Abstract
Background
Hyperuricemia appeared to be a common symptom in IgA nephropathy (IgAN), even in those with normal eGFR. IgAN was characterized by variation of pathological features, especially variable tubulointerstitial lesions. Since tubular reabsorption and excretion appeared to be more important in determination of plasma uric acid levels in persons without obvious decrease of glomerular filtration rate, we took advantage of our IgAN cohort to investigate whether plasma uric acid level associated with tubular interstitial lesions, and could be considered as a maker for tubular interstitial lesions, especially at early stage with normal eGFR.
Methods
623 IgAN patients were involved in the present study. Morphological changes were evaluated with Oxford classification scoring system as well as Beijing classification system of IgAN. Statistical analysis was done with SPSS 13.0.
Results
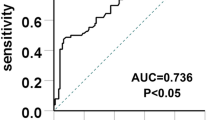
We found that plasma uric acid level associated with percentage of interstitial fibrosis/tubular atrophy. Higher plasma uric acid levels indicated higher tubulointerstitial scores, either with Oxford system (P = 0.012) or with Beijing classification system (P = 4.8*10-4) in the whole cohort. We also found that in the subgroup of 258 IgAN cases with normal baseline eGFR (eGFR > =90 ml/min/1.73 M2), higher plasma uric acid associated with more severe tubulointerstitial lesions with Beijing scoring system (P = 3.4*10-5). The risk of having more than 10% tubulointerstitial lesions in patients with hyperuricemia increased 58% compared with normal uric acid level. In subgroup with normal eGFR, only hyperuricemia predicted tubulointerstitial leisions, and the risk of having more tubulointerstitial changes increased 100%. Among these patients, hyperuricemia was associated with more tubulointerstitial lesions with a specificity of 60.3%. Specificity increased to 65% among those patients with eGFR > =90 ml/min/1.73 m2.
Conclusions
Plasma uric acid levels indicate tubular interstitial lesions in IgAN and hyperuricemia may be considered as a marker for tubulointerstitial lesions.
Similar content being viewed by others
Background
Hyperuricemia appeares to be a common manifestation in IgA nephropathy (IgAN) patients, even when their glomerular filtration rate (GFR) are normal and hyperuricemia has been considered as a risk factor of chronic kidney disease progression [1–4]. However, why plasma uric acid level elevated in part of IgAN patients with young age and normal GFR did not get enough attention.
It is well known that renal handling of uric acid excretion is a major denominator of plasma uric acid levels in adults with hyperuricemia [5–7]. Renal uric acid excretion is regulated by multiple factors such as glomerular filtration rate, tubular re-absorption and excretion [5, 7]. Tubular reabsorption and excretion appear to be more important in determination of plasma uric acid levels in persons without obvious decrease of GFR.
IgAN is characterized by variation of pathological features, especially variable tubulointerstitial lesions from almost normal to diffuse tubular atrophy and interstitial fibrosis [8], and tubulointerstitial damage has been reported to be an important risk factor on progression of IgAN [9–12]. So we took advantage of our IgAN cohort to investigate whether plasma uric acid level associated with tubular interstitial lesions, and furthermore could be considered as a maker for tubular/interstitial lesions, especially at early stage with normal eGFR.
Methods
Subjects
623 individuals (342 males and 281 females), were randomizely recruited from the IgA Nephropathy database at Renal Division of Peking University First Hospital. Among them, 258 individuals with estimated GFR (eGFR) > = 90 ml/min/1.73 m2. Characteristics of the population were listed in Table 1. eGFR was calculated with the equation developed from data the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [13].
The protocal for this study was approved by the Medicial Ethics Committee of Peking University and informed written consent for this study was obtained from every participant.
Evaluation of renal pathological changes
Two pathologists re-evaluated the renal biopsy slides with Oxford classification system of IgAN. Briefly, microscopic slides of all cases were reviewed independently by each of the pathologists. Histological parameters were evaluated as: 1) Mesangial hypercellularity score (If more than half the glomeruli have more than three cells in a mesangial area, this is categorized as M1, otherwise as M0; M0 ≤ 0.5/M1 > 0.5); 2) Endocapillary hypercellularity (hypercellularity due to increased number of cells within glomerular capillary lumina causing narrowing of the lumina, E0 as absent/E1 as present); 3) Segmental glomerulosclerosis (any amount of the tuft involved in sclerosis, but not involving the whole tuft or the present of an adhesion, S0 means absent /S1 means present); 4) Interstitial fibrosis/ tubular atrophy (percentage of cortical area involved by the tubular atrophy or interstitial fibrosis, T0: 0-25%/T1: 26-50%/T2: >50%) [14, 15]. Meanwhile, the histological changes of interstitial fibrosis/tubular atrophy were also graded by Beijing classification system of IgAN in present study (T0: 0%/T1: < 10%/T2: 10-24%/T3:25-49%/T4: >= 50%) [16].
Statistical analyses
Statistical Package for the Social Sciences (SPSS v13.0; Chicago, IL) was used. Baseline characteristics were reported as mean ± SEM or median (inter-quartile range [IQR]) for continuous variables and proportions for categorical variables. Association of plasma uric acid levels and tubular atrophy/interstitial fibrosis was analyzed by univariate analysis with covariates (age, gender, BMI, proteinuria, systolic blood pressure, diastolic blood pressure and other pathological parameters) adjustment. Correlation among plasma uric acid levels, eGFR and tubulointerstitial lesions were analyzed with Spearman's correlation in the whole cohort and the subgroup with different eGFR. Logistic regression was performed to detect whether hyperuricemia was a predictor of tubulointerstitial changes. In each Model, physical and biochemical traits including age, gender, eGFR, urinary protein, systolic blood pressure, diastolic blood pressure and hyperuricemia were analyzed by single factor analysis first, and significant factors were put into multiple factor model. Chi-square was used to calculate sensitivity and specificity of hyperuricemia prediction of tubulointerstitial change.
Results
General data
General baseline data within one week before renal biopsy were collected. Among the 623 IgAN, 258 cases presented with normal eGFR (> = 90 ml/min/1.73 m2). The pathological changes of all cases were evaluated with Oxford classification system, and tubulointerstitial changes were re-evaluated with Beijing classification system as well (Table 1).
Plasma uric acid levels associated with tubular atrophy and interstitial fibrosis
We found that plasma uric acid level associated with percentage of interstitial fibrosis/tubular atrophy. Higher plasma uric acid levels indicated higher tubulointerstitial scores, either with Oxford system (P = 0.012; Table 2) or with Beijing system (P = 4.8*10-4; Table 2).
We did the same analyses in the subgroup of 258 IgAN cases with normal baseline eGFR (eGFR > =90 ml/min/1.73 M2, Table 2). With Beijing classification system we also found that tubulointerstitial scores associated with plasma uric acid levels in the subgroup of normal eGFR (P = 3.4*10-5; Table 2), higher plasma uric acid associated with more severe tubulointerstitial lesions.
In Beijing scoring system, tubular atrophy/interstitial fibrosis more than 10% was identified as cut-off point of higher risk for ESRD [16]. Similarly more than 25% of tubular atrophy/interstitial fibrosis also increased risk of GFR decline in Oxford system. So here we re-attributed all individuals into two groups, by 10% interstitial fibrosis/tubular atrophy (Tadj_Bj0 < 10%, Tadj_Bi1 >= 10%), then by 25% interstitial fibrosis/tubular atrophy (Tadj_Ox0 < =25%, Tadj_Ox1 > 25%) and re-analyzed the association of interstitial fibrosis/tubular atrophy with plasma uric acid level, both in the whole cohort and subgroup with normal eGFR. Again, tubulointerstitial changes divided by 25% or 10% interstitial fibrosis/tubular atrophy associated with plasma uric acid levels. Patients with more tubular atrophy/interstitial fibrosis had higher plasma uric acid level (Table 2).
Hyperuricemia as a clinical marker of tubular atrophy/interstitial fibrosis
Since higher plasma uric acid levels associated with more tubular atrophy/interstitial fibrosis, we further analyzed whether hyperuricemia was a predictor of tubular atrophy/interstitial fibrosis in IgAN. It showed that eGFR correlated better with tubulointerstitial score than plasma uric acid levels, both in the whole group and the subgroup with eGFR less than 90 ml/min. But in the subgroup with higher eGFR (> = 90 ml/min), only plasma uric acid level correlated with tubulointerstitial score (Table 3). In the logistic multivariate analysis model, variables assessed as potential predictors included age, gender, blood pressure, BMI, proteinuria, eGFR. A combined model of eGFR and hyperuricemia status predicted tubular atrophy/interstitial fibrosis in the whole cohort. The risk of having more than 10% tubulointerstitial lesions in patients with hyperuricemia increased 58% compared with normal uric acid level. In subgroup with higher eGFR (> = 90 ml/min), only hyperuricemia predicted tubulointerstitial lesions, and the risk of having more tubulointerstitial changes increased 100% (Table 4). However in the subgroup with GFR less than 90 ml/min, decrease of eGFR is the main risk factor for tubulointerstitial lesions.
Among these patients, hyperuricemia associated with more tubulointerstitial lesions with a specificity of 60.3%. Specificity increased to 65% among those patients with eGFR > =90 ml/min/1.73 m2 (Table 5).
Discussion
Tubulointerstitial damage has been reported to be an important risk factor for progression of IgAN [9–12]. Many IgAN patients have tubular atrophy and interstitial fibrosis even with normal eGFR. And it appeared that pathological examination was the only method to detect renal tubulointerstitial lesions. Hyperuricemia has been an interesting clinical symptom in IgAN patients and considered to be associated with prognosis of the disease [1, 17, 18]. As early as 1975, Berger et al. has reported that 20-60% patients with gout also have mild to moderate renal dysfunction [19]. Besides influence of glomerular filtration rate on elimination of uric acid, several studies suggested that renal tubules play crucial roles in the regulation of uric acid balance in the body [20, 21]. Thus we systematically evaluated the pathological changes of 623 individuals with IgAN by Oxford classification system [14, 15], as well as the classification for tubulointerstitial changes in Beijing system [16] and found that tubular atrophy/interstitial fibrosis associated with plasma uric acid levels. The higher plasma uric acid levels indicated higher tubulointerstitial scores. And we also found similar association in the subgroup with normal eGFR. Finally, we identified that hyperuricemia could be a marker to predict tubular atrophy/interstitial fibrosis in IgAN, especially in those with normal eGFR.
In our study, we took advantage of the new pathological classification system, Oxford system [14, 15] as well as Beijing system [16] and did analysis in whole cohort of 623 IgAN patients, then in subgroup with eGFR > =90 ml/min/1.73 m2 and subgroup with eGFR < 90 ml/min. Our result showed that eGFR correlated with tubulointerstitial score better than plasma uric acid levels in the whole cohort and in the subgroup with eGFR less than 90 ml/min. Lower eGFR correlated with severe tubulointerstitial lesions. However in the subgroup with eGFR > = 90 ml/min, eGFR did not correlate with tubulointerstitial score anymore, only uric acid levels presented correlation with the scores. Previously published reports also found that plasma uric acid correlated with pathological changes in 202 cases of IgAN, in which the best correlation coefficients were 0.42 for interstitial fibrosis and 0.39 for tubular atrophy [17]. Although our study did not identified such high correlation coefficients for plasma uric acid and tubulointerstitial lesions, we found that plasma uric acid but not eGFR was an factor associated with tubulointerstitial changes in patients with obviously normal eGFR. However when eGFR decreased, eGFR itself may be correlating with tubulointerstitial changes.
Since tubular atrophy/interstitial fibrosis more than 10% was identified as cut-off point of higher risk for ESRD in Beijing scoring system [16] and more than 25% tubular atrophy/interstitial fibrosis increased risk of GFR decline in Oxford system, we collapsed individuals into two groups by cut-off point at 10% for Beijing scoring system, then by 25% interstitial fibrosis/tubular atrophy for Oxford scoring system. We set up two models of multivariate logistic regression, in which dependent variable was tubulointerstitial lesion by 10% cut off in model 1 and tubulointerstitial lesion by 25% cut off in model 2. For model 1, the risk of having more than 10% tubulointerstitial lesions in IgAN patients with hyperuricemia increased compared with normouricemia in whole cohort as well as in subgroup with normal eGFR. For model 2, none of the clinical signs predicted tubulointerstitial changes. These may be explained by the time of renal biopsy. Most patients accepted renal biopsy were attributed to less than 25% tubulointerstital lesions group, especially those with normal eGFR.
Our study showed that hyperuricemia has specificity of 60-64% to be associated with tubulointerstitial lesions in IgAN. Although it is much less than accepted levels of specificity 80% to consider hyperuricemia a predictor for tubulointerstitial lesions, the specificity still indicated the close association of hyperuricemia with tubulointerstitial score.
In summary, this observation study at an IgAN cohort revealed that interstitial fibrosis/tubular atrophy was associated with plasma uric acid levels in IgAN and plasma uric acid level was a hopeful clinical marker indicating tubulointerstitial lesions especially in patients with normal eGFR.
Conclusions
Hyepruricemia appeared to be a common symptom in IgA nephropathy. We took advantage of our IgAN cohort and found that plasma uric acid level indicates tubulointerstitial lesions in IgAN and hyperuricemia may be considered as a marker for tubulointerstitial lesions.
References
Syrjanen J, Mustonen J, Pasternack A: Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant. 2000, 15: 34-42.
Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S: Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004, 44: 642-650.
Chonchol M, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, Kestenbaum B, Carney JK, Fried LF: Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007, 50: 239-247. 10.1053/j.ajkd.2007.05.013.
Chang HY, Tung CW, Lee PH, Lei CC, Hsu YC, Chang HH, Yang HF, Lu LC, Jong MC, Chen CY, Fang KY, Chao YS, Shih YH, Lin CL: Hyperuricemia as an independent risk factor of chronic kidney disease in middle-aged and elderly population. Am J Med Sci. 2010, 339: 509-515.
Aringer M, Graessler J: Understanding deficient elimination of uric acid. Lancet. 2008, 372: 1929-1930. 10.1016/S0140-6736(08)61344-6.
Kutzing MK, Firestein BL: Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2008, 324: 1-7.
Mount DB, Kwon CY, Zandi-Nejad K: Renal urate transport. Rheum Dis Clin North Am. 2006, 32: 313-331. 10.1016/j.rdc.2006.02.006.
Barratt J, Feehally J: IgA Nephropathy. J Am Soc Nephrol. 2005, 16: 2088-2097. 10.1681/ASN.2005020134.
Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B, Hemmelgarn B: Histopathologic features aid in prediciton risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010, 5: 425-430. 10.2215/CJN.06530909.
Lv J, Zhang H, Zhou Y, Li G, Zou W, Wang H: Natural history of immunoglobulin A nephropathy and predictive factors of prognosis: a long-term follow up of 204 cases in China. Nephrology (Carlton). 2008, 13: 242-246. 10.1111/j.1440-1797.2007.00898.x.
Reich HN, Troyanov S, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry: Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007, 18: 3177-3183. 10.1681/ASN.2007050526.
D'Amico G: Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000, 36: 227-237. 10.1053/ajkd.2000.8966.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009, 150: 604-612. 10.7326/0003-4819-150-9-200905050-00006.
Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D'Agati V, D'Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, et al: The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76: 546-556. 10.1038/ki.2009.168.
Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D'Agati V, D'Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, et al: The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009, 76: 534-545. 10.1038/ki.2009.243.
Jiang L, Liu G, Lv J, Huang C, Chen B, Wang S, Zou W, Zhang H, Wang H: Concise semiquantitative histological scoring system for immunoglobulin A nephropathy. Nephrology (Carlton). 2009, 14: 597-605. 10.1111/j.1440-1797.2008.01083.x.
Myllymäki J, Honkanen T, Syrjänen J, Helin H, Rantala I, Pasternack A, Mustonen J: Uric acid correlates with the severity of histopathological parameters in IgA nephropathy. Nephrol Dial Transplant. 2005, 20: 89-95. 10.1093/ndt/gfh584.
Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M: Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron. 2001, 87: 333-339. 10.1159/000045939.
Berger L, Yu TF: Renal function in gout. IV. An analysis of 524 gouty subjects including long-term follow-up studies. Am J Med. 1975, 59: 605-613. 10.1016/0002-9343(75)90222-3.
Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Gorgoni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, et al: SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008, 40: 437-442. 10.1038/ng.106.
Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H: Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002, 417: 447-452.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2369/15/11/prepub
Acknowledgements
We appreciate the assistance of A Grant of the Medical Development of the Capital (2009–2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interest.
Authors’ contributions
CYQ conceived of the study, and participated in its design and coordination and helped to draft the manuscript. ZJJ collected the clinical data and drafted the manuscript. LY participated in the collection of clinical data. SUF and WSX re-evaluated the renal biopsy slides with Oxford classification system and Beijing classification system of IgA nephropathy. LXY helped to perform the statistical analysis. ZH participated in the design of the study. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhou, J., Chen, Y., Liu, Y. et al. Plasma uric acid level indicates tubular interstitial leisions at early stage of IgA nephropathy. BMC Nephrol 15, 11 (2014). https://doi.org/10.1186/1471-2369-15-11
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2369-15-11