10 trials respectively). Blinding of personnel and women was not possible in any of the trials; none of the trials reported that outcomes were assessed in a blinded manner.
Comparison 1: magnesium sulphate versus placebo or no treatment
This comparison included 11 trials with 15,709 women [16–26]. In six trials, the indication for use of magnesium sulphate was the prevention or treatment of eclampsia; for three trials, the indication was fetal neuroprotection, and for two trials, the prevention of preterm birth (see Table 1 and Additional file 5 for effect estimates and forest plots for outcomes in Comparison 1).
Life-threatening adverse effects of treatment
No significant differences were seen between magnesium sulphate and placebo/no treatment for maternal death (RR 0.53; 95% CI 0.26 to 1.09; 5 trials, 14,662 women; Analysis 1.1), cardiac arrest (RR 0.80; 95% CI 0.21 to 2.98; 4 trials, 13,977 women; Analysis 1.2) or respiratory arrest (RR 2.50; 95% CI 0.49 to 12.88; 4 trials 13,977 women; Analysis 1.3).
Interventions to limit adverse effects
Women receiving magnesium sulphate experienced a significantly increased (almost three times) risk of discontinuing treatment due to associated adverse effects (RR 2.77; 95% CI 2.32 to 3.30; 5 trials 13,666 women; Analysis 1.4). There were no significant differences between groups in the outcomes calcium gluconate administration (RR 1.35; 95% CI 0.63 to 2.88; 2 trials, 10,795 women; Analysis 1.5) and intensive care unit admission (RR 0.97; 95% CI 0.72 to 1.30; 2 trials, 11,172 women; Analysis 1.6).
Adverse effects associated with treatment
Women receiving magnesium sulphate were almost five times more likely to experience 'any side effects’ in the four included trials (RR 4.62; 95% CI 2.42 to 8.83; 13,322 women; Analysis 1.7). Women receiving magnesium sulphate compared with women receiving no treatment/placebo experienced an approximately 50% increased risk of hypotension (RR 1.52; 95% CI 1.10 to 2.11; 3 trials, 1,782 women; Analysis 1.11) and tachycardia (RR 1.53; 95% CI 1.03 to 2.29; 1 trial, 1,062 women; Analysis 1.12). Compared with women receiving no treatment/placebo, women receiving magnesium sulphate experienced an approximately 50% increased risk of problems at the IM injection site (RR 1.49; 95% CI 1.25 to 1.79; 1 trial, 4,553 women; Analysis 1.25), and more than six times the risk of problems/discomfort at the IV site (RR 6.34; 95% CI 3.10 to 12.98; 3 trials, 8,704 women; Analysis 1.24).
Women receiving magnesium sulphate had an approximately 50% increased risk of respiratory depression (RR 1.41; 95% CI 1.07 to 1.86; 5 trials, 14,098 women; Analysis 1.8), more than two times the risk of drowsiness/confusion (RR 2.46; 95% CI 1.83 to 3.29; 3 trials, 11,189 women; Analysis 1.16), headache (RR 2.21; 95% CI 1.27 to 3.86; 2 trials, 10,556 women; Analysis 1.17), dizziness (RR 2.62; 95% CI 1.63 to 4.21; 2 trials, 11,054 women; Analysis 1.19), mouth dryness or thirst (RR 2.38; 95% CI 1.59 to 3.56; 2 trials, 11,054 women; Analysis 1.18) and blurred vision (RR 2.34; 95% CI 1.32 to 4.14; 1 trial, 1,062 women; Analysis 1.22), more than five times the risk of nausea and/or vomiting (RR 5.50; 95% CI 2.29 to 13.22; 4 trials; 13,821 women; Analysis 1.14), nearly seven times the risk of flushing and warmth (RR 6.94; 95% CI 4.19 to 11.49; 5 trials, 13,956 women; Analysis 1.13) and sweating (RR 6.37; 95% CI 1.96 to 20.65; 2 trials, 3,265 women; Analysis 1.20), nearly 15 times the risk of itching and tingling (RR 14.98; 95% CI 1.98 to 113.38; 1 trial, 9,992 women; Analysis 1.21), and more than 15 times the risk of muscle weakness (RR 15.81; 95% CI 7.36 to 33.96; 3 trials, 10,212 women; Analysis 1.15).
There were no significant differences between groups for the outcomes absent/reduced tendon reflexes (RR 1.01; 95% CI 0.71 to 1.44; 3 trials; 11,241 women; Analysis 1.9) and slurred speech (RR 3.04; 95% CI 0.13 to 73.42; 1 trial, 135 women; Analysis 1.23).
Other outcomes
For women receiving magnesium sulphate compared to no treatment/placebo, a small significant increased risk of caesarean section was shown (RR 1.04; 95% CI 1.00 to 1.08; 10 trials, 14,105 women; Analysis 1.26). No differences were seen between groups for the outcomes postpartum haemorrhage (RR 0.94; 95% CI 0.87 to 1.04; 4 trials, 10,535 women; Analysis 1.27) and pulmonary oedema (RR 1.12; 95% CI 0.72 to 1.74; 4 trials, 12,787 women; Analysis 1.28).
Subgroup analysis by indication for use
When considering indication for use, the subgroup interaction tests for the majority of outcomes were non-significant, indicating no differential effects according to the different reasons for administration (see Table 1 for effect estimates for indication for use subgroups and Additional file 5 for forest plots). While substantial statistical heterogeneity (I2 > 90%) was observed for the outcomes 'any side effects’, flushing and/or warmth and nausea and/or vomiting, this could not be explained by considering the indication for use of treatment. In each case the test for subgroup differences was non-significant ('any side effects’: Chi2 = 1.68, P = 0.43, I2 = 0%; Analysis 1.7) (flushing and/or warmth: Chi2 = 0.07, P = 0.79, I2 = 0%; Analysis 1.13) (nausea and/or vomiting: Chi2 = 1.16, P = 0.28, I2 = 13.9%; Analysis 1.14).
For the outcome problems at the IV site and/or arm discomfort, the subgroup interaction test indicated a significant difference between indication for use subgroups, and a possible differential effect in favour of receiving treatment for pre-eclampsia, with women receiving treatment for fetal neuroprotection being more likely to experience arm discomfort (Chi2 = 25.80, P = < 0.00001, I2 = 96.1%; Analysis 1.24). It is possible, however, that the methods used to collect information on arm discomfort/problems at the IV site differed substantially between trials, and could help to explain this observed differential effect.
Subgroup analysis by regimen for administration
To explore the effect of aspects of the regimen for administration of magnesium sulphate on adverse effects, the trials from Comparison 1 were grouped as pre-specified where possible according to their dosage and/or route of administration (loading dose only; loading plus maintenance dose; 4 g IV loading dose plus any maintenance; 5-6 g IV loading dose plus any maintenance; 1 g/hour IV maintenance; 2-3 g/hour IV maintenance; IM maintenance).
For the outcomes maternal death, cardiac arrest, respiratory arrest and use of calcium gluconate, no significant differences were shown between the magnesium sulphate and no magnesium sulphate groups for any of the subgroups, and the subgroup interaction tests for all outcomes indicated no significant differential effects across the treatment subgroups (see Table 1 for effect estimates for regimen subgroups and Additional file 5 for forest plots). The significantly increased risk of discontinuing treatment due to adverse effects and experiencing 'any side effects’ for the magnesium sulphate group was seen across all of the different regimen subgroups (see Analyses 1.4 and 1.7); for both outcomes, the subgroup interaction tests did not indicate differential effects according to the subgroups.
Comparison 2: lower dose versus higher dose IM maintenance: prevention or treatment of eclampsia
This comparison included two trials with 176 women with both trials assessing magnesium sulphate for eclampsia, or 'imminent eclampsia’ (see Table 2 and Additional file 5 for effect estimates and forest plots for Comparison 2). One trial compared a lower dose 'Dhaka’ regimen from Bangladesh: 4 g IV and 6 g IM as a loading dose, and 2.5 g IM every four hours as maintenance, with a higher dose 'Bhalla’ regimen: 4 g IV and 8 g IM as a loading dose, and 4 g IM every four hours as maintenance [27]. The second trial compared a loading dose of 4 g IV, and 2 g IM every three hours as maintenance, with Pritchard’s regimen (a loading dose of 4 g IV and 10 g IM, and 5 g IM every four hours as maintenance) [28].
Life-threatening adverse effects of treatment
No significant difference between groups was shown for the risk of 'maternal death due to toxicity’ in one trial of 126 women (RR 0.25, 95% CI 0.01 to 6.05; Analysis 2.1). No other primary review outcomes were reported.
Interventions to limit adverse effects
Women allocated to the lower dose regimen were significantly less likely to have treatment stopped due to 'toxicity’ in one trial, an approximate 95% relative risk reduction (RR 0.05; 95% 0.01 to 0.39; 126 women; Analysis 2.2). Women allocated to the lower dose regimen were significantly less likely to have a maintenance dose deferred or skipped due to adverse effects, an approximate 64% relative risk reduction (RR 0.36; 95% CI 0.20 to 0.63; 2 trials, 176 women; Analysis 2.3). No clear difference was shown for the need for calcium gluconate in one trial (RR 0.25; 95% CI 0.06 to 1.06; 1 trial, 50 women; Analysis 2.4).
Adverse effects associated with treatment
Women allocated to the lower dose regimen were significantly less likely to have absent tendon reflexes during treatment, an approximate 79% relative risk reduction (RR 0.21; 95% CI 0.10 to 0.46; 2 trials, 176 women; Analysis 2.6). There were insufficient data for reliable conclusions about the differential effects on respiratory depression in one trial of 126 women (RR 0.25; 95% CI 0.01 to 6.05; Analysis 2.5). There were no cases of gluteal abscess in one trial of 126 women measuring this outcome (Analysis 2.7).
Other outcomes
No significant differences were shown between groups in one trial of 126 women for the outcomes postpartum haemorrhage (RR 0.38; 95% CI 0.03 to 4.03; Analysis 2.8) and pulmonary oedema (RR 0.25; 95% CI 0.01 to 6.05; Analysis 2.9).
Comparison 3: IV maintenance versus IM maintenance: prevention or treatment of eclampsia
This comparison included three trials with 361 women (see Table 2 and Additional file 5 for effect estimates and forest plots for Comparison 3); all trials assessed magnesium sulphate for the prevention or treatment of eclampsia. Two trials compared Pritchard’s IM regimen (a loading dose of 4 g IV and 10 g IM (5 g in each buttock), and 5 g IM in alternative buttocks every four hours as maintenance) with either a loading dose of 6 g IV, and 2 g/hour IV maintenance [30], or a loading dose of 4 g IV, and 0.75 g/hour IV maintenance [29]. The third trial did not describe its regimens, and compared the use of an IV Springfusor pump with standard hospital practice (IV loading dose; IM maintenance) [31].
Life-threatening adverse effects of treatment
In one trial, no significant difference was seen for the outcome maternal death (RR 0.35, 95% CI 0.04 to 3.27; 1 trial, 137 women; Analysis 3.1). There were no data on the other primary outcomes.
Interventions to limit adverse effects
No clear difference was seen for the outcome discontinuation or modification of treatment due to adverse effects (RR 1.46, 95% CI 0.83 to 2.58; 2 trials, 317 women; Analysis 3.2).
Adverse effects associated with treatment
No significant difference was seen in two trials for the outcome 'clinical signs of toxicity’ (RR 0.82; 95% CI 0.05 to 12.56; 154 women; Analysis 3.3). In one trial of 300 women, women allocated to the IV regimen were almost five times more likely to report their pain level as 'acceptable,’ compared with women allocated to the IM regimen (RR 4.93; 95% CI 3.56 to 6.78; Analysis 3.4).
Other outcomes
There were insufficient data for reliable conclusions about the differential effects on caesarean section (RR 1.03; 95% CI 0.78 to 1.35; 2 trials, 154 women; Analysis 3.5) and postpartum haemorrhage (RR 0.35; 95% CI 0.04 to 3.27; 1 trial, 137 women; Analysis 3.6).
Comparison 4: short versus standard (24 hour) postpartum maintenance therapy: prevention of eclampsia
This comparison included two trials with 260 women which assessed magnesium sulphate for the prevention of eclampsia (see Table 2 and Additional file 5 for effect estimates and forest plots for Comparison 4). The trials compared individualised (short) versus standard (24 hour) postpartum maintenance therapy [32, 33]. One trial compared 2 g/hour IV maintenance for 12 hours versus for 24 hours [32], whilst the other trial compared individualised maintenance (based on clinical criteria) with 24 hours maintenance; the regimens were unclear [33].
Life-threatening adverse effects of treatment
There were no data on the primary outcomes.
Adverse effects associated with treatment
There were insufficient data for reliable conclusions about the differential effects on 'toxicity’ in two trials (RR 0.23; 95% CI 0.06 to 1.08; 256 women; Analysis 4.1), or 'side effects’ in one trial (RR 0.17; 95% CI 0.02 to 1.30; 60 women; Analysis 4.2). There were no cases of 'intolerance’ among women in either group in the one trial of 196 women reporting this outcome (Analysis 4.3).
Comparison 5: lower dose versus higher dose IV maintenance: prevention of preterm birth
This comparison included two trials with 260 women (see Table 3 and Additional file 5 for effect estimates and forest plots for Comparison 5). Both trials assessed magnesium sulphate for the prevention of preterm labour, comparing a 4 g loading dose and 2 g/hour maintenance, with either a 6 g loading dose and ≥ 2 g/hour maintenance [34] or a 4 g loading dose and 5 g/hour maintenance [35].
Life-threatening adverse effects of treatment
The two trials did not report on the review’s primary outcomes.
Interventions to limit adverse effects
There was no cessation due to adverse effects in either trial (Analysis 5.1).
Adverse effects associated with treatment
No significant difference was shown for the outcome 'no side effects’ when data for the two trials were pooled (RR 1.55; 95% CI 0.94 to 2.84; 248 women; Analysis 5.2), however there was a substantial degree of statistical heterogeneity for this outcome (I2 = 63%), and the subgroup interaction test indicated a potential differential effect based on the comparison regimen (Chi2 = 2.73, P = 0.10, I2 = 63.4%). In one trial, women receiving the lower dose IV maintenance regimen (2 g/hour) were significantly more likely to experience 'no side effects’ than women receiving the higher dose maintenance regimen (5 g/hour) (RR 1.96; 95% CI 1.35 to 2.84; 148 women; Analysis 5.2.2). No significant difference was shown between the low and high dose IV maintenance groups for flushing (RR 0.61; 0.33 to 1.12; 2 trials, 248 women; Analysis 5.3), however moderate statistical heterogeneity was also observed for this outcome, which may be in part explained by the differing high dose comparison regimens.
There were insufficient data for reliable conclusions about the differential effects on the risk of nausea and vomiting (RR 0.79; 95% CI 0.45 to 1.37; 1 trial, 100 women; Analysis 5.4) and headache (RR 0.56; 95% CI 0.30 to 1.05; 2 trials. 248 women; Analysis 5.5).
Other outcomes
No significant differences were shown between groups for the outcomes caesarean section (RR 1.11; 95% CI 0.73 to 1.70; 2 trials, 248 women; Analysis 5.6) and pulmonary oedema (RR 0.21; 95% CI 0.03 to 1.76; 2 trials, 260 women; Analysis 5.7).
Comparison 6: ready to use solution versus reference drug requiring dilution: prevention of preterm birth
This comparison included one trial of 46 women (see Table 3). The trial compared a pre-mixed 'ready-to-use’ solution of magnesium sulphate, with a reference drug, a commercially available infusion solution concentrate requiring dilution [36]. All women were given a 4 g IV loading dose followed by 1-2 g/hour IV maintenance.
Life-threatening adverse effects of treatment
There were no maternal deaths, or 'serious’ adverse events in either group (Analysis 6.1 and 6.2). There were no data on other primary outcomes.
Interventions to limit adverse effects
There were insufficient data for reliable conclusions about the differential effects on 'withdrawing from the study due to adverse effects’ (Analysis 6.3) and one or two injection site changes (Analysis 6.5).
Adverse effects associated with treatment
There were insufficient data for reliable conclusions about the differential effects on any of the adverse effects reported in the trial (adverse events of severe intensity, poor general tolerability, respiratory depression, warmth, nausea and/or vomiting, tiredness, headache, dry mouth, dizziness, sweating, skin redness, burning at injection site, palpitations, constipation, dyspnoea, heart pain, agitation) (Analyses 6.4 and 6.6 to 6.21).
Other outcomes
The trial did not assess other outcomes of interest.
Evidence from non-randomised comparative studies with concurrent controls
Fourteen non-randomised comparative studies with concurrent controls were included (3,615 women) [37–51]: four non-randomised clinical trials (969 women) [46–49], four prospective before and after studies (78 women) [40–43], five retrospective cohort studies (2,502 women) [37–39, 45, 51] and one retrospective case-control study (66 women) [44]. Additionally, one historical control study was included (76 women) [50]. The detailed characteristics of the studies are presented in Additional file 4, with the risk of bias assessment presented in Figures 5 and 6. For the four non-randomised trials, sequence generation, allocation concealment and blinding were not considered adequate (Figure 5). In regards to other comparative studies, for the majority of studies, selection, according to principles of the Newcastle-Ottawa Scale [14], was considered adequate (7/11), whilst comparability was largely unclear or not considered adequate, and outcome or exposure assessment was largely unclear (Figure 6).
Risk of bias for non-randomised comparative studies with concurrent controls~. Risk of bias summary showing review authors’ judgements about each risk of bias item for included non-randomised comparative studies with concurrent controls. Each risk of bias item is judged as at a low risk of bias, unclear risk of bias or high risk of bias. ~This includes one historical control study.
Results from these studies largely supported those from the randomised trials (see Tables 4 and 5), with no major maternal complications (including death and cardiac arrest) in the studies that reported these outcomes. For one retrospective study reporting on the cessation of treatment due to adverse effects [45], the percentage of women stopping treatment was similar to the percentages reported in the randomised trials (Table 5). Effect estimates from non-randomised studies for the risk of caesarean section for women receiving magnesium sulphate versus no magnesium sulphate, were notably higher than the pooled effect estimate from the randomised trials (Table 4). In two retrospective cohort studies, women receiving magnesium sulphate were significantly more likely to experience failed labour induction [39], and undergo caesarean section due to failure to progress [37] (Table 4).
No significant increase in neuromuscular weakness among women receiving nifedipine as their antihypertensive during magnesium sulphate therapy, compared with women receiving an alternative or no antihypertensive agent, was shown in one retrospective cohort [45] (Table 4). Significantly increased risks of thirst, respiratory problems and minor bleeding, were however observed among women receiving nifedipine, compared with no antihypertensive agent; a significantly increased risk of neuromuscular blockade was observed among women receiving an alternative antihypertensive, compared with nifedipine [45] (Table 4). Whilst two prospective before and after studies showed a significant increase in bleeding time for women receiving magnesium sulphate [40, 41] (Table 4), this was not supported by an increased risk of postpartum haemorrhage in the randomised trials (Table 1). Similarly, whilst in one retrospective case control study, pregnant women with pulmonary oedema were significantly more likely to have received magnesium sulphate as compared with women without pulmonary oedema [44] (Table 4), the randomised trials did not support an increased risk of pulmonary oedema overall (Table 1).
In one retrospective study, women receiving magnesium sulphate for greater than 48 hours compared with for less than 48 hours had a significantly increased risk of experiencing more than one adverse effect [51] (Table 5). No significant differences were seen, however, in the risk of discontinuing therapy due to adverse effects, or for any other adverse effects [51] (Table 5). In one non-randomised trial, women allocated to a loading dose only, compared with women receiving Pritchard’s regimen (a loading dose of 4 g IV and 10 g IM, and 5 g IM every four hours as maintenance), were significantly less likely to experience nausea and vomiting, dizziness, irritation at the injection site, and undergo a caesarean section [49] (Table 5). Similar to the randomised trials, a significantly increased risk of pain was experienced among women receiving IM versus IV maintenance therapy [46] (Table 5). Supporting the findings from one randomised trial, no significant differences in adverse effects or medication errors were shown in the historical control study that assessed two different magnesium sulphate solutions using an identical dosage regimen [50] (Table 5).
Evidence from case series
Thirty-two studies [52–83] (3,276 women), 20 prospective and 12 retrospective in nature, reporting maternal adverse effects were included; the characteristics of the studies and the quality assessment are presented in Additional file 4. Adverse effects have been presented in Table 6 as overall mean and median percentage estimates calculated from individual study results, with the range of percentages reported in the studies also presented. For the quality assessment of case series, we predominately considered participant selection, along with the collection/reporting of adverse effect information, as detailed in Additional file 4.
Adverse effect estimates reported in case series were largely similar to those reported in the randomised trials in Comparison 1 (see Additional file 6). Mean/median percentage estimates of serious outcomes, respiratory arrest, the use of calcium gluconate and discontinuation of therapy due to adverse effects, were slightly higher in the case series. The estimates for the outcome 'any maternal adverse effects’ reported in case series were however notably lower than those in the randomised trials. The case series estimates for nausea and/or vomiting, blurred vision and hypotension were higher than estimates from the randomised trials.
Evidence from case reports
Seventy-five studies describing a total of 137 case reports of maternal adverse effects were included [84–158] (see Table 7; the more detailed characteristics of cases are presented in Additional file 7).
Iatrogenic overdoses (16 reports) [84–100] and rapid administration of magnesium sulphate (one report) [86] were associated with a range of serious adverse events, such as respiratory arrest, cardiac arrest, cardiopulmonary arrest and death. Of note was a detailed account of 52 errors associated with magnesium sulphate administration; including seven cases resulting in death, or women remaining in a persistent vegetative state [87]. Additional errors, relating to the epidural or intrathecal administration of magnesium sulphate were associated with pain, inadequate pain relief, or temporary paralysis (four reports) [101–104].
Women at an increased risk of adverse effects were described (11 reports) (105-115), including women with renal failure (three reports) [113–115], and women with neuromuscular junction disorders, myopathies, and neuropathologies (eight reports) [105–112]. For the neurological cases, magnesium sulphate administration, according to recommended regimens, was associated with muscle pain, weakness or temporary paralysis, and associated respiratory problems. The interaction of magnesium sulphate with agents used in general anaesthesia (10 reports) [116–125] and with antihypertensive agents including nifedipine (six reports) [126–131] was associated with varying adverse effects, most commonly muscular weakness or paralysis, and altered respiratory function – associated with prolonged neuromuscular blockade.
A variety of unusual maternal adverse effects, not previously attributed to magnesium sulphate, were described (11 reports) [132–142], including bilateral progressive labial swelling [132], marked osteoporotic change [136], and urinary tract stone formation [139]; in such cases women had received prolonged magnesium sulphate tocolysis therapy. Additional adverse effects, such as hypotension and fatigue as reported in randomised trials, were detailed (16 reports) [143–158]. Of note were five reports where adverse effects such as delirium [156], diplopia [153], tetany and paraesthesia [152–154] associated with serum hypocalcaemia, were described.
Evidence from patient safety organisations
Whilst the majority of patient safety organisations did not provide free access to their adverse event or equivalent databases, information regarding medication errors and maternal adverse effects associated with antenatal magnesium sulphate was available from the Institute for Safe Medication Practices USA. The Institute provided accessible information in their electronic medication safety newsletters. Three cases of serious iatrogenic overdose were reported in the 12 February 1997 issue [159]. Whilst the women survived, one suffered a respiratory arrest, and one, temporary paralysis of her extremities, ICU admission and the need for prolonged ventilation. Additional cases were described in the 30 June 1999, 15 June 2005, 20 October 2005, and 3 June 2010 issues.
The February 2010 issue of the Patient Safety Newsletter 'Sharing Lessons Leaned’ produced by the Office of Safety and Quality in Health Care, Western Australia, reported 12 incidents involving magnesium sulphate overdose from October 2001 to October 2009, identified through searches of state-wide relevant databases [160]; the consequent maternal adverse effects were not however discussed.
Women’s experiences – from personal blogs and discussion forum threads
(See Additional file 8 for blog and discussion forum thread sources).
Before treatment
Women considered information regarding others’ experiences with magnesium sulphate important, with a particular desire for knowledge regarding adverse effects and their severity; a number of women expressed an explicit wish for “the truth, no matter how bad or scary.” Women expressed expectations of treatment benefits, relating to the different indications for use, and also concern and a sense of fear regarding the possible adverse effects of treatment; one woman exclaimed “What?!! NO!!! ANYTHING BUT MAG!!!”
During treatment
Whilst variations in experiences existed, women described a variety of adverse effects, the predominant being a sensation of heat: “MY GOD, THE HEAT! I felt so hot and it came from within.” Other commonly described adverse effects were similar to those frequently reported adverse effects in randomised trials – muscle weakness, lethargy, blurred vision, nausea or vomiting, headaches, confusion, arm discomfort, thirst, feeling sweaty and dizzy. Less common adverse effects women attributed to magnesium sulphate treatment included a failed induction of labour and consequent need for a caesarean section, the delayed onset of lactation, and the development of pulmonary oedema. A number of women described their request or the need for treatment cessation due to adverse effects, with two women highlighting their experience of being “accidentally overdosed.” Many women portrayed their experience as terrifying, revealing a sense of anguish.
After treatment
Women expressed relief following treatment cessation, and great concerns regarding the possibility of treatment in future pregnancies: “I pray I never have to do it again.” A number of women felt information regarding the possible adverse effects of treatment was miscommunicated and misleading. Although many women recalled adverse effects of severe intensity, they were generally very thankful, and there was agreement among commenting women that the potential perceived benefits of treatment outweighed discomforts experienced, with magnesium sulphate being described as “a necessary evil.”
Discussion
It is widely acknowledged that the use of antenatal magnesium sulphate, for the prevention of eclampsia in women with pre-eclampsia and the treatment of women with eclampsia [1–4, 9], to delay or prevent preterm birth [5, 6], and for neuroprotection of the fetus when given to women at risk of preterm birth [7], may be associated with adverse effects for the mother. The risk of individual adverse effects, and how such adverse effects vary according to different regimens, has however not been clear. Determining this is of great importance given the significant number of women who may be eligible for treatment with magnesium sulphate during pregnancy – with approximately 2-8% of pregnancies complicated by pre-eclampsia [161], and an estimated 1-2% of births being very preterm (before 32 weeks’ gestation) [162].
Summary of main results
We were able to include 21 randomised trials in this systematic review; 11 comparing magnesium sulphate with placebo or no treatment, and 10 comparing different magnesium sulphate regimens. Whilst 'good’ data on well recognised and easily detectable adverse effects may be potentially available from randomised trials, the often small numbers of participants, who may differ from individuals given the treatment in everyday practice, or the short periods of time that the participants are studied for, can reduce the possibility of unpredictable, rare or delayed adverse effects being observed and reported [12, 163, 164]. For such reasons, it was considered important to include other study designs. We included 15 non-randomised comparative studies with concurrent controls, 32 case series, and 75 papers describing individual case reports.
Evidence from the randomised trials assessing magnesium sulphate versus placebo or no treatment confirmed the expected higher rates of 'minor’ maternal adverse effects among women receiving treatment, without an increase in major complications (death, cardiac arrest, respiratory arrest). The four randomised trials reporting on 'any adverse effects’ of treatment, showed an absolute risk of 38% (2,521/6,642) for women exposed to antenatal magnesium sulphate compared with 8.5% (567/6,680) for women unexposed. Five randomised trials reported on the need for cessation of treatment due to maternal adverse effects, with significantly more women receiving magnesium sulphate having their therapy stopped compared with women not receiving magnesium sulphate (6.6% versus 2.4%). The most frequently reported adverse effects included warmth or flushing, sweating, and arm discomfort or problems at the IV site.
When considering indication for use and regimens for administration, very few differences were observed between the pre-specified subgroups. Considering indication for use, no significant subgroup interactions were identified, expect for when considering arm discomfort, which suggested a possible increase for women receiving treatment for fetal neuroprotection (compared with women receiving treatment for pre-eclampsia). When considering regimen for administration for the trials comparing magnesium sulphate with placebo or no treatment, the subgroup interaction tests did not indicate differential effects by treatment subgroups. For the majority of outcomes, the subgroups contained very few trials (many contained only one), making comparisons between subgroups difficult.
The 10 randomised trials comparing different magnesium sulphate regimens were mostly too small to provide reliable evidence about the comparative effects of different regimens on maternal adverse effects. Whilst this review did not formally assess efficacy, none of the trials demonstrated significantly decreased effectiveness with the lower dose regimens. One trial reporting on the cessation of treatment due to adverse effects showed significantly more women receiving higher dose IM maintenance having their therapy ceased as compared with women receiving lower dose IM maintenance (25.9% versus 1.4%). This was supported by one small non-randomised trial, reporting significantly higher rates of adverse effects for women receiving Pritchard’s IM regimen versus a loading dose only. One randomised trial showed significantly more women receiving magnesium sulphate via an IV pump reporting 'acceptable’ pain as compared with women receiving magnesium sulphate via the IM route (100% versus 20%). This was supported by a non-randomised trial that showed a significantly higher risk of pain among women receiving magnesium sulphate via the IM route versus via an IV infusion.
The data from the 32 case series largely supported that from the randomised trials. Whilst serious outcome estimates were broadly similar, differences between procedures for collecting information on adverse events between studies may help to explain the variations observed between estimates. Higher estimates for 'any adverse effects’ in randomised trials may have been influenced by the use of more rigorous methods for data collection, such as the use of trial-specific check-lists [19]. The notably higher estimates in case series for blurred vision and nausea and/or vomiting similarly may have been influenced by the use of specific questioning and interviewing in regards to adverse effects [68], and incomplete reporting, such as the use of generic statements [72].
As the establishment of a causal relationship between a treatment and a subsequent adverse effect through individual cases is difficult, data presented in case reports should be interpreted with a degree of caution. That said, the significant harm that may result from accidental overdose of magnesium sulphate is unquestionable. The 16 reports, including Simpson’s account of 52 errors associated with administration [87], highlight the potentially life-threatening consequences of magnesium sulphate overdose.
Factors contributing to iatrogenic overdoses were identified [87]. These included: the inaccurate or inadequate mixing of an IV magnesium sulphate solution, leading to a higher concentration of solution than intended or an undesirable concentration gradient between the infusion bag and tubing; the use of infusion bags containing a high total dose of magnesium sulphate, and the consequent danger associated with infusion rate programming error; the use of the same infusion bag for administration of the loading and maintenance infusions, and the failure to reduce the infusion rate following the loading dose; the removal of an IV line from an IV pump temporarily, and the accidental free-flow of solution; the handover of clinical responsibilities in busy units, and the transfer of women between units. Fortunately in most reported cases, the error was recognised before permanent adverse outcomes occurred. Monitoring of maternal status (before, during and after administration of magnesium sulphate), to allow signs and symptoms of toxicity to be recognised, and for potential consequences of errors to be mitigated in a timely manner, is imperative. Common recommendations for monitoring include: regular assessment and documentation of vital signs (pulse, blood pressure, respiratory rate), level of consciousness, patellar reflexes and urinary output; and the provision of 1:1 nursing care during loading administration (and 1:2-3 care during maintenance or where 1:1 care is not possible) [87].
Case reports also highlighted the potential for an increased risk of adverse effect occurrence among particular groups of women – those with renal insufficiency or failure, and those with neuromuscular disorders such as myasthenia gravis, Friedreich’s ataxia, myotonic dystrophy, and other rare myopathies, such as mitochondrial myopathy. The potential for prolonged/enhanced neuromuscular blockade when magnesium sulphate is used in conjunction with general anaesthetic agents and antihypertensive agents including nifedipine was also shown. Whilst this potential interaction between magnesium sulphate and nifedipine was highlighted, reassuringly no increased neuromuscular blockade was shown in the retrospective cohort study that compared the use of nifedipine versus other/no antihypertensive during magnesium sulphate treatment (Table 4). Though an excess of respiratory problems and minor bleeding was shown among women receiving nifedipine, compared with no antihypertensive, the condition of women receiving nifedipine was considered comparatively more severe (more severe hypertension, more frequent pre-eclampsia symptoms), which was suggested to account for this difference [45].
Adverse effects not previously reported in randomised trials that were discussed in case reports included marked bilateral labial swelling, severe paralytic ileus, marked osteoporotic change, and urinary tract stone formation [132, 135, 136, 139]. In each case, magnesium sulphate had been used as a tocolytic agent for a prolonged period (nine, three, 101 and 21 days respectively). The relevant Cochrane reviews have shown magnesium sulphate to be ineffective at delaying or preventing preterm birth [5, 6]. Acknowledging this and the maternal adverse effects of magnesium sulphate, from minor to unpleasant to potentially fatal, its use as a tocolytic appears inappropriate, as has been strongly stated by Grimes and Nanda [165]. Furthermore, the Food and Drug Administration in the United States recently advised health care professionals against the use of magnesium sulphate as a tocolytic for more than five to seven days due to associated harm to developing fetal bones (the use of the drug for tocolysis is 'off-label’ (not approved by the Food and Drug Administration)) [166]. In view of the extremely widespread use of antenatal magnesium sulphate in obstetric practice, the potential neonatal and infant adverse effects of antenatally administered magnesium sulphate additionally require comprehensive evaluation, such as by systematic review.
Overall completeness, applicability and quality of the evidence
This review was based on a comprehensive search strategy, and whilst no language restrictions were applied, inclusion was restricted to those studies written in English or for which a translation was readily available, and to those studies published in the databases that were searched, which may have limited available studies. Additionally, there were a number of articles that were excluded as we were unable to obtain the abstract and/or full-text. The included studies were however conducted in both high-income and low-income countries, and are therefore may considered widely applicable to the treatment of pregnant women.
The main limitations include the small number of studies with relatively small sample sizes comparing different antenatal magnesium sulphate regimens, and the missing data for several important outcomes in almost all trials. Additionally, a great number of trials could not be included in this review, as they did not provide any information regarding maternal adverse effects of treatment. This supports previous reports, that many trials do not report harms, or do so in a fragmented or suboptimal way [167].
The studies included in the review were of mixed quality and we emphasise the need to consider the risk of bias as outlined in Figures 2, 3, 4, 5 and 6, and the more detailed quality assessment in Additional file 4, which includes consideration of the different procedures used to collect information on adverse effects and reporting, when interpreting results.
Potential biases in the review process
We recognise the potential for bias associated with the second reviewer reviewing a random sample of the search records (10% of the total), and independently extracting data from a random sample of included studies (10% of the total, and all 21 randomised controlled trials). We attempted to minimise bias however in a number of ways, for example by using a comprehensive search strategy. Although this was extensive, it is possible that some studies conducted in low- and middle-income countries may not have been identified, if they were not published, or published in journals not indexed in the bibliographic databases searched.
Agreements and disagreements with other reviews
We are not aware of any other reviews specifically assessing maternal adverse effects of antenatal magnesium sulphate, and comparing the adverse effects of different regimens for the various indications for use. The findings of this review are broadly consistent with results from relevant Cochrane reviews, comparing magnesium sulphate with alternative drugs for women with eclampsia [1–3], evaluating magnesium sulphate for women with pre-eclampsia [4], assessing magnesium sulphate for preventing preterm birth in and after threatened preterm labour [5, 6], and evaluating magnesium sulphate for neuroprotection of the fetus for women at risk of preterm birth [7].
These Cochrane reviews similarly concluded no increased risk of major maternal complications (death, cardiac arrest, respiratory arrest) for women receiving magnesium sulphate according to those regimens used in the trials [4, 5, 7], increased risks of many comparatively minor adverse effects [4, 6, 7], and an increased risk of women ceasing treatment due to adverse effects [7]. One Cochrane review that assessed magnesium sulphate for women with pre-eclampsia had previously shown a small increased risk of caesarean section for women receiving magnesium sulphate, compared with placebo/no treatment [4], as was suggested in this review.
The findings of this review largely support the methodological conclusions from the systematic review by Golder et al. [10] – that there are often limited differences in the risk estimates of adverse effects derived from randomised trials and observational studies. Further, they highlight the importance of including a broad range of study designs to ensure that the most complete picture of potential harms associated with an intervention can be drawn [10, 163, 167].
Conclusions
Implications for practice
By considering adverse effects alone, this review was not designed to guide the choice of magnesium sulphate regimen for the different antenatal indications for use. Healthcare providers must therefore weigh the potential risks of magnesium sulphate for the mother against the benefits, for each known beneficial indication separately.
Vigilance in the use of magnesium sulphate is required to promote and ensure safety for women. Certainly, errors associated with the administration of magnesium sulphate represent a significant and perhaps unappreciated risk of harm. An important step in improving the safety for women is knowledge and recognition of the risk of error; this review, along with articles such as that published by Simpson [87], may help in increasing awareness. Individual hospitals should consider common precursors, or contributing factors, to errors associated with antenatal magnesium sulphate occurring in their obstetric units, and ensure safety procedures are in place to help prevent such accidents.
It is important, where ever possible, that women receive a full explanation, not only of why antenatal magnesium sulphate is in their case needed, but also of the nature of symptoms that may be experienced during treatment. An understanding of these benefits, the possible adverse effects, and the vigilant care and monitoring that will be received during treatment, may help to decrease or relieve anxiety during treatment that may otherwise be caused by unexpected adverse effects. Health professionals should seek to make their approach to information provision open and honest.
Implications for research
Fewer maternal adverse effects may be a benefit of lower dose magnesium sulphate regimens, and whilst this review did not formally assess effectiveness, no trial included in this review showed compromised efficacy with such lower dose regimens. Further trials comparing different regimens are however required, to determine the optimal regimens that achieve the desired clinical effectiveness with minimal maternal adverse effects and cessation, for each of the known beneficial indications for use. Such trials must be of a high quality, and of sufficient sample size to assess the comparative effects on relevant maternal and/or infant efficacy and safety related outcomes. For all future randomised trials, consideration of the extension for harms of the CONSORT statement [15] including during the study design phase, is crucial to ensure the better collection and reporting of adverse effects [167].
A number of important questions remain regarding maternal adverse effects of magnesium sulphate. Common reasons for ceasing treatment, and how serious adverse effects and treatment cessation vary according to total dose of magnesium sulphate received, require evaluation. Such questions may be addressed in an individual patient data meta-analysis. Additional well-designed and sufficiently powered trials or observational studies are required to address the need to reduce errors associated with magnesium sulphate administration. Such studies may, for example, consider comparing the use of a pre-mixed solution with a concentrate requiring dilution, or the use of separate infusion bags for the loading and maintenance dose infusions, compared with the use of the same infusion bag with the need to reprogram infusion rates.
Abbreviations
- CI:
-
Confidence interval
- ICU:
-
Intensive care unit
- IM:
-
Intramuscular
- IV:
-
Intravenous
- OR:
-
Odds ratio
- RR:
-
Risk ratio.
References
Duley L, Gülmezoglu A, Chou D: Magnesium sulphate versus lytic cocktail for eclampsia. Cochrane Database Syst Rev. 2010, 9: CD002960
Duley L, Henderson-Smart D, Chou D: Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev. 2010, 10: CD000128
Duley L, Henderson-Smart D, Walker G, Chou D: Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010, 12: CD000127
Duley L, Gulmezoglu A, Henderson-Smart D, Chou D: Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010, 11: CD000025
Crowther CA, Hiller JE, Doyle LW: Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev. 2002, 4: CD001060
Han S, Crowther CA, Moore V: Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database Syst Rev. 2010, 7: CD000940
Doyle LW, Crowther CA, Middleton P, Marret S, Rouse DJ: Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009, 1: CD004661
Lu J, Nightingale C: Magnesium sulfate in eclampsia and pre-eclampsia: pharmacokinetic principles. Clin Pharmacokinet. 2000, 38 (4): 305-314. 10.2165/00003088-200038040-00002.
Duley L, Matar H, Almerie M, Hall D: Alternative magnesium sulphate regimens for women with pre-eclampsia and eclampsia. Cochrane Database Syst Rev. 2010, 8: CD007388
Golder S, Loke YK, Bland M: Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med. 2011, 8 (5): e1001026-10.1371/journal.pmed.1001026.
Papanikolaou PN, Christidi GD, Ioannidis JPA: Comparison of evidence on harms of medical interventions in randomized and nonrandomized studies. CMAJ. 2006, 174 (5): 635-641. 10.1503/cmaj.050873.
Loke Y, Price D, Herxheimer A, Cochrane Adverse Effects Methods Group: Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res Methodol. 2007, 7: 1-9. 10.1186/1471-2288-7-1.
Higgins J, Green S: Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. 2011, The Cochrane Collaboration, Available at: http://handbook.cochrane.org
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell M: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm,
Ioannidis JPA, Evans SJW, Gotzsche PC, O’Neill RT, Altman DG, Schulz K, Moher D, CONSORT Group: Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004, 141 (1): 781-788.
Chen FP, Chang SD, Chu KK: Expectant management in severe preeclampsia: does magnesium sulfate prevent the development of eclampsia?. Acta Obstet Gynecol Scand. 1995, 74: 181-185. 10.3109/00016349509008935.
Coetzee EJ, Dommisse J, Anthony J: A randomised controlled trial of intravenous magnesium sulphate versus placebo in the management of women with severe pre-eclampsia. Br J Obstet Gynaecol. 1998, 105 (3): 300-303. 10.1111/j.1471-0528.1998.tb10090.x.
Cox SM, Sherman ML, Leveno KJ: Randomized investigation of magnesium sulfate for prevention of preterm birth. Am J Obstet Gynecol. 1990, 163 (3): 767-772. 10.1016/0002-9378(90)91065-K.
Crowther CA, Hiller JE, Doyle LW, Haslam RR, Australasian Collaborative Trial of Magnesium Sulphate Collaborative Group: Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2006, 290 (2): 2669-2676.
The Magpie Trial Collaborative Group: Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: A randomised placebo-controlled trial. Lancet. 2002, 359 (9321): 1877-1890.
Livingston JC, Livingston LW, Ramsey R, Mabie BC, Sibai BM: Magnesium sulfate in women with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2003, 101 (2): 217-220. 10.1016/S0029-7844(02)03053-3.
Ma L: Magnesium sulfate in prevention of preterm labor. Zhong hua yi xue za zhi. 1992, 72 (3): 158-161.
Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Leveque C, Hellot M-F, Benichou J, PREMAG trial group: Magnesium sulfate given before very-preterm birth to protect infant brain: the randomized controlled PREMAG trial. Br J Obstet Gynaecol. 2007, 114 (3): 310-318.
Moodley J, Moodley VV: Prophylactic anticonvulsant therapy in hypertensive crises of pregnancy the need for a large, randomized trial. Hypertens Pregnancy. 1994, 13 (3): 245-252. 10.3109/10641959409072226.
Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, Iams JD, Wapner RJ, Sorokin Y, Alexander JM, Harper M, Thorp JM, Ramin SM, Malone FD, Carpenter M, Miodovnik M, Moawad A, O’Sullivan MJ, Peaceman AM, Hankins GDV, Langer O, Caritis SN, Roberts JM, For the Eunice Kennedy Shiver NICHD Maternal-Fetal Medicine Units Network: A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008, 359: 895-905. 10.1056/NEJMoa0801187.
Witlin AG, Friedman SA, Sibai BM: The effect of magnesium sulfate therapy on the duration of labor in women with mild preeclampsia at term: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 1997, 176 (3): 623-627. 10.1016/S0002-9378(97)70558-1.
Shilva Saha SC, Kalra J, Prasad R: Safety and efficacy of low-dose MgSO4 in the treatment of eclampsia. Int J Gynaecol Obstet. 2007, 97 (2): 150-151. 10.1016/j.ijgo.2007.01.008.
Malapaka SVN, Ballal PK: Low-dose magnesium sulfate versus Pritchard regimen for the treatment of eclampsia imminent eclampsia. Int J Gynaecol Obstet. 2011, 115 (1): 70-72. 10.1016/j.ijgo.2011.05.013.
Bhattacharjee N, Saha SP, Ganguly RP, Patra KK, Dhali B, Das N, Barui G: A randomised comparative study between low-dose intravenous magnesium sulphate and standard intramuscular regimen for treatment of eclampsia. J Obstet Gynaecol. 2011, 31 (4): 298-303. 10.3109/01443615.2010.549972.
Chissell S, Botha JH, Moodley J, McFadyen L: Intravenous and intramuscular magnesium sulphate regimens in severe pre-eclampsia. S Afr Med J. 1994, 84 (9): 607-610.
Mundle S, Regi A, Biswas B, Bracken H, Easterling T, Winikoff B: Preeclampsia in low-resource settings: a randomized trial of IV MgSO4 via flow controlled pump. Int J Gynaecol Obstet. 2009, 107 (2): S278-
Ehrenberg HM, Mercer BM: Abbreviated postpartum magnesium sulfate therapy for women with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006, 108 (4): 833-838. 10.1097/01.AOG.0000236493.35347.d8.
Suneja A, Sinha S, Vaid N, Ahuja S: A prospective randomized controlled trial to individualize the duration of post partum magnesium sulfate therapy. Hypertens Pregnancy. 2008, 27: 504-
Behrad V, Moossavifar N, Mojtahedzadeh M, Esmaili H, Moghtadeii P: A prospective, randomized, controlled trial of high and low doses of magnesium sulfate for acute tocolysis. Acta Med Iran. 2003, 41 (2): 126-131.
Terrone DA, Rinehart BK, Kimmel ES, May WL, Larmon JE, Morrison JC: A prospective, randomized, controlled trial of high and low maintenance doses of magnesium sulfate for acute tocolysis. Am J Obstet Gynecol. 2000, 182 (6): 1477-1482. 10.1067/mob.2000.107334.
Zygmunt M, Heilmann L, Berg C, Wallwiener D, Grischke E, Munstedt K, Spindler A, Lang U: Local and systemic tolerability of magnesium sulphate for tocolysis. Eur J Obstet Gynecol Reprod Biol. 2003, 107 (2): 168-175. 10.1016/S0301-2115(02)00368-8.
Ales KL, Charlson ME: Prognosis of hypertension first documented during labor. Am J Perinatol. 1987, 4 (4): 317-323. 10.1055/s-2007-999799.
Seyb ST, Berka RJ, Socol ML, Dooley SL: Risk of cesarean delivery with elective induction of labor at term in nulliparous women. Obstet Gynecol. 1999, 94 (4): 600-607. 10.1016/S0029-7844(99)00377-4.
Park KH, Cho YK, Lee CM, Choi H, Kim BR, Lee HK: Effect of preeclampsia, magnesium sulfate prophylaxis, and maternal weight on labor induction: a retrospective analysis. Gynecol Obstet Invest. 2006, 61 (1): 40-44. 10.1159/000088424.
Assaley J, Baron JM, Cibils LA: Effects of magnesium sulfate infusion upon clotting parameters in patients with pre-eclampsia. J Perinat Med. 1998, 26 (2): 115-119. 10.1515/jpme.1998.26.2.115.
Kynczl-Leisure M, Cibils LA: Increased bleeding time after magnesium sulfate infusion. Am J Obstet Gynecol. 1996, 175 (5): 1293-1294. 10.1016/S0002-9378(96)70043-1.
Ramanathan J, Sibai BM, Duggirala V, Maduska AL: Pulmonary function in preeclamptic women receiving MgSO4. J Reprod Med. 1988, 33 (5): 432-435.
Ramanathan J, Sibai BM, Pillai R, Angel JJ: Neuromuscular transmission studies in preeclamptic women receiving magnesium sulfate. Am J Obstet Gynecol. 1988, 158 (1): 40-46. 10.1016/0002-9378(88)90772-7.
Poggi SH, Barr S, Cannum R, Collea JV, Landy HJ, Kezsler M, Ghidini A: Risk factors for pulmonary edema in triplet pregnancies. J Perinatol. 2003, 23 (6): 462-465. 10.1038/sj.jp.7210968.
Magee LA, Miremadi S, Li J, Cheng C, Ensom MHH, Carleton B, Cote A-M, Von-Dadelszan P: Therapy with both magnesium sulfate and nifedipine does not increase the risk of serious magnesium-related maternal side effects in women with preeclampsia. Am J Obstet Gynecol. 2005, 193 (1): 153-163. 10.1016/j.ajog.2004.11.059.
Chowdhury JR, Chaudhuri S, Bhattacharyya N, Biswas PK, Panpalia M: Comparison of intramuscular magnesium sulfate with low dose intravenous magnesium sulfate regimen for treatment of eclampsia. J Obstet Gynaecol Res. 2009, 35 (1): 119-125. 10.1111/j.1447-0756.2008.00842.x.
Mahajan NN, Thomas A, Soni RN, Gaikwad NL, Jain SM: 'Padhar regime’ - a low-dose magnesium sulphate treatment for eclampsia. Gynecol Obstet Invest. 2009, 67 (1): 20-24. 10.1159/000158647.
Young BK, Weinstein HM: Effects of magnesium sulfate on toxemic patients in labor. Obstet Gynecol. 1977, 49 (6): 681-685.
Shoaib T, Khan S, Javed I, Bhutta SZ: Loading dose of magnesium sulphate versus standard regime for prophylaxis of pre-eclampsia. J Coll Physicians Surg Pak. 2009, 19 (1): 30-33.
Palmer L, Newby BD: Development of a simplified protocol for administration of 20% magnesium sulphate for prophylaxis and treatment of eclampsia. Can J Hosp Pharm. 2009, 62 (6): 490-495.
Nassar AH, Sakhel K, Maarouf H, Naassan GR, Usta IM: Adverse maternal and neonatal outcome of prolonged course of magnesium sulfate tocolysis. Acta Obstet Gynecol Scand. 2006, 85 (9): 1099-1103. 10.1080/00016340600756896.
Aali BS, Khazaeli P, Ghasemi F: Ionized and total magnesium concentration in patients with severe preeclampsia-eclampsia undergoing magnesium sulfate therapy. J Obstet Gynaecol Res. 2007, 33 (2): 138-143. 10.1111/j.1447-0756.2007.00508.x.
Adewole IF, Oladokun A, Okewole AI, Omigbodun AO, Afolabi A, Ekele B, Audu LR, Obed Y: Magnesium sulphate for treatment of eclampsia: the Nigerian experience. Afr J Med Med Sci. 2000, 29 (3–4): 239-241.
Ekele BA, Badung SLH: Is serum magnesium estimate necessary in patients with eclampsia on magnesium sulphate?. Afr J Reprod Health. 2005, 9 (1): 128-132. 10.2307/3583167.
Pritchard JA, Cunningham FG, Pritchard SA: The Parkland Memorial Hospital protocol for treatment of eclampsia: evaluation of 245 cases. Am J Obstet Gynecol. 1984, 148 (7): 951-963. 10.1016/0002-9378(84)90538-6.
Raman NV, Rao CA: Magnesium sulfate as an anticonvulsant in eclampsia. Int J Gynaecol Obstet. 1995, 49 (3): 289-298. 10.1016/0020-7292(95)02388-S.
Elliott JP: Magnesium sulfate as a tocolytic agent. Am J Obstet Gynecol. 1983, 147 (3): 277-284.
Girard B, Beucher G, Muris C, Simonet T, Dreyfus M: Magnesium sulphate and severe preeclampsia: its use in current practice. J Gynecol Obstet Biol Reprod. 2005, 34 (1 Pt 1): 17-22.
Harding K, Erskine K, Howell P: Audit of the impact of magnesium sulphate on the management of severe pre-eclampsia in a London university hospital. Eur J Anaesthesiol. 1997, 14 (5): 527-528. 10.1097/00003643-199709000-00019.
Thapa K, Jha R: Magnesium sulphate: a life saving drug. J Nepal Med Assoc. 2008, 47 (171): 104-108.
Dasari P, Habeebullah S: Maternal mortality due to hypertensive disorders of pregnancy in a tertiary care center in Southern India. Int J Gynaecol Obstet. 2010, 110 (3): 271-273. 10.1016/j.ijgo.2010.04.021.
Donovan EF, Tsang RC, Steichen JJ, Strub RJ, Chen IW, Chen M: Neonatal hypermagnesemia: effect on parathyroid hormone and calcium homeostasis. J Pediatr. 1980, 96 (2): 305-310. 10.1016/S0022-3476(80)80835-3.
Mojadidi Q, Thompson RJ: Five years' experience with eclampsia. South Med. 1973, 66 (4): 414-416. 10.1097/00007611-197304000-00004.
Tukur J, Muhammad Z: Management of eclampsia at AKTH: before and after magnesium sulphate. Niger J Med. 2010, 19 (1): 104-107.
Getaneh W, Kumbi S: Use of magnesium sulfate in pre-eclampsia and eclampsia in teaching hospitals in Addis Ababa: a practice audit. Ethiop Med J. 2010, 48 (2): 157-164.
Ahmed R: Magnesium sulphate as an anticonvulsant in the management of eclampsia. J Coll Physicians Surg Pak. 2004, 14 (10): 605-607.
Begum R, Begum A, Johanson R, Ali MN, Akhter S: A low dose ("Dhaka") magnesium sulphate regime for eclampsia. Acta Obstet Gynecol Scand. 2001, 80 (11): 998-1002.
Digre KB, Varner MW, Schiffman JS: Neuroophthalmologic effects of intravenous magnesium sulfate. Am J Obstet Gynecol. 1990, 163 (6 Pt 1): 1848-1852.
Hales KA, Matthews JP, Rayburn WF, Atkinson BD: Intravenous magnesium sulfate for premature labor: comparison between twin and singleton gestations. Am J Perinatol. 1995, 12 (1): 7-10. 10.1055/s-2007-994388.
Sass N, Mesquita M, Kenji G, Goncalves F, Arauj F: Do women with preeclampsia benefit from an exclusively intravenous magnesium sulphate regimen? Is 2.0 grams/hour effective? (abstract). Hypertens Pregnancy. 2007, 4: S591-
Omu AE, Al-Harmi J, Vedi HL, Mlechkova L, Sayed AF, Al-Ragum NS: Magnesium sulphate therapy in women with pre-eclampsia and eclampsia in Kuwait. Med Princ Pract. 2008, 17 (3): 227-232. 10.1159/000117797.
Cotton DB, Gonik B, Dorman KF: Cardiovascular alterations in severe pregnancy-induced hypertension: acute effects of intravenous magnesium sulfate. Am J Obstet Gynecol. 1984, 148 (2): 162-165. 10.1016/S0002-9378(84)80169-6.
Yeast JD, Halberstadt C, Meyer BA, Cohen GR, Thorp JA: The risk of pulmonary edema and colloid osmotic pressure changes during magnesium sulfate infusion. Am J Obstet Gynecol. 1993, 169 (6): 1566-1571. 10.1016/0002-9378(93)90438-O.
Jirapinyo M, Thuvasethakul P, Leelaphiwat S: Prospective study on premature labor with magnesium sulfate. Asia Oceania J Obstet Gynaecol. 1990, 16 (2): 91-96.
Kfuri TA, Morlock L, Hicks RW, Shore AD: Medication errors in obstetrics. Clin Perinatol. 2008, 35 (1): 101-117. 10.1016/j.clp.2007.11.015.
Little JA, Velazquez MB, Rayburn WF: Reported medication errors in obstetric inpatients in 1 hospital. J Reprod Med. 2003, 48 (10): 818-820.
Bilgin T, Cengiz C, Ozan H: Alterations in respiratory functions during magnesium sulfate infusion in severe preeclampsia and eclampsia. Int J Gynaecol Obstet. 1994, 45 (1): 59-60. 10.1016/0020-7292(94)90768-4.
Herpolsheimer A, Brady K, Yancey MK, Pandian M, Duff P: Pulmonary function of preeclamptic women receiving intravenous magnesium sulfate seizure prophylaxis. Obstet Gynecol. 1991, 78 (2): 241-244.
Ghia N, Spong CY, Starbuck VN, Scialli AR, Ghidini A: Magnesium sulfate therapy affects attention and working memory in patients undergoing preterm labor. Am J Obstet Gynecol. 2000, 183 (4): 940-944. 10.1067/mob.2000.109045.
Fuentes A, Rojas A, Porter KB, Saviello G, O'Brien WF: The effect of magnesium sulfate on bleeding time in pregnancy. Am J Obstet Gynecol. 1995, 173 (4): 1246-1249. 10.1016/0002-9378(95)91363-7.
Guzin K, Goynumer G, Gokdagli F, Turkgeldi E, Gunduz G, Kayabasoglu F: The effect of magnesium sulfate treatment on blood biochemistry and bleeding time in patients with severe preeclampsia. J Matern Fetal Neonatal Med. 2010, 23 (5): 399-402. 10.3109/14767050903156684.
Yazdani M, Jahvani FA, Tabei SZ: Effects of magnesium sulfate on bleeding time in premature labor. Iran J Med Sci. 2004, 29 (4): 172-174.
Moghadas FRS, Motevalian M, Rezai Z, Ehteshamipour E: The effect of magnesium sulfate therapy on bleeding time in women with threatened preterm labor. Iran J Pharmacol Ther. 2007, 6 (1): 55-57.
Anon: Magnesium overdose kills pregnant R.Ph.; her twins saved. Drug Top. 1990, 134: 11-12.
Cohen MR, Davis NM: Free flow associated with electronic infusion devices: underestimated danger. Hosp Pharm. 1992, 27 (5): 384-390.
Richards A, Stather-Dunn L, Moodley J: Cardiopulmonary arrest after the administration of magnesium sulphate. A case report. S Afr Med J. 1985, 67 (4): 145-
Simpson KR, Knox GE: Obstetrical accidents involving intravenous magnesium sulfate: recommendations to promote patient safety. MCN Am J Matern Child Nurs. 2004, 29 (3): 161-169. 10.1097/00005721-200405000-00006.
McCubbin JM, Sibai BM, Ardella TN, Anderson GD: Cardiopulmonary arrest due to acute maternal hypermagnesaemia. Lancet. 1981, 1 (8228): 1058-
McDonnell NJ: Cardiopulmonary arrest in pregnancy: two case reports of successful outcomes in association with perimortem Caesarean delivery. Br J Anaesth. 2009, 103 (3): 406-409. 10.1093/bja/aep176.
Morisaki H, Yamamoto S, Morita Y, Kotake Y, Ochiai R, Takeda J: Hypermagnesemia-induced cardiopulmonary arrest before induction of anesthesia for emergency cesarean section. J Clin Anesth. 2000, 12 (3): 224-226. 10.1016/S0952-8180(00)00142-2.
Rabinerson D, Gruber A, Kaplan B, Royburt M, Ovadia J: Accidental cardiopulmonary arrest following magnesium sulphate overdose. Eur J Obstet Gynecol Reprod Biol. 1994, 55 (2): 149-150. 10.1016/0028-2243(94)90071-X.
Swartjes JM, Schutte MF, Bleker OP: Management of eclampsia: cardiopulmonary arrest resulting from magnesium sulfate overdose. Eur J Obstet Gynecol Reprod Biol. 1992, 47 (1): 73-75. 10.1016/0028-2243(92)90217-M.
Bohman VR, Cotton DB: Supralethal magnesemia with patient survival. Obstet Gynecol. 1990, 76 (5 Pt 2): 984-986.
Cao Z, Bideau R, Valdes R, Elin RJ: Acute hypermagnesemia and respiratory arrest following infusion of MgSO4 for tocolysis. Clin Chim Acta. 1999, 285 (1–2): 191-193.
McKenna D: That's our drip counter!. Lancet. 1873, 2006: 367(9525)-
Wax JR, Segna RA, Vandersloot JA: Magnesium toxicity and resuscitation–an unusual cause of postcesarean evisceration. Int J Gynaecol Obstet. 1995, 48 (2): 213-214. 10.1016/0020-7292(94)02251-S.
Bruhwiler H, Hafliger M, Luscher KP: Severe accidental magnesium poisoning in a twins pregnancy in the 32nd week of pregnancy. Geburtshilfe Frauenheilkd. 1994, 54 (3): 184-186. 10.1055/s-2007-1023579.
Hayashi K, Oshiro M, Takara I, Iha H, Sugahara K: Coma caused by hypermagnesemia in a pregnant woman complicated with HELLP syndrome. Masui. 2003, 52 (7): 783-785.
McDonnell NJ, Muchatuta NA, Paech MJ: Acute magnesium toxicity in an obstetric patient undergoing general anaesthesia for caesarean delivery. Int J Obstet Anesth. 2010, 19 (2): 226-231. 10.1016/j.ijoa.2009.09.009.
Buettner AU: Two cases of inadvertent magnesium sulphate overdose. Int J Obstet Anesth. 2011, 20 (1): 92-93. 10.1016/j.ijoa.2010.08.003.
Dror A, Henriksen E: Accidental epidural magnesium sulfate injection. Anesth Analg. 1987, 66 (10): 1020-1021.
Goodman EJ, Haas AJ, Kantor GS: Inadvertent administration of magnesium sulfate through the epidural catheter: report and analysis of a drug error. Int J Obstet Anesth. 2006, 15 (1): 63-67. 10.1016/j.ijoa.2005.06.009.
Lejuste MJ: Inadvertant intrathecal administration of magnesium sulfate. S Afr Med J. 1985, 68 (6): 367-368.
Lewis-Younger C, Speranza V, Gaar G: Temporary paralysis resulting from medical error. J Toxicol Clin Toxicol. 2004, 42 (5): 732-
Bashuk RG, Krendel DA: Myasthenia gravis presenting as weakness after magnesium administration. Muscle Nerve. 1990, 13 (8): 708-712. 10.1002/mus.880130808.
Bruner JP, Yeast JD: Pregnancy associated with Friedreich ataxia. Obstet Gynecol. 1990, 76 (5 Pt 2): 976-977.
Catanzarite V, Gambling D, Bird LM, Honold J, Perkins E: Respiratory compromise after MgSO4 therapy for preterm labor in a woman with myotonic dystrophy: a case report. J Reprod Med. 2008, 53 (3): 220-222.
Hosono T, Suzuki M, Chiba Y: Contraindication of magnesium sulfate in a pregnancy complicated with late-onset diabetes mellitus and sensory deafness due to mitochondrial myopathy. J Matern Fetal Med. 2001, 10: 355-356. 10.1080/jmf.10.5.355.356-20.
Cohen BA, London RS, Goldstein PJ: Myasthenia gravis and preeclampsia. Obstet Gynecol. 1976, 48 (1 Suppl): 35S-37S.
Mueksch JN, Stevens WA: Undiagnosed myasthenia gravis masquerading as eclampsia. Int J Obstet Anesth. 2007, 16 (4): 379-382. 10.1016/j.ijoa.2007.03.012.
Robins K, Lyons G: Opioid-related narcosis in a woman with myopathy receiving magnesium. Int J Obstet Anesth. 2007, 16 (4): 367-369. 10.1016/j.ijoa.2007.01.013.
Moriarty KT, McFarland R, Whittaker R, Burch J, Turnbull HE, Taylor RW, Turnbull DM: Pre-eclampsia and magnesium toxicity with therapeutic plasma level in a woman with m.3243A > G melas mutation. J Obstet Gynaecol. 2008, 28 (3): 349-10.1080/01443610802048545.
Archer TL, Heitmeyer JD: Perioperative hemodynamics obtained by pulse contour analysis facilitated the management of a patient with chronic hypertension, renal insufficiency, and superimposed preeclampsia during cesarean delivery. J Clin Anesth. 2010, 22 (4): 274-279. 10.1016/j.jclinane.2009.03.019.
Chan SM, Lu CC, Ho ST, Liaw WJ, Cherng CH, Chen WH, Lin TC: Eclampsia following cesarean section with HELLP syndrome and multiple organ failure. Acta Anaesthesiol Taiwan. 2008, 46 (1): 46-48. 10.1016/S1875-4597(08)60021-1.
Nethravathi M, Panicker JN, Taly AB, Arunodaya GR, Sinha S: Therapeutic magnesium for eclampsia: an unusual cause for antepartum flaccid paralysis. J Postgrad Med. 2007, 53 (1): 79-80. 10.4103/0022-3859.30341.
Saitoh K, Motegi R, Hirabayashi Y, Shimizu R: Cardiac arrests probably induced by hypermagnesemia during anesthesia for caesarean section. Masui. 1994, 43 (3): 388-391.
Baraka A, Dajani A, Jabagi S: Magnesium-induced respiratory arrest in a parturient recovering from general anesthesia-a case report. Middle East J Anesthesiol. 1984, 7 (6): 437-440.
Nguyen TTT, Koenders MEF, Wierda JMKH: Drug interaction in a pregnancy complicated by pre-eclampsia and cesarean section. Ned Tijdschr Anesthesiol. 2001, 14 (1): 21-25.
Fay TN, Bogod D: Magnesium sulphate: the time for reckoning. Br J Obstet Gynaecol. 1996, 103 (8): 852-
Funai Y, Ikeda Y, Takahashi R, Asada A: Prolonged apnea from rocuronium in a patient with hypermagnesemia after cesarean section: a case report. Masui. 2010, 59 (6): 721-723.
Hino H, Kaneko I, Miyazawa A, Aoki T, Ishizuka B, Kosuqi K, Amemiya A: Prolonged neuromuscular blockade with vecuronium in patient with triple pregnancy treated with magnesium sulfate. Masui. 1997, 46 (2): 266-270.
Kwan WF, Lee C, Chen BJ: A noninvasive method in the differential diagnosis of vecuronium-induced and magnesium-induced protracted neuromuscular block in a severely preeclamptic patient. J Clin Anesth. 1996, 8 (5): 392-397. 10.1016/0952-8180(96)00087-6.
Sinatra RS, Philip BK, Naulty JS, Ostheimer GW: Prolonged neuromuscular blockade with vecuronium in a patient treated with magnesium sulfate. Anesth Analg. 1985, 64 (12): 1220-1222.
Yoshida A, Itoh Y, Nagaya K, Takino K, Sugawara J-I, Murakami T, Okamura K, Takahashi M: Prolonged relaxant effects of vecuronium in patients with deliberate hypermagnesemia: time for caution in cesarean section. J Anesth. 2006, 20 (1): 33-35. 10.1007/s00540-005-0354-9.
Sloan PA, Rasul M: Prolongation of rapacuronium neuromuscular blockade by clindamycin and magnesium. Anesth Analg. 2002, 94 (1): 123-124.
Ben-Ami M, Giladi Y, Shalev E: The combination of magnesium sulphate and nifedipine: a cause of neuromuscular blockade. Br J Obstet Gynaecol. 1994, 101 (3): 262-263. 10.1111/j.1471-0528.1994.tb13126.x.
Snyder SW, Cardwell MS: Neuromuscular blockade with magnesium sulfate and nifedipine. Am J Obstet Gynecol. 1989, 161 (1): 35-36. 10.1016/0002-9378(89)90226-3.
Wu Q-A, Ye Y-Q: Neuromuscular blockade after therapy with magnesium sulfate and amlodipine. Eur J Obstet Gynecol Reprod Biol. 2010, 149 (2): 225-
Pittman JA: Magnesium sulphate for pre-eclampsia and a sudden bradycardia. Br J Anaesth. 2000, 85 (2): 327-328.
Scardo JA, Brost BC, Sola E, Newman RB, Tate SB: Severe hypotension with nifedipine-magnesium in severe preeclampsia: A hemodynamic observation. J Matern Fetal Investig. 1997, 7 (3): 152-154.
Waisman GD, Mayorga LM, Camera MI, Vignolo CA, Martinotti A: Magnesium plus nifedipine: potentiation of hypotensive effect in preeclampsia?. Am J Obstet Gynecol. 1988, 159 (2): 308-309. 10.1016/S0002-9378(88)80072-3.
Awwad JT, Khalil AM, Aswad NK, Suidan FJ, Karam KS: Labial edema in pregnancy. A case report. J Reprod Med. 1994, 39 (11): 921-922.
Basaran A, Bozdag G, Aksu AT, Deren O: Twin pregnancy complicated with acute appendicitis and cholecystitis in the same gestational period. Arch Gynecol Obstet. 2007, 276 (3): 291-293. 10.1007/s00404-007-0331-7.
Haldeman W: Can magnesium sulfate therapy impact lactogenesis?. J Hum Lact. 1993, 9 (4): 249-252. 10.1177/089033449300900426.
Hill WC, Gill PJ, Katz M: Maternal paralytic ileus as a complication of magnesium sulfate tocolysis. Am J Perinatol. 1985, 2 (1): 47-48. 10.1055/s-2007-999911.
Hung J-W, Tsai M-Y, Yang B-Y, Chen J-F: Maternal osteoporosis after prolonged magnesium sulfate tocolysis therapy: a case report. Arch Phys Med Rehabil. 2005, 86 (1): 146-149. 10.1016/j.apmr.2004.02.016.
Lurie S, Rotmensch S, Feldman N, Glezerman M: Breast engorgement and galactorrhea during magnesium sulfate treatment of preterm labor. Am J Perinatol. 2002, 19 (5): 239-240. 10.1055/s-2002-33086.
Riggs JE, Hogg JP: Central pontine myelinolysis: association with parenteral magnesium administration. Mil Med. 2000, 165 (6): 494-495.
Sameshima H, Higo T, Kodama Y, Ikenoue T: Magnesium tocolysis as the cause of urinary calculus during pregnancy. J Matern Fetal Med. 1997, 6 (5): 296-297.
Spital A, Greenwell R: Severe hyperkalemia during magnesium sulfate therapy in two pregnant drug abusers. South Med J. 1991, 84 (7): 919-921. 10.1097/00007611-199107000-00026.
Roberts D, Haslett E, Hickey-Dwyer M, McCormack J: Eclampsia complicated by bilateral retinal detachments and abnormal eye movements. Lancet. 1998, 351 (9105): 803-804. 10.1016/S0140-6736(05)78930-3.
Thorp JM, Katz VL, Campbell D, Cefalo RC: Hypersensitivity to magnesium sulfate. Am J Obstet Gynecol. 1989, 161 (4): 889-890. 10.1016/0002-9378(89)90744-8.
Bourgeois FJ, Thiagarajah S, Harbert GM, DiFazio C: Profound hypotension complicating magnesium therapy. Am J Obstet Gynecol. 1986, 154 (4): 919-920. 10.1016/0002-9378(86)90485-0.
Rodis JF, Vintzileos AM, Campbell WA, Deaton JL, Nochimson DJ: Maternal hypothermia: an unusual complication of magnesium sulfate therapy. Am J Obstet Gynecol. 1987, 156 (2): 435-436. 10.1016/0002-9378(87)90300-0.
Cardosi RJ, Chez RA: Magnesium sulfate, maternal hypothermia, and fetal bradycardia with loss of heart rate variability. Obstet Gynecol. 1998, 92 (4 Pt 2): 691-693.
Hennessy A, Hill I: A case of maternal bradycardia at therapeutic doses of magnesium sulphate in preeclampsia. Aust N Z J Obstet Gynaecol. 1999, 39 (2): 256-257. 10.1111/j.1479-828X.1999.tb03387.x.
Oettinger M, Perlitz Y: Asymptomatic paroxysmal atrial fibrillation during intravenous magnesium sulfate treatment in preeclampsia. Gynecol Obstet Invest. 1993, 36 (4): 244-246. 10.1159/000292638.
Pritchard JA: The use of magnesium sulfate in preeclampsia-eclampsia. J Reprod Med. 1979, 23 (3): 107-114.
Herschel M, Mittendorf R: Tocolytic magnesium sulfate toxicity and unexpected neonatal death. J Perinatol. 2001, 21 (4): 261-262. 10.1038/sj.jp.7200498.
Tang F, Xiao B, Xiong Q, Yang M: A case report: magnesium intoxication occurring in the process of total serum magnesium decrease. J Obstet Gynaecol Res. 2010, 36 (1): 174-177. 10.1111/j.1447-0756.2009.01084.x.
Sherer D, Cialone P, Abramowicz J, Woods J: Transient symptomatic subendocardial ischemia suring magnesium sulfate tocolytic therapy. Am J Obstet Gynecol. 1992, 166 (1 Pt 1): 33-35.
Koontz SL, Friedman SA, Schwartz ML: Symptomatic hypocalcemia after tocolytic therapy with magnesium sulfate and nifedipine. Am J Obstet Gynecol. 2004, 190 (6): 1773-1776. 10.1016/j.ajog.2004.02.050.
Mayan H, Hourvitz A, Schiff E, Farfel Z: Symptomatic hypocalcaemia in hypermagnesaemia-induced hypoparathyroidism, during magnesium tocolytic therapy - Possible involvement of the calcium-sensing receptor. Nephrol Dial Transplant. 1999, 14 (7): 1764-1766. 10.1093/ndt/14.7.1764.
Monif GR, Savory J: Iatrogenic maternal hypocalcemia following magnesium sulfate therapy. JAMA. 1972, 219 (11): 1469-1470. 10.1001/jama.1972.03190370059013.
Nassar AH, Salti I, Makarem NN, Usta IM: Marked hypocalcemia after tocolytic magnesium sulphate therapy. Am J Perinatol. 2007, 24 (8): 481-482. 10.1055/s-2007-986696.
Ganzevoort JW, Hoogerwaard EM, van der Post JAM: Hypocalcemic delirium due to magnesium sulphate therapy in a pregnant woman with pre-eclampsia]. Ned Tijdschr Geneeskd. 2002, 146 (31): 1453-1456.
Elliott JP, O'Keeffe DF, Greenberg P, Freeman RK: Pulmonary edema associated with magnesium sulfate and betamethasone administration. Am J Obstet Gynecol. 1979, 134 (6): 717-719.
Worrell JA, Brunner JP, O'Donnell DM, Carroll FE: Pulmonary edema associated with tocolytic therapy. AJR Am J Roentgenol. 1992, 158 (6): 1356-1357. 10.2214/ajr.158.6.1590140.
Institute for Safe Medical Practices: ISMP Medication Safety Alert. Enhance systems to ensure safe IV magnesium use. http://www.ismp.org/Newsletters/acutecare/articles/19970212.asp,
Office of Safety & Quality in Healthcare Government of Western Australia: Sharing Lessons Learned -. 2010, http://www.safetyandquality.health.wa.gov.au/home/patient_news.cfm, Feb . Preventing Magnesium Sulphate Overdose in Obstetric Settings,
Duley L: The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009, 33 (3): 130-137. 10.1053/j.semperi.2009.02.010.
Tucker J, McGuire W: Epidemiology of preterm birth. BMJ. 2004, 329: 675-678. 10.1136/bmj.329.7467.675.
Loke YK, Golder SP, Vandenbroucke JP: Comprehensive evaluations of the adverse effects of drugs: importance of appropriate study selection and data sources. Ther Adv Drug Saf. 2011, 2 (2): 59-68. 10.1177/2042098611401129.
Kremer L, Phillips R, Van-Dalen E: Tips and tricks for understanding and using SR results - no 13: adverse effects in systematic reviews. Evidence-Based Child Health. 2009, 4: 384-385. 10.1002/ebch.305.
Grimes D, Nanda K: Magnesium sulfate tocolysis: time to quit. Obstet Gynecol. 2006, 108 (4): 986-989. 10.1097/01.AOG.0000236445.18265.93.
U.S. Food and Drug Administration: FDA recommends against prolonged use of magnesium sulfate to stop pre-term labor due to bone changes in exposed babies. http://www.fda.gov/Drugs/DrugSafety/ucm353333.htm,
Ioannidis JPA: Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Arch Intern Med. 2009, 169 (19): 1737-1739. 10.1001/archinternmed.2009.313.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2393/13/195/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
CAC was the principal investigator for the Australasian Collaborative Trial of Magnesium Sulphate. Two review authors (CAC and PFM) were co-authors of the Cochrane review 'Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus’. The three review authors (CAC, ESB and PFM) were authors of the Cochrane review 'Different magnesium sulphate regimens for neuroprotection of the fetus’. CAC was an author of the Cochrane reviews 'Magnesium sulphate for preventing preterm birth in threatened preterm labour’ and 'Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour’.
Authors’ contributions
ESB, CAC and PFM designed the study. ESB and PFM undertook searches, extracted and analysed data. ESB wrote the first draft of the paper. All authors supported the interpretation of results, provided comments on subsequent drafts and approved the final version.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bain, E.S., Middleton, P.F. & Crowther, C.A. Maternal adverse effects of different antenatal magnesium sulphate regimens for improving maternal and infant outcomes: a systematic review. BMC Pregnancy Childbirth 13, 195 (2013). https://doi.org/10.1186/1471-2393-13-195
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2393-13-195