Abstract
Background
Mild cognitive impairment (MCI) is a heterogeneous clinical entity that comprises the prodromal phase of Alzheimer's disease (Pr-AD). New biomarkers are useful in detecting Pr-AD, but they are not universally available. We aimed to investigate baseline clinical and neuropsychological variables that might predict progression from MCI to AD dementia.
Methods
All patients underwent a complete clinical and neuropsychological evaluation at baseline and every 6 months during a two-year follow-up period, with 54 out of 109 MCI patients progressing to dementia (50 of them progressed to AD dementia), and 55 remaining as stable MCI (S-MCI).
Results
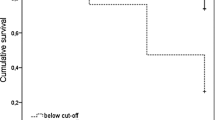
A combination of MMSE and California Verbal Learning Test Long Delayed Total Recall (CVLT-LDTR) constituted the best predictive model: subjects scoring above 26/30 on MMSE and 4/16 on CVLT-LDTR had a negative predictive value of 93.93% at 2 years, whereas those subjects scoring below both of these cut-off scores had a positive predictive value of 80.95%.
Conclusions
Pr-AD might be distinguished from S-MCI at baseline using the combination of MMSE and CVLT-LDTR. These two neuropsychological predictors are relatively brief and may be readily completed in non-specialist clinical settings.
Similar content being viewed by others
Background
Different biomarkers have been thoroughly studied in order to establish which ones best predict the progression from mild cognitive impairment (MCI) to Alzheimer's disease (AD) dementia. With current evidence, medial temporal lobe and hippocampal atrophy on MRI, glucose metabolism reduction measured by FDG-PET and CSF biomarkers reflecting AD pathology, are useful in detecting prodromal AD (Pr-AD) patients [1]. Nevertheless, these new biomarkers are still not universally available in routine clinical practice. A neuropsychological profile differentiating Pr-AD from stable MCI (S-MCI) has been investigated, and episodic memory impairment seems to be a strong predictor of progression to dementia [2–4], although it is not specific as some patients will not develop AD. Impairment in other cognitive domains (executive function and language) has been described in Pr-AD, but there are discrepancies about which additional impaired area will most improve sensitivity and specificity for detecting Pr-AD [5–7]. We aim to investigate the clinical variables and neuropsychological measures at the moment of MCI diagnosis, which will better predict the progression from MCI to early AD dementia in a two-year follow-up period.
Methods
A total of 115 patients (63.3% females; mean age at diagnosis 74.4 years, SD 6.8; education, 63.3% primary school) were consecutively recruited from the Memory Clinic of University Hospital "Marqués de Valdecilla" (Santander, Spain) between April 2007 and April 2008. All of them initially fulfilled the Petersen criteria for MCI [8]. We excluded subjects who met criteria for dementia (DSM-IV), AD (NINCDS-ADRDA), depressive episode (IDC-10), subjects with significative cerebrovascular disease (Hachinski scale score ≥4), and those with any other medical or psychiatric identifiable cause accounting for their complaints. All patients underwent a complete clinical and neuropsychological evaluation at baseline and every 6 months during a two-year follow-up period. General cognitive function was assessed using MMSE, data on activities of daily living were collected using the Interview for Deterioration in Daily living activities in Dementia (IDDD), and symptoms of depression were measured using the Hamilton Rating Scale for Depression. Neuropsychological battery included test for the assessment of memory (California Verbal Learning Test-CVLT), language and semantic memory (15-items short-form of the Boston Naming Test, category fluency), praxis and visuospatial skills (Rey complex figure copy and WAIS block design subtest), attention and executive function (Symbol Digit Modalities Test, Trail Making part A and B, Stroop interference Test, Frontal Assessment Battery, category and letter fluency). A cognitive domain was judged as impaired when subjects scored 1.5 SD below values for age and education matched controls in at least one test. According to the results of the neuropsychological exploration, subjects were classified as: 1/ subjective memory complaints (SMC), patients performing normally on neuropsychological examination; 2/ pure amnestic MCI (a-MCI), patients fulfilling Petersen's criteria for amnestic MCI, with memory being the only affected domain; 3/ multidomain MCI (md-MCI), patients fulfilling a-MCI criteria and with one or more non-memory domain performance being under the cut-off value; 4/ non-amnesic MCI (na-MCI), patients with intact memory performance but scoring below the cut-off score on one or more non-memory tests.
The study was approved by the ethical committee of the University Hospital "Marqués de Valdecilla", and written informed consent was obtained from all the patients.
Statistical analyses were performed with SPSS software v.14.0. Differences in demographic, baseline clinical characteristics and neuropsychological measures between groups (S-MCI versus Pr-AD) were determined using Student's t-test for quantitative and χ2 for categorical variables. We included in a logistic regression model all variables associated with disease progression (p < 0.05), plus gender, age and ApoE e4 status, in order to select those of them that were independent predictors of dementia. ROC curve analysis was performed to evaluate the discriminating power of the predictive model for the progression to dementia. The area under the curve (AUC) was used as a measure of the overall performance. Optimum cut-off points of selected variables were calculated by choosing the point on the ROC curve that maximized both sensitivity and specificity.
Results
From the 115 patients included in the study, 6 patients were missed early in time and they did not complete the first year of follow-up, so they were excluded. The patients that ended the follow-up (n = 109) were classified in MCI subgroups according to their neuropsychological performance at baseline: 29 a-MCI (27%), 55 md-MCI (50%), 15 na-MCI (14%) and 10 SMC (9%). During the 2 years follow-up, 54 patients (49.54%) progressed to dementia, and 55 remained as S-MCI without progressing to dementia. Two patients were diagnosed as Lewy bodies dementia (LBD) and two as vascular dementia (VD), the remaining 50 patients being diagnosed as AD dementia. Given the initial classification in MCI subgroups, 62% (n = 18) of a-MCI and 58% (n = 32) of md-MCI patients evolved to AD dementia, whereas only 13% (n = 2) of na-MCI patients progressed to dementia (1 LBD and 1 VD). Among md-MCI subjects, one patient was diagnosed as VD and another was considered LBD, and none patient evolved to dementia in the SMC group.
We searched for differences in demographic, past medical history and neuropsychological assessments at baseline between S-MCI and Pr-AD (Table 1). Patients that evolved to non-AD dementia were excluded. In the univariate analysis, Pr-AD patients were significantly older than S-MCI, and Pr-AD scored significantly lower than S-MCI in MMSE, CVLT scores (short and long delay recall, both free and cued), category fluency and Frontal Battery Assessment (FAB). As expected, ApoE e4 allele was much more frequent in Pr-AD than in S-MCI. Conversely, active smoking and history of previous stroke were more frequent in S-MCI than Pr-AD, and S-MCI subjects scored slightly higher in Hamilton Rating Scale for depression. In our multivariate analysis, CVLT long delay total recall score (CVLT-LDTR) and MMSE turned out to be the only two variables independently associated to Pr-AD. In order to assess their discriminative power, we performed a ROC curve analysis that showed an area under the curve of 0.773 for MMSE (95% CI = 0.674-0.873, P < 0.001) and 0.775 for CVLT-LDTR (95% CI = 0.689-0.861, P < 0.001). The optimal thresholds for MMSE and CVLT-to predict progression from MCI to early AD were 26.5/30 and 4.5/16 respectively: those subjects that scored above 26/30 on MMSE and 4/16 on CVLT-LDTR had a negative predictive value of 93.93% at 2 years; inversely, those scoring below on both tests had a positive predictive value of 80.95%. For those subjects with just only one test below the cut-off score (Table 2), a firm prognosis could not be done as misclassifications were frequent. APOE ε4 status did not reach statistical significance (P = 0.168) when it was added to the multivariate predictive model.
Discussion
As a whole, our progression rate is approximately 25% per year, within the range of conversion from MCI to AD previously reported [2, 5, 6, 8–11]. In contrast with the literature indicating that md-MCI is the subgroup with the higher progression rate to dementia and that pure amnestic forms have lower rates of progression [6, 10, 12–14], in our series MCI of the amnestic type (regardless of other cognitive domains impairment) showed the higher risk of progression to dementia, with approximately 60% evolving to dementia during the 2 years follow-up. Amyloid imaging in MCI subtypes [15] showed that the highest proportion (approximately 80%) of amyloid-positive patients belonged to the md-MCI subgroup, although nearly 50% of a-MCI subjects were also amyloid-positive, thus reflecting subjacent AD pathology in a high proportion of a-MCI cases. Therefore, not only md-MCI but also a-MCI should be considered as high-risk of progression to dementia subgroups, and this is specially valid for those subjects with a poorer memory performance.
MMSE and CVLT-LDTR were the only measures that arose from multivariate analysis as independently associated with progression risk from MCI to early AD. According to our results, subjects scoring below 26/30 on MMSE and 4/16 on CVLT-LDTR constitute a MCI subgroup at high risk of progressing to early AD. MMSE is one of the most used tests for screening of cognitive impairment worldwide, and it has been reported that decline in MMSE starts approximately three years before the diagnosis of dementia [16]. Other tests used to determine general cognitive status (ADAS-cog, Addenbrooke's Cognitive Examination) have been reported as good predictors, independently [9] or associated with other neuropsychological measures [5, 12]; however, these tests are not used so widespread in general neurology clinics. Episodic memory tests have been widely described as good predictors for AD in MCI subjects, although usually they lacked specificity [17]. Several types of memory test have been used [2–6, 12, 18], but comparisons between them are limited [17]. The long delay scores of verbal episodic memory tests that are based on learning across multiple trials (i.e. CVLT) seemed to provide the most sensitive index for initial diagnosis of MCI and for detecting subjects which most likely will evolve to AD, as their performance is highly dependent on entorhinal and hippocampal systems [17]. The amnestic syndrome of the hippocampal type described in AD [1] is specifically characterized by a decreased total recall due to little effect of cueing. This feature differs from that seen in frontotemporal dementia, vascular dementia, other causes of fronto-subcortical dementia and psychiatric diseases, such as depression, that usually benefit from cueing. Therefore, CVLT-LDTR is more sensitive to detect Pr-AD than other CVLT scores because it captures the characteristic AD amnestic syndrome. Semantic memory (measured usually with category fluency) and attention and executive functions (measured usually with TMT-A or Symbol Digit Modalities Test) have been described as predictors of clinical progression in several studies [5–7, 13, 19], mainly when combined with other episodic memory scores or general cognitive assessments. However, in our multivariate analysis, category fluency and FAB lost significance, indicating they did not add information to that given by MMSE and CVLT-LDTR.
Conclusions
Pr-AD might be distinguished from S-MCI at baseline using the combination of MMSE and CVLT-LDTR. The clinical relevance of the findings is that these two neuropsychological predictors are relatively brief and may be readily completed in non-specialist clinical settings (where CSF biomarkers, MRI or PET are not available).
References
Dubois B, Albert ML: Amnestic MCI or prodromal Alzheimer's disease?. Lancet Neurol. 2004, 3: 246-248. 10.1016/S1474-4422(04)00710-0.
Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, Michel B, Puel M, Volteau M, Touchon J, Verny M, Dubois B: Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007, 69: 1859-1867. 10.1212/01.wnl.0000279336.36610.f7.
Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, Petersen RC, Shaw LM, Trojanowski JQ, Jack CR, Weiner MW, Jagust WJ, Alzheimer's Disease Neuroimaging Initiative: Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010, 75: 230-238. 10.1212/WNL.0b013e3181e8e8b8.
Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Moss M, Albert M: Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007, 64: 862-871. 10.1001/archneur.64.6.862.
Fleisher AS, Sowell BB, Taylor C, Gamst AC, Petersen RC, Thal LJ: Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007, 68: 1588-1595. 10.1212/01.wnl.0000258542.58725.4c.
Rami L, Gomez-Anson B, Sanchez-Valle R, Bosch B, Monte GC, Llado A, Molinuevo JL: Longitudinal study of amnesic patients at high risk for Alzheimer's disease: clinical, neuropsychological and magnetic resonance spectroscopy features. Dement Geriatr Cogn Disord. 2007, 24: 402-410. 10.1159/000109750.
Molinuevo JL, Gomez-Anson B, Monte GC, Bosch B, Sanchez-Valle R, Rami L: Neuropsychological profile of prodromal Alzheimer's disease (Prd-AD) and their radiological correlates. Arch Gerontol Geriatr. 2010
Petersen RC: Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004, 256: 183-194. 10.1111/j.1365-2796.2004.01388.x.
Rozzini L, Chilovi BV, Conti M, Bertoletti E, Delrio I, Trabucchi M, Padovani A: Conversion of amnestic Mild Cognitive Impairment to dementia of Alzheimer type is independent to memory deterioration. Int J Geriatr Psychiatry. 2007, 22: 1217-1222. 10.1002/gps.1816.
Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG: Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006, 67: 2176-2185. 10.1212/01.wnl.0000249117.23318.e1.
Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, Krampla W, Tragl KH: Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007, 68: 288-291. 10.1212/01.wnl.0000252358.03285.9d.
Mitchell J, Arnold R, Dawson K, Nestor PJ, Hodges JR: Outcome in subgroups of mild cognitive impairment (MCI) is highly predictable using a simple algorithm. J Neurol. 2009, 256: 1500-1509. 10.1007/s00415-009-5152-0.
Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, Zamora D, Goodkind M, Bell K, Stern Y, Devanand DP: Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006, 63: 916-924. 10.1001/archpsyc.63.8.916.
Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL: Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001, 58: 411-416. 10.1001/archneur.58.3.411.
Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, Klunk WE, De Kosky ST: Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009, 65: 557-568. 10.1002/ana.21598.
Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF: Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008, 64: 492-498. 10.1002/ana.21509.
Rabin LA, Pare N, Saykin AJ, Brown MJ, Wishart HA, Flashman LA, Santulli RB: Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer's disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2009, 16: 357-376.
Shankle WR, Romney AK, Hara J, Fortier D, Dick MB, Chen JM, Chan T, Sun X: Methods to improve the detection of mild cognitive impairment. Proc Natl Acad Sci USA. 2005, 102: 4919-4924. 10.1073/pnas.0501157102.
Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR: Detecting dementia: novel neuropsychological markers of preclinical Alzheimer's disease. Dement Geriatr Cogn Disord. 2004, 17: 42-48. 10.1159/000074081.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2377/11/78/prepub
Acknowledgements and funding
C. Sánchez-Quintana was involved in the DNA sample collections and genotyping analysis. This work was made possible by the generous participation of the patients and their families. This study was supported by a grant from "Obra Social de Caja Cantabria" (2007-2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AP performed the neuropsychological evaluations and reviewed critically the manuscript. ERR, JLVH and IM carried out the neurological evaluation and follow-up and reviewed critically the manuscript. PSJ performed the statistical analyses and reviewed critically the manuscript. SGP and JB reviewed critically the manuscript. OC drafted the manuscript and contributed to its final version. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pozueta, A., Rodríguez-Rodríguez, E., Vazquez-Higuera, J.L. et al. Detection of early Alzheimer's disease in MCI patients by the combination of MMSE and an episodic memory test. BMC Neurol 11, 78 (2011). https://doi.org/10.1186/1471-2377-11-78
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2377-11-78