Abstract
Background
Facioscapulohumeral muscular dystrophy (FSHD) is an autosomal dominant disorder characterized by the weakness of facial, shoulder-girdle and upper arm muscles. Most patients with FSHD have fewer numbers of tandem repeated 3.3-kb KpnI units on chromosome 4q35. Chromosome 10q26 contains highly homologous KpnI repeats, and inter-chromosomal translocation has been reported.
Methods
To clarify the influence on the deletion of the repeats, we surveyed three different ethnic populations and FSHD patients using the BglII/BlnI dosage test.
Results
The frequency of translocation in 153 Japanese, 124 Korean, 114 Chinese healthy individuals and 56 Japanese 4q35-FSHD patients were 27.5%, 29.8%, 19.3%, and 32.1%, respectively. The ratio of '4 on 10' (trisomy and quatrosomy of chromosome 4) was higher than that of '10 on 4' (nullsomy and monosomy of chromosome 4) in all populations.
Conclusions
The inter-chromosomal exchange was frequently observed in all four populations we examined, and no significant difference was observed between healthy and diseased groups.
Similar content being viewed by others
Background
Facioscapulohumeral muscular dystrophy (FSHD) is a common form of muscular disorder with an autosomal dominant trait. FSHD is characterized by weakness and atrophy of facial, shoulder-girdle and humeral muscles, with occasional subsequent pelvic-girdle and lower limb involvement. More than 95% of patients with FSHD have a smaller (< 35 kb) EcoRI fragment on chromosome 4q35 detected by probe p13E-11 and are called 4q35-FSHD [1–3]. This EcoRI fragment in normal individuals contains tandem repeated 3.3-kb KpnI units (D4Z4) varying from 11 to 150 in number, while 4q35-FSHD patients showed less than ten units [2, 3]. No responsible gene has been isolated within the FSHD gene region.
Probe p13E-11 cross-hybridizes with chromosome 10q26, which contains highly homologous 3.3-kb KpnI repeated units. Since the BlnI restriction enzyme site exists exclusively within each unit derived from 10q26, but not in D4Z4 (an unit from 4q35), double enzyme digestion using EcoRI and BlnI can discriminate as 4q35 (BlnI-resistant) and 10q26 (BlnI-sensitive) units [4]. In a Dutch control population, subtelomeric translocations between chromosomes 4 and 10 were seen in about 20% of individuals [5–7]. Furthermore, somatic mosaicism was found in 40% of de novo FSHD families and 46% of these individuals had BlnI-resistant units on chromosome 10 [8]. These findings imply that frequent recombination between the subtelomeric region of chromosomes 4 and 10 has some roles for deletion of the FSHD region. In this study, we examined the frequency of subtelomeric translocation among three different ethnic populations of Japanese, Korean and Chinese, and compared with Japanese 4q35-FSHD patients.
Methods
Blood samples were obtained with informed consent. Genomic DNA was extracted from peripheral blood lymphocytes using a standard technique.
The BglII/BlnI dosage test was accomplished using the probe p13E-11 as previously reported [9]. We examined 153 Japanese, 124 Korean and 114 Chinese healthy individuals, and unrelated 56 Japanese 4q35-FSHD patients. All individuals were classified into five groups according to the number of chromosomes with BlnI-resistant 4q-type KpnI units; nullsomy (N: 0), monosomy (M: 1), disomy (D: 2), trisomy (T: 3) and quatrosomy (Q: 4) (Figure 1A). Less than 5% of individuals showed unexpected ratios of chromosome 4q;10q, and excluded from this study. The frequency of translocation in each group was estimated and statistical analysis was performed using the chi-square test. We also tested 30 Japanese healthy individuals using pulsed field gel electrophoresis (PFGE) to certify the results obtained by the dosage test. For PFGE, we used agarose embedded "plug" DNA samples [10].
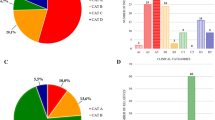
PFGE and the Bgl II/ Bln I dosage test A) Patterns of translocation between chromosome 4q35 and 10q26 KpnI units. Subtelomeric translocation changes the number of BlnI-resistant (from chromosome 4q35) and BlnI-sensitive (from chromosome 10q26) fragments. According to the number of units from chromosome 4, each individual is classified as nullsomy, monosomy, disomy, trisomy or quatrosomy. B) BglII/BlnI dosage test. Double enzyme digestion with BglII and BlnI characterizes the first KpnI unit as a 4.0-kb fragment from chromosome 4q35 or a 1.8-kb fragment from chromosome 10q26. The ratio estimated from the intensity of the two fragments are; nullsomy (N) = 0 (0/4), monosomy (M) = 0.3 (1/3), disomy (D) = 1 (2/2), trisomy (T) = 3 (3/1), and quatrosomy (Q) = infinity (4/0). C) Comparison of the dosage test with PFGE. Thirty Japanese individuals were examined using both PFGE and the dosage test. The ratio from the dosage test was consistent with the results of PFGE in all samples. D: disomy, T: trisomy E/H: EcoRI/HindIII, E/B: EcoRI/BlnI, M: Marker
Results
Results of PFGE and the dosage test
The results of PFGE were completely identical to those of the BglII/BlnI dosage test in 30 Japanese individuals examined (Figure 1C), and we used the dosage test for further analysis.
The frequency of 4q;10q translocations
The frequency of individuals having translocated KpnI units in Japanese was 27.5% (42/153), Korean 29.8% (37/124), Chinese 19.3% (22/114), and 4q35-FSHD patients 32.1% (18/56) (Table). There was no significant difference between each population and the Dutch population reported previously [7]. No gender difference was observed in any population (data not shown).
The ratio of chromosome 10 with 4-type repeats (4 on 10) in Japanese was 19.6%, Korean 25.8%, Chinese 14.0% and 4q35-FSHD 23.2%. On the other hand, the ratio of chromosome 4 with 10-type units (10 on 4) in Japanese was 7.9%, Korean 4.0%, Chinese 5.3%, and 4q35-FSHD 8.9% (Figure 2). The frequency of '4 on 10' was higher than that of '10 on 4' in all populations examined, although the ratio of '4 on 10' and '10 on 4' were similar in the Dutch population [5, 7]. We could not find any differences between healthy populations and Japanese 4q35-FSHD patients.
Frequency of the translocation between chromosomes 4q35 and 10q26 The 4 on 10 (trisomy and quatrosomy) is more frequently observed than 10 on 4 (nullsomy and monosomy) in all populations examined, although these were similar in the Dutch population. The findings from the Dutch population were estimated from the results of PFGE [7].
Discussion
The molecular size of EcoRI fragments on chromosomes 4 and 10 detected by probe p13E-11 varies markedly from 10 to 300 kb. Since fragments longer than 50 kb are difficult to detect by conventional Southern blot analysis, PFGE analysis using agarose embedded plug DNA is often necessary to identify all four fragments from chromosome 4 and 10. However, we cannot always obtain such high quality DNA samples. The BglII/BlnI dosage test used in this study is a useful and easy method to reveal translocation between chromosome 4 and 10, which characterizes the first KpnI repeat unit as a BlnI-resistant 4.0-kb (chromosome 4-type) or a BlnI-sensitive 1.8-kb (chromosome 10-type) fragment. It should be noted, however, the dosage test cannot detect all inter-chromosomal exchanges, i.e., if one had exchanged KpnI repeats at the distal portion following the standard repeats, this exchange will be missed and judged as standard. van Overveld et al. reported that approximately 4.3% (9 among 208) of individuals with standard first repeat showed a hybrid consisting of both BlnI-resistant and -sensitive repeats [7]. Therefore, the dosage test may underestimate the exchange ratio, although the present results of the PFGE and dosage tests were identical in 30 Japanese individuals. In the present study, less than 5% of individuals showed unclassified ratios of chromosome 4q;10q. These individuals may have complicated chromosomal rearrangements, or a deletion of the probe p13E-11 region as previously described [9]. The limitation of the densitometric analysis should be also considered. We are currently examining in detail on these individuals.
The subtelomeric exchange between chromosomes 4 and 10 was frequently observed in all four populations we examined, and their ratios were similar to the Dutch population previously reported [5, 7]. The inter-chromosomal exchange may contribute to the deletion ofKpnI repeats on chromosome 4q35, although there was no difference between healthy and diseased individuals. The ratio of '4 on 10' (trisomy and quatrosomy of chromosome 4) was higher than that of '10 on 4' (nullsomy and monosomy) in all populations we examined. Translocations of chromosome ends have been reported to cause several disorders, such as alpha-thalassemia mental retardation syndrome, Wolf-Hirschhorn syndrome and Miller-Dieker syndrome. Further studies will be needed to clarify the influence of the subtelomeric exchange to the deletion of the repeated units on FSHD.
Conclusions
The frequency of translocation was 27.5% (Japanese), 29.8% (Korean), 19.3% (Chinese), and 32.1% (Japanese 4q35-FSHD patients). The ratio of '4 on 10' (trisomy and quatrosomy of chromosome 4) was higher than that of '10 on 4' (nullsomy and monosomy of chromosome 4) in all populations. In our study, there was no difference between healthy and diseased groups. Further studies will be needed to clarify the influence of the subtelomeric exchange to the deletion of the repeated units on FSHD.
References
Wijmenga C, Hewitt JE, Sandkuijl LA, Clark LN, Wright TJ, Dauwerse HG, Gruter AM, Hofker MH, Moerer P, Williamson R, et al: Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nature Genet. 1992, 2: 26-30.
van Deutekom JCT, Wijmenga C, van Tienhoven EA, Gruter AM, Hewitt JE, Padberg GW, van Ommen GJB, Hofker MH, Frants RR: FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum Mol Genet. 1993, 2: 2037-2042.
Goto K, Lee JH, Matsuda C, Hirabayashi K, Kojo T, Nakamura A, Mitsunaga Y, Furukawa T, Sahashi K, Arahata K: DNA rearrangements in Japanese facioscapulohumeral muscular dystrophy patients: clinical correlations. Neuromusc Disord. 1995, 5: 201-208. 10.1016/0960-8966(94)00055-E.
Deidda G, Caccuri S, Piazzo N, Felicetti L: Direct detection of 4q35 rearrangements implicated in facioscapulohumeral muscular dystrophy (FSHD). J Med Genet. 1996, 33: 361-365.
van Deutekom JCT, Bakker E, Lemmers RJLF, van der Wielen MJR, Bik E, Hofker MH, Padberg GW, Frants RR: Evidence for subtelomeric exchange of 3.3 kb tandemly repeated units between chromosomes 4q35 and 10q26: implications for genetic counseling and etiology of FSHD1. Hum Mol Genet. 1996, 5: 1997-2003. 10.1093/hmg/5.12.1997.
Lemmers RJLF, van der Maarel SM, van Deutekom JCT, van der Wielen MJR, Deidda G, Dauwerse HG, Hewitt J, Hofker M, Bakker E, Padberg GW, Frants RR: Inter- and intrachromosomal sub-telomeric rearrangements on 4q35: implications for facioscapulohumeral muscular dystrophy (FSHD) aetiology and diagnosis. Hum Mol Genet. 1998, 7: 1207-1214. 10.1093/hmg/7.8.1207.
van Overveld PGM, Lemmers RJLF, Deidda G, Sandkuijl L, Padberg GW, Frants RR, van der Maarel SM: Interchromosomal repeat array interactions between chromosomes 4 and 10: a model for subtelomeric plasticity. Hum Mol Genet. 2000, 9: 2879-2884. 10.1093/hmg/9.19.2879.
van der Maarel SM, Deidda G, Lemmers RJLF, van Overveld PGM, van der Wielen M, Hewitt JE, Sandkuijl L, Bakker B, van Ommen GJB, Padberg GW, Frants RR: De novo facioscapulohumeral muscular dystrophy: frequent somatic mosaicism, sex-dependent phenotype, and the role of mitotic transchromosomal repeat interaction between chromosomes 4 and 10. Am J Hum Genet. 2000, 66: 26-35. 10.1086/302730.
van der Maarel SM, Deidda G, Lemmers RJLF, Bakker E, van der Wielen MJR, Sandkuijl L, Hewitt JE, Padberg GW, Frants RR: A new dosage test for subtelomeric 4; 10 translocations improves conventional diagnosis of facioscapulohumeral muscular dystrophy (FSHD). J Med Genet. 1999, 36: 823-828.
Kenwrick S, Patterson M, Speer A, Fischbeck K, Davies K: Molecular analysis of the Duchenne muscular dystrophy region using pulsed field electrophoresis. Cell. 1987, 48: 351-357.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2377/2/7/prepub
Acknowledgements
This work was supported in part by the Research Grants-in-Aid for Nervous and Mental Disorders from the Ministry of Health, Labor and Welfare, Japan, and CREST from the Science and Technology Agency, Japan. We thank Prof. Rune R. Frants and his colleagues for helpful assistance and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors' contributions
TM participated in the design of the study and performed the statistical analysis. KG and GY carried out the molecular genetic studies including the dosage test and PFGE. JHL and CZ obtained control DNA samples of Korean and Chinese. YKH and KA conceived of the study, and participated in its design and coordination.
All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Matsumura, T., Goto, K., Yamanaka, G. et al. Chromosome 4q;10q translocations; Comparison with different ethnic populations and FSHD patients. BMC Neurol 2, 7 (2002). https://doi.org/10.1186/1471-2377-2-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2377-2-7