Abstract
Background
Family members of patients with end stage renal disease were reported to have an increased prevalence of chronic kidney disease (CKD). However, studies differentiated genetic and non-genetic family members are limited. We sought to investigate the prevalence of CKD among fist-degree relatives and spouses of dialysis patients in China.
Methods
Seventeen dialysis facilities from 4 cities of China including 1062 first-degree relatives and 450 spouses of dialysis patients were enrolled. Sex- and age- matched controls were randomly selected from a representative sample of general population in Beijing. CKD was defined as decreased estimated glomerular (eGFR < 60 mL/min/1.73 m2) or albuminuria.
Results
The prevalence of eGFR less than 60 mL/min/1.73 m2, albuminuria and the overall prevalence of CKD in dialysis spouses were compared with their counterpart controls, which was 3.8% vs. 7.8% (P < 0.01), 16.8% vs. 14.6% (P = 0.29) and 18.4% vs. 19.8% (P = 0.61), respectively. The prevalence of eGFR less than 60 mL/min/1.73 m2, albuminuria and the overall prevalence of CKD in dialysis relatives were also compared with their counterpart controls, which was 1.5% vs. 2.4% (P = 0.12), 14.4% vs. 8.4% (P < 0.01) and 14.6% vs. 10.5% (P < 0.01), respectively. Multivariable Logistic regression analysis indicated that being spouses of dialysis patients is negatively associated with presence of low eGFR, and being relatives of dialysis patients is positively associated with presence of albuminuria.
Conclusions
The association between being family members of dialysis patients and presence of CKD is different between first-degree relatives and spouses. The underlying mechanisms deserve further investigation.
Similar content being viewed by others

Background
Chronic kidney disease (CKD) is a global public health problem [1, 2], and it affects 10-16% of the adult population in Asia, Australia, Europe and the United States [3, 4]. A recent national survey in China [3] indicates that the prevalence of CKD in China is 10.8%, and the number of patients with CKD is estimated to 119.5 million. CKD has been associated with high morbidity and mortality [5], hence it is important to launch programs aiming at reducing the burden of CKD. It is reported that screening for proteinuria among high-risk population is cost-effective [6]. However, who constitute high-risk population for CKD remains to be answered.
Recent studies revealed that family members of patients with end stage renal disease (ESRD) have an increased prevalence of CKD [7–12]. Differences in ethnicities, lifestyles and screening methods may cause high variability in results [13, 14]. Furthermore, some studies did not differentiate the genetic and non-genetic family members of patients with ESRD. A recent study from Taiwan revealed that both relatives and spouses of hemodialysis patients were found to have high prevalence of CKD [15]. The limited number of participants and the limited representativeness of controls constraint the power of that study. The present study was conducted to investigate the prevalence of CKD among the first-degree relatives and spouses of dialysis patients, and to compare that with controls from a representative sample of general population in Beijing.
Methods
Study population
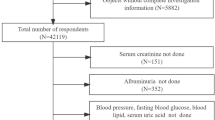
Seventeen dialysis facilities from 4 cities of China were enrolled (12 in Beijing, 3 in Tianjin, 1 in Dalian and 1 in Shijiazhuang). ESRD Patients with inherited kidney disease, such as autosomal dominant polycystic kidney disease or Alport’s syndrome were excluded for this study. All family members, including first-degree relatives (including parent, sibling and child) and spouses of these patients were invited to participate in the study from October 2006 to August 2007. Altogether 1642 family members of ESRD patients participated in this study on a voluntary basis. Among them, 130 members who didn’t have either complete questionnaire or complete lab results were excluded. Finally, 1062 relatives and 450 spouses from 715 hemodialysis and 127 peritoneal dialysis families were eligible for present analysis. The ethics committee of Peking University First Hospital approved the study, which covers all participating institutions. All participants gave written informed consent before data collection.
Controls were selected from a representative sample of the general population of adults in Beijing, which is described in details elsewhere [16].
Screening protocol and assessment criteria
Data were collected in examination centers at local health stations. All subjects completed a questionnaire documenting their sociodemographic status (e.g., age, sex, and educational level), health status (renal disease, diabetes mellitus, or hypertension), history of nephrotoxic medications (non-steroids anti-inflammatory drugs, [NSAIDS] or Chinese herbs containing aristolochic acid, [AA]), lifestyle behaviors (e.g., smoking), and the primary causes of renal failure of dialysis patients (glomerular disease, hypertension, diabetes, interstitial nephritis, and ‘all other’ causes).
Anthropometric measurements were obtained. Indicators of kidney damage and possible risk factors then were examined. All blood samples and urinary samples were tested in the central laboratory of Beijing University First Hospital.
Definitions of CKD
Albumin and creatinine were measured from a fresh morning spot urine sample or morning urine sample stored at 4°C for less than 1 week. Albuminuria was measured using immunoturbidimetic methods (Audit Diagnostics, Cork, Ireland). Urinary creatinine was measured by means of Jaffe’s kinetic method on a Hitachi 7170 autoanalyzer (Hitachi, Tokyo, Japan). Urinary albumin-creatinine ratio (ACR; milligrams per gram) was calculated. Patients with ACR determinations that ranged from 17 to 250 mg/g (1.9 to 28.3 mg/mmol) for males and 25 to 355 mg/g (2.8 to 40.2 mg/mmol) for females were classified as having microalbuminuria, and participants with ACR values greater than the microalbuminuria range were classified as having macroalbuminuria. Albuminuria was defined as the presence of either microalbuminuria or macroalbuminuria. Women during menstruation were excluded from analyses for albuminuria.
Blood was collected by venipuncture after an overnight fast of at least 10 hours. Serum creatinine was measured by the same methods as was urinary creatinine. eGFR was calculated with an equation developed by modifying the Modification of Diet in Renal Disease (MDRD) equation based on data from Chinese CKD patients [17]. And decreased kidney function was defined as eGFR < 60 ml/min/1.73 m2 (1.00 ml/s/1.73 m2):
where Scr is serum creatinine concentration (in mg/dL) and age in years.
The CKD was defined as decreased kidney function or albuminuria based on the classification system established by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) [18].
Definition of other conditions
Blood pressure was measured by sphygmomanometer, three times at 1 minute intervals. The mean of the three readings was calculated, unless the difference between readings was greater than 10 mmHg, in which case the mean of the two closet of the three measurements was used. Hypertension was defined as systolic blood pressure of 140 mmHg or greater or diastolic blood pressure of 90 mmHg or greater or use of antihypertensive medications in past 2 weeks irrespective of blood pressure, or any self-reported history of hypertension. Fasting blood glucose was measured enzymatically by means of a glucose oxidase method using the Hitachi 7170 autoanalyzer. Diabetes was defined as fasting plasma glucose of 7.0 mmol/L or more, by hypoglycaemic agents despite fasting plasma glucose, or any self-reported history of diabetes.
Serum total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides and uric acid were measured with commercially available reagents using a Hitachi 7170 autoanalyzer.
The body mass index (BMI) was calculated as weight (in kilograms) divided by height squared (in meters squared). Overweight is defined as BMI greater than 24 kg/m2. BMI = weight (kg)/height (m2) × 100%. Dyslipidemia was defined as present if total cholesterol was ≥ 5.72 mmol/L (220 mg/dL), or if low density lipoprotein (LDL) was ≥ 3.64 mmol/L (140 mg/dL), or triglyceride was ≥ 1.70 mmol/L (150 mg/dL) or high density lipoprotein (HDL) was < 0.91 mmol/L (35 mg/dL). Hyperuricaemia was defined as serum uric acid > 422 μmol/L for males and > 363 μmol/L for females.
Statistics analysis
All analyses and calculations were performed by SPSS statistical package, version 16.0 (SPSS Inc., Chicago, IL, USA). Controls were selected from the cross-sectional survey of CKD in a representative sample of the general adults in Beijing [16]. Sample sizes of each age-stratified group of ≤ 30, 31–40, 41–50, 51–60, and > 60 years were 1814, 2816, 4206, 3002, and 2097 participants, respectively. In selecting controls for spouses, 900 sex- and age-stratified matched participants were randomly selected as controls. 2124 sex- and age-stratified matched participants were randomly selected as controls for relatives.
Data were presented as the mean ± standard deviation for continuous variables and as proportions for categorical variables. Descriptive analysis were used to characterize the participant population by sociodemographic data (eg. age, sex and education status) and health status (eg. hypertension and diabetes). Differences in variables between the two groups were analyzed using chi-square statistics for categorical variables or independent t-test for continuous variables. The unadjusted odds ratios (OR) between family members of dialysis patients and indicators of kidney damage were determined by univariate Logistic regression analysis. McNemar’s test was used to test univariate associations. A multivariate Logistic regression analysis was then performed to adjust for confoundings including age, gender, diabetes, hypertension, nephrotoxic medications, dyslipidemia, overweight, and chronic respiratory tract infection. Odds ratio (OR) was calculated and 95% confidence interval (CI) was provided. A P value of 0.05 or less was considered to be statistically significant.
Results
Spouses of dialysis patients
Characteristics of spouses and matched controls are listed in Table 1. eGFR was significantly higher in dialysis spouses than controls (86.9 ± 16.8 vs. 83.7 ± 18.1 mL/min/1.73 m2, P < 0.01). A significantly lower prevalence of low eGFR was found in dialysis spouses compared with controls (3.8% vs. 7.8%, P < 0.01). There were no differences in the prevalence of albuminuria (16.8% vs. 14.6%, P = 0.29) and CKD (18.4% vs. 19.8%, P = 0.61) between these two groups.
Among spouses, the prevalence of nephrotoxic medications use was higher and the prevalence of hypertension was lower compared with that of controls. There were no differences in prevalence of diabetes and overweight between these two groups. After adjusting for potential confounders, being spouses of dialysis patients was negatively associated with presence of decreased eGFR, with an OR of 0.50 (95% CI 0.28–0.88; Table 2).
First-degree relatives of dialysis patients
Characteristics of relatives and matched controls are listed in Table 3. A significantly higher prevalence of albuminuria (14.4% vs. 8.4%, P < 0.001) was found in relatives compared with controls. There was no difference in the prevalence of reduced eGFR between two groups (1.5% vs. 2.4%, P = 0.12).
The prevalence of diabetes and nephrotoxic medication use were higher among relatives compared with those of controls, while the prevalence of hypertension was lower among relatives compared with that of controls. After adjusting for potential confounders, being relatives of dialysis patients was positively associated with presence of albuminuria, with an OR of 2.02 (95%CI 1.57–2.59; Table 2).
Discussion
Our study revealed a higher prevalence of albuminuria among first-degree relatives of dialysis patients, and the positive association is independent of various potential confounders. Furthermore, we observed a lower prevalence of decreased renal function among spouses of dialysis patients compared with controls. A major strength of our study is the large sample size and the representativeness of controls.
The high prevalence of CKD among first-degree relatives of dialysis patients in our study is consistent with previous studies [19, 20]. Compared with previous screening programs for high-risk populations, the prevalence of albuminuria in relatives of dialysis patients (14.4%) in this study was lower than that from the Kidney Early Evaluation Program (KEEP) study (29%) [21], but similar to that from Tsai’s study (10.7%) [15]. This figure was higher than the observed rate of albuminuria in a general population-based screening program in China (9.4%)3 and in Europe and the United States (5.9% – 7.4%) [22, 23]. One possible explanations might be familial clustering of metabolic disorders, such as hypertension and diabetes, which are known risk factors of albuminuria. Previous studies suggested that, of these renal risk factors, hypertension and diabetes mellitus are multifactorial disease under the influence of both genetic traits and environmental factors [10, 24]. Environmental factors, such as low socioeconomic status and lifestyle of inactivity and smoking, also should be considered as potential contributing for CKD [25, 26]. However, in our analyses, after adjusting for potential confounders, the positive association between being relatives of dialysis patients and albuminuria still exist, indicating that there might be genetic susceptibility of CKD for relatives.
Genetic traits may contribute to the development of CKD in relatives of dialysis patients. It is well known that familial focal segmental glomerulosclerosis (FSGS) is a significant and growing cause of CKD. Given the progress in understanding the biology and pathology of podocyte, mutation of associated genes, such as ACTN4, TRPC6 and NPHS2, contribute to the damage of podocyte and podocyte dysfunction [27, 28]. The latter was associated to the development of proteinuria and FSGS [29]. These genes were recognized to be the genetic basis of FSGS. Meanwhile, more related genes or chromosomal regions were identified in diabetic (3q, 18q22.3-23), non-diabetic nephropathy (chromosome 10), systemic lupus erythematosus and familial IgA nephropathy (6q22-23) [30–32].
Spouses of patients with ESRD were considered to be nongenetic controls for studying the family clustering of CKD [11]. Spousal concordance of health risks and behaviors such as cardiovascular disease, hypertension, metabolic syndrome and high fasting glucose levels has been observed in many disease [33–35]. In this study, we also found higher rates of some health risks in dialysis spouses compared with controls, such as dyslipidemia and use of nephrotoxic medications. However, this study demonstrated a significantly lower prevalence of low eGFR, but no differences in the prevalence of albuminuria and CKD in dialysis spouses compared with controls. Meanwhile, in our analyses, after adjusting for potential confounders, the negative association between being spouses of dialysis patients and low eGFR still exist. These results were enhanced by the sufficient number of participants from multicentric facilities and the ample representativeness of sex- and age- matched controls, which were randomly selected on a ratio of 2:1 from a representative sample of the general population of adults in Beijing [16]. The exact reason was unknown. We assumed that for being spouses of dialysis patients, perceived and objective CKD knowledge are likely to impact risk-modifying behavior in different ways. Multi-component structured empowerment intervention is effective in pre-dialysis CKD patients and may lead to a delay in the progression of kidney disease [36–40].
Our study has limitations that deserve mention. Firstly, it was implemented on a voluntary bias within the dialysis units. Additionally, ESRD patients who didn’t start dialysis treatment were not included in present study. There were kinds of selecting bias in the study which limited the extension of the results from this study. Secondly, urine and blood results were based on a single measurement. It should be noticed that it might overestimate the prevalence of albuminuria based on one measurement. Finally, a cross-sectional study design has several inherent weaknesses, such as lack of long-term observation for outcome and difficulty interpreting the association of exposure with outcome.
Conclusions
In conclusion, the association between being family members of dialysis patients and presence of CKD is different between first-degree relatives and spouses. Genetic susceptibility may account for the phenomenon of family clustering of CKD. However, non-genetic environmental factors also should be considered as potential contributing for CKD. The underlying mechanisms deserve further investigation. Strategies aimed at intervention of hypertension and other metabolic disorders might prove effective in controlling the pandemic of CKD in family members of dialysis patients.
References
Meguid El Nahas A, Bello AK: Chronic kidney disease: the global challenge. Lancet. 2005, 365 (9456): 331-340.
Nugent RA, Fathima SF, Feigl AB, Chyung D: The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract. 2011, 118 (3): 269-277. 10.1159/000321382.
Zhang L, Wang F, Wang L, et al: Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012, 379 (9818): 815-822. 10.1016/S0140-6736(12)60033-6.
Matsushita K, van der Velde M, Astor BC, et al: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010, 375 (9731): 2073-2081.
Levey AS, Atkins R, Coresh J, et al: Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from kidney disease improving global outcomes. Kidney Int. 2007, 72 (3): 247-259. 10.1038/sj.ki.5002343.
Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR: Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003, 290 (23): 3101-3114. 10.1001/jama.290.23.3101.
Freedman BI, Soucie JM, Kenderes B, et al: Family history of end-stage renal disease does not predict dialytic survival. Am J Kidney Dis. 2001, 38 (3): 547-552. 10.1053/ajkd.2001.26851.
Freedman BI, Volkova NV, Satko SG, et al: Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol. 2005, 25 (6): 529-535. 10.1159/000088491.
Gumprecht J, Zychma MJ, Moczulski DK, Gosek K, Grzeszczak W: Family history of end-stage renal disease among hemodialyzed patients in Poland. J Nephrol. 2003, 16 (4): 511-515.
Lei HH, Perneger TV, Klag MJ, Whelton PK, Coresh J: Familial aggregation of renal disease in a population-based case–control study. J Am Soc Nephrol. 1998, 9 (7): 1270-1276.
O’Dea DF, Murphy SW, Hefferton D, Parfrey PS: Higher risk for renal failure in first-degree relatives of white patients with end-stage renal disease: a population-based study. Am J Kidney Dis. 1998, 32 (5): 794-801. 10.1016/S0272-6386(98)70135-0.
Spray BJ, Atassi NG, Tuttle AB, Freedman BI: Familial risk, age at onset, and cause of end-stage renal disease in white Americans. J Am Soc Nephrol. 1995, 5 (10): 1806-1810.
McClellan W, Speckman R, McClure L, et al: Prevalence and characteristics of a family history of end-stage renal disease among adults in the United States population: Reasons for Geographic and Racial Differences in Stroke (REGARDS) renal cohort study. J Am Soc Nephrol. 2007, 18 (4): 1344-1352. 10.1681/ASN.2006090952.
Bello AK, Peters J, Wight J, de Zeeuw D, El Nahas M: A population-based screening for microalbuminuria among relatives of CKD patients: the Kidney Evaluation and Awareness Program in Sheffield (KEAPS). Am J Kidney Dis. 2008, 52 (3): 434-443. 10.1053/j.ajkd.2007.12.034.
Tsai JC, Chen SC, Hwang SJ, Chang JM, Lin MY, Chen HC: Prevalence and risk factors for CKD in spouses and relatives of hemodialysis patients. Am J Kidney Dis. 2010, 55 (5): 856-866. 10.1053/j.ajkd.2009.12.021.
Zhang L, Zhang P, Wang F, et al: Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis. 2008, 51 (3): 373-384. 10.1053/j.ajkd.2007.11.009.
Ma YC, Zuo L, Chen JH, et al: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006, 17 (10): 2937-2944. 10.1681/ASN.2006040368.
National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002, 39 (Suppl 1): S1-S266.
Bergman S, Key BO, Kirk KA, Warnock DG, Rostant SG: Kidney disease in the first-degree relatives of African-Americans with hypertensive end-stage renal disease. Am J Kidney Dis. 1996, 27 (3): 341-346. 10.1016/S0272-6386(96)90356-X.
Jurkovitz C, Franch H, Shoham D, Bellenger J, McClellan W: Family members of patients treated for ESRD have high rates of undetected kidney disease. Am J Kidney Dis. 2002, 40 (6): 1173-1178. 10.1053/ajkd.2002.36866.
Brown WW, Peters RM, Ohmit SE, et al: Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2003, 42 (1): 22-35. 10.1016/S0272-6386(03)00405-0.
Coresh J, Selvin E, Stevens LA, et al: Prevalence of chronic kidney disease in the United States. JAMA. 2007, 298 (17): 2038-2047. 10.1001/jama.298.17.2038.
Hallan SI, Coresh J, Astor BC, et al: International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006, 17 (8): 2275-2284. 10.1681/ASN.2005121273.
Satko SG, Sedor JR, Iyengar SK, Freedman BI: Familial clustering of chronic kidney disease. Semin Dial. 2007, 20 (3): 229-236. 10.1111/j.1525-139X.2007.00282.x.
Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL: Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology. 2003, 14 (4): 479-487.
Sabanayagam C, Shankar A, Saw SM, Lim SC, Tai ES, Wong TY: Socioeconomic status and microalbuminuria in an Asian population. Nephrol Dial Transplant. 2009, 24 (1): 123-129.
Mukerji N, Damodaran TV, Winn MP: TRPC6 and FSGS: the latest TRP channelopathy. Biochim Biophys Acta. 2007, 1772 (8): 859-868. 10.1016/j.bbadis.2007.03.005.
Rood IM, Deegens JK, Wetzels JF: Genetic causes of focal segmental glomerulosclerosis: implications for clinical practise. Nephrol Dial Transplant. 2012, 27 (3): 882-890. 10.1093/ndt/gfr771.
D’Agati VD: The spectrum of focal segmental glomerulosclerosis: new insights. Curr Opin Nephrol Hypertens. 2008, 17 (3): 271-281. 10.1097/MNH.0b013e3282f94a96.
de Borst MH, Benigni A, Remuzzi G: Primer: strategies for identifying genes involved in renal disease. Nat Clin Pract Nephrol. 2008, 4 (5): 265-276. 10.1038/ncpneph0785.
Gharavi AG, Yan Y, Scolari F, et al: IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22-23. Nat Genet. 2000, 26 (3): 354-357. 10.1038/81677.
Satko SG, Freedman BI: The importance of family history on the development of renal disease. Curr Opin Nephrol Hypertens. 2004, 13 (3): 337-341. 10.1097/00041552-200405000-00012.
Jurj AL, Wen W, Li HL, et al: Spousal correlations for lifestyle factors and selected diseases in Chinese couples. Ann Epidemiol. 2006, 16 (4): 285-291. 10.1016/j.annepidem.2005.07.060.
Kim HC, Kang DR, Choi KS, Nam CM, Thomas GN, Suh I: Spousal concordance of metabolic syndrome in 3141 Korean couples: a nationwide survey. Ann Epidemiol. 2006, 16 (4): 292-298. 10.1016/j.annepidem.2005.07.052.
Di Castelnuovo A, Quacquaruccio G, Donati MB, de Gaetano G, Iacoviello L: Spousal concordance for major coronary risk factors: a systematic review and meta-analysis. Am J Epidemiol. 2009, 169 (1): 1-8.
Byrne J, Khunti K, Stone M, Farooqi A, Carr S: Feasibility of a structured group education session to improve self-management of blood pressure in people with chronic kidney disease: an open randomised pilot trial. BMJ Open. 2011, 1 (2): e000381-10.1136/bmjopen-2011-000381.
Jia T, Bi SH, Lindholm B, Wang T: Effect of multi-dimensional education on disease progression in pre-dialysis patients in China. Ren Fail. 2012, 34 (1): 47-52. 10.3109/0886022X.2011.623560.
Chen SH, Tsai YF, Sun CY, Wu IW, Lee CC, Wu MS: The impact of self-management support on the progression of chronic kidney disease–a prospective randomized controlled trial. Nephrol Dial Transplant. 2011, 26 (11): 3560-3566. 10.1093/ndt/gfr047.
Wu IW, Wang SY, Hsu KH, et al: Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality–a controlled cohort study based on the NKF/DOQI guidelines. Nephrol Dial Transplant. 2009, 24 (11): 3426-3433. 10.1093/ndt/gfp259.
Tuot DS, Plantinga LC: What patients don’t know may hurt them: knowledge and the perception of knowledge among patients with CKD. Kidney Int. 2011, 80 (12): 1256-1257. 10.1038/ki.2011.269.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2369/14/19/prepub
Acknowledgment
The authors thank all of the physicians and nurses in all participant hospitals. We also appreciate all of the dialysis patients and their family members for their generous corporation.
Support
This work was funded by the development and validation of new markers for diagnosis and evaluation of CKD (09050704310902) from the Beijing Science and Technology Committee and the grants for the Early Detection and Prevention of Non-communicable Chronic Diseases from the International Society of Nephrology Research Committee, and the grants from the China Health and Medical Development Foundation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KX and ZL participated in the study, analyzed the data, interpreted the results, and drafted the manuscript. LL, ZL, YP, LZ, LW, CM, CX, JA, LG, XJ, LH, WS, HW, WY, GY, CP, WH and JQ participated in the survey and study design and collected the data. ZL and WM formed the study concept, interpreted the results, and revised the manuscript. WH revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kong, X., Liu, L., Zuo, L. et al. Association between family members of dialysis patients and chronic kidney disease: a multicenter study in China. BMC Nephrol 14, 19 (2013). https://doi.org/10.1186/1471-2369-14-19
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2369-14-19