Abstract
Background
The associations between the polymorphisms in Cytotoxic T lymphocyte-associated molecule-4 (CTLA-4) gene and Graves’ disease (GD) have been extensively investigated in Chinese population. However, the results were inconsistent. The objective of this study is to investigate the associations between the polymorphisms in CTLA-4 gene and the risk of GD by meta-analysis.
Methods
We searched Pubmed database, Medline (Ovid) database, CNKI database and Wanfang database, covering all studies until August 11, 2012. Statistical analysis was performed by using the Revman4.2 software and the Stata10.0 software.
Results
A total of 28 case–control studies concerning the most widely studied three polymorphisms [+49A/G(rs231775), -318C/T(rs5742909) and CT60(rs3087243)] for Chinese population in 21 publications were included. The results suggested that the G allele carriers (GG+GA) might have an increased risk of GD when compared with the AA homozygote carriers for the +49A/G polymorphism (GG+GA vs. AA: OR = 2.57, 95%CI = 1.87-3.52). However, as to the -318C/T polymorphism and CT60 polymorphism, the results indicated that the variant allele carriers might have decreased risks of GD when compared with the homozygote carriers (−318C/T: TT+TC vs. CC: OR = 0.78, 95%CI = 0.62-0.97; CT60: AA+AG vs. GG: OR = 0.64, 95%CI = 0.52-0.78).
Conclusions
The current meta-analysis indicated that the polymorphisms in the CLTA-4 gene might be risk factors for GD in the Chinese population. In future, more large-scale case–control studies are needed to validate these results.
Similar content being viewed by others
Background
Graves’ disease (GD) is a complex autoimmune thyroid disease, which is caused by excessive production of thyroid hormone and characterized by an enlarged thyroid gland, protrusion of the eyeballs, a rapid heartbeat and nervous excitability [1]. It is reported that GD occurs in about 1.2% in Western population and 0.25–1.09% in Chinese population [2]. It is widely accepted that GD is caused by complex interactions between many genetic factors and environmental factors. Numerous studies have been published focusing on the topic of genetic factors of GD risk in the Chinese population. Many genes involved in the inception and evolution of GD have been identified as GD candidate genes, such as ADRB2 [3], TSHR [4], CTLA-4 [5] and IL-13 gene [6]. And among them, the CTLA-4 gene is one of the most extensively studied.
Cytotoxic T lymphocyte-associated molecule-4 (CTLA-4) is a T cell surface molecule [7]. It is a negative regulator of T cell activation and plays an important role in the pathogenesis of GD. The CTLA-4 gene is localized on chromosome 2q33. Many polymorphisms have been identified in the CTLA-4 gene. It is reported that the polymorphisms in CTLA-4 gene might influence the expression of the protein, and might play important roles in the pathogenesis of GD [8]. Up to now, many studies have been performed to investigate the associations between the polymorphisms in the CTLA-4 gene and the risk of GD. Among them, the +49A/G, -318C/T and CT60 polymorphisms were the most widely studied. To this day, the associations between polymorphisms of the CTLA-4 gene and the risk of GD have been widely investigated in the Chinese population. However, the results were inconsistent, and the associations were not yet formally evaluated. In order to derive a more precise conclusion, we performed a meta-analysis to assess the associations between the polymorphisms in the CTLA-4 gene and the risk of GD in the Chinese population. To our knowledge, this is the first comprehensive genetic meta-analysis performed in the Chinese population for Graves’ disease.
Methods
Study identification and selection
A literature search in Pubmed database, Medline (Ovid) database, CNKI database and Wanfang database was carried out to identify studies investigating the association between the Graves’ disease risk and the CTLA-4 polymorphisms on Aug 11th, 2012. The search terms were as follows: Graves’ disease or GD in combination with polymorphism or variant or mutation and in combination with CTLA-4 or Cytotoxic T lymphocyte-associated molecule-4. All languages were included. The inclusion criteria were: (a) studies evaluating the association between the (+49A/G, -318C/T and CT60) polymorphisms in the CTLA-4 gene and Graves’ disease risk in the Chinese population, (b) the design should be a case–control design, (c) sufficient data (genotype distributions of cases and controls) available to calculate an odds ratio (OR) with its 95%CI (confidence interval), (d) genotype distributions in control group should be consistent with Hardy-Weinberg equilibrium (HWE). Studies were excluded if one of the following existed: (a) the studied populations were based on family or sibling pairs, (b) genotype frequencies or numbers were not presented in the original studies, (c) reviews and abstracts. If more than one study was published by the same authors using the same case series or overlapping case series, studies with the largest size of samples were included.
Data extraction
Two investigators independently extracted the data and reached a consensus on all items. The following items were extracted from each study if available: first author’s name, publication year, province of origin, age of cases, genotype number in cases and controls and genotyping method.
Statistical analysis
The strength of associations between the polymorphisms in the CTLA-4 gene and Graves’ disease risk was assessed by odds ratios (OR) with the corresponding 95% confidence intervals (CI). The genetic models evaluated for the pooled OR of the polymorphisms were dominant models (GG+GA vs. AA for the +49A/G, TT+TC vs. CC for the -318C/T, and AA+AG vs. GG for the CT60). OR was analyzed by a fixed-effects model (the Mantel-Haenszel method) or a random-effects model (the DerSimonian and Laird method) according to the heterogeneity. Heterogeneity was assessed by a X 2 based Q statistic and was considered statistically significant at p-value <0.10. When the P value was more than 0.10, the pooled OR was calculated by the fixed-effects model, otherwise, a random-effects model was used. The significance of the pooled OR was determined by the Z-test and p-value less than 0.05 was considered as statistically significant. Sensitivity analysis was conducted by sequential excluding a single study each time in an attempt to identify the potential influence of the individual data set to the pooled ORs. In addition, the possible publication bias was investigated with the Begg’s funnel plot. Funnel plot asymmetry was assessed by Egger’s linear regression test [9]. For each polymorphism, other genetic models were also used to assess the association with the risk of Graves’ disease (for the +49A/G polymorphism: GG vs. AA+GA, GG vs. AA, GA vs. AA, G vs. A; for the -318C/T polymorphism: TT vs. CC+TC, TT vs. CC, TC vs. CC, T vs. C; for the CT60 polymorphism: AA vs. AG+GG, AA vs. GG, AG vs. GG, A vs. G). HWE was tested by Person’s X 2 test. Statistical analysis was performed using Revman4.2 software and Stata10.0 software.
Results
Studies selection and characteristics
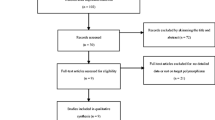
The selection process of studies was as follows. Briefly, a total of 429 results were identified after an initial search from the Pubmed, Medline (Ovid), CNKI and Wanfang databases. After reading the titles and abstracts, 302 results were excluded for being irrelevant to CTLA-4 polymorphisms and Graves’ disease risk, abstracts, reviews or duplications of search results. After reading full-texts of the remaining 127 studies, 68 studies were excluded for not relevant to the GD risk in the Chinese population, and 9 studies were excluded for not relevant to the investigated polymorphisms (+49A/G, -318C/T and CT60). Thus, 50 studies were left for data extraction. And then, a total of 54 case–control studies were extracted for these three polymorphisms. Among 54 case–control studies, genotype numbers for control group in 7 studies were not consistent with HWE, data in 19 studies were overlapped. So these 27 case–control studies were excluded. Finally, a total of 28 case–control studies in 21 publications were identified for meta-analysis [2, 10–27]. Summary of the properties of the studies are listed in Table 1. Overall, there were 17 case-controls studies for the +49A/G polymorphism [2, 5, 11–13, 15, 17–25, 27, 28], 7 case–control studies for the -318C/T polymorphism [2, 10–12, 14, 26, 28] and 4 case–control studies for the CT60 polymorphism [2, 10, 16, 18]. The genotype distributions for these polymorphisms are listed in Table 2.
Quantitative synthesis
The +49A/G polymorphism
A total of 4009 cases and 3651 controls from 17 case–control studies were included for data synthesis. As is shown in Figure 1, we analyzed the heterogeneity of GG+GA vs. AA for all 17 studies and the value of X 2 was 47.22 with 16 degrees of freedom and p-value < 0.00001 in a random-effects model. Additionally, I-square value is another index of the test of heterogeneity. In Figure 1, the I-square was 66.1%, suggesting a moderate of heterogeneity. Thus, we chose the random-effects model to synthesize the data. Overall, OR was 2.57 (95%CI = 1.87-3.52) and the test for overall effect Z value was 5.83 (p-value < 0.00001). The results suggested that the G allele carriers might have an increased risk of Graves’ disease compared with those individuals with the AA homozygote. Statistically similar results were obtained after sequential excluding each case–control study for the GG+GA vs. AA comparative, suggesting the stability of our meta-analysis. Significant publication bias was detected in the funnel plot (figure not shown), and in the Egger’s test, the result was: t = 2.82, p-value = 0.013, which also indicated considerable publication bias. Summary of the results of other genetic comparisons are listed in Table 3.
The -318C/T polymorphism
A total of 999 cases and 702 controls from 7 case–control studies were included for data synthesis. As is shown in Figure 2, we analyzed the heterogeneity of TT+TC vs. CC for all 7 studies and the value of X 2 was 2.56 with 6 degrees of freedom and p-value = −0.86 in a fixed-effects model. Additionally, I-square value is another index of the test of heterogeneity. In Figure 2, the I-square was 0%, suggesting an absent of heterogeneity. Thus, we chose the fixed-effects model to synthesize the data. Overall, OR was 0.78 (95%CI = 0.62-0.97) and the test for overall effect Z value was 2.18 (p-value = 0.03). The results suggested that the T allele carriers might have a decreased risk of Graves’ disease compared with those individuals with the CC homozygote. Statistically similar results were obtained after sequential excluding each case–control study for the TT+TC vs. CC comparative, suggesting the stability of our meta-analysis. No publication bias was detected with either the funnel plot (figure not shown) or Egger’s test (t = 0.09, p-value = 0.929). Summary of the results of other genetic comparisons are listed in Table 3.
The CT60 polymorphism
A total of 818 cases and 1159 controls from 4 case–control studies were included for data synthesis. As is shown in Figure 3, we analyzed the heterogeneity of AA+AG vs. GG for all 4 studies and the value of X 2 was 0.91 with 3 degrees of freedom and p-value = 0.82 in a fixed-effects model. Additionally, I-square value is another index of the test of heterogeneity. In Figure 3, the I-square was 0%, suggesting an absent of heterogeneity. Thus, we chose the fixed-effects model to synthesize the data. Overall, OR was 0.64 (95%CI = 0.52-0.78) and the test for overall effect Z value was 4.34 (p-value = 0.001). The results suggested that the A allele carriers might have a decreased risk of Graves’ disease compared with those individuals with the GG homozygote. Statistically similar results were obtained after sequential excluding each case–control study for the AA+AG vs. GG comparative, suggesting the stability of our meta-analysis. No publication bias was detected with either the funnel plot (figure not shown) or Egger’s test (t = 0.19, p-value = 0.864). Summary of the results of other genetic comparisons are listed in Table 3.
Discussion
Graves' disease (GD) is a thyroid-specific autoimmune disease affecting 0.25–1.09% of the Chinese population [2]. To this day, the mechanisms of GD have been widely studied from the environmental factors to the genetic factors [29]. However, the results are inconsistent and the exact mechanisms are still unrevealed. Among genetic risk factors, the cytotoxic T lymphocyte associated-4 (CTLA-4) gene is one of the widely investigated. CTLA-4 gene, which encodes a vital negative regulatory molecule of the immune system [30], has been demonstrated as candidate gene of GD [31, 32]. To date, three polymorphisms (+49A/G, -318C/T and CT60) have been suggested as GD risk factors in the Chinese population. However, the results were inconsistent. Therefore, we performed a comprehensive meta-analysis to assess the association and to get more conclusive results.
This meta-analysis, including a total of 28 case–control studies in 21 publications, investigated three most widely studied polymorphisms in the CTLA-4 gene. We found that the +49A/G polymorphism was associated with an increased risk of GD in the Chinese population, and the G allele carriers might have a higher risk of disease than the AA homozygote carriers. The results suggested a significant association between this polymorphism in the Chinese population, which is consistent with some other populations, such as the UK population [33] and the Iranian population [34]. Our results indicated that the increase in the risk is more evident in the Chinese population than in other populations, suggesting possible roles of ethnic differences in genetic backgrounds and the environment. In addition, the +49A/G polymorphism is located in exon 1, and results in a threonine-to-alanine conversion at codon 17 in the peptide leader sequence of the CTLA-4 protein. It reported that this polymorphism was associated with lower mRNA levels of the soluble alternative splice form of CTLA-4 [35]. Thus, our results could be partly explained that the variant carriers might have lower mRNA levels of the protein of the CTLA-4, and then have increased risk of the disease. In future, more studies should be performed in the Chinese population to validate these results.
A total of 999 cases and 702 controls from 7 case–control studies were included for the -318C/T polymorphism. The results suggested that the T allele carriers might be associated with a decreased risk of GD compared with CC homozygote carriers. As for the CT60 polymorphism, 818 cases and 1159 controls from 4 case–control studies were included, and the results also indicated a decrease in the risk of GD. Considering the included case–control studies for both polymorphisms were relatively small, larger number of relevant studies are needed in future to validate these results.
Hitherto, many studies have already been published focusing on the genetic risk factors of the GD among the Chinese population. For instance, Chu reported that a non-synonymous single-nucleotide polymorphism rs40401 (P27S) of the interleukin 3 (IL3) gene was associated with increased risk of GD [36]; Guo found the rs568408 polymorphism in the interleukin-12 (IL-12) gene was also associated with increased risk of GD [37]. In addition, polymorphisms in the ADRB2 gene [3], interleukin-10 (IL-10) gene [38], TNF-α gene [39] were also found to be associated with GD in Chinese population. These genes were all suggested as the candidate genes for GD in Chinese population. In future, the associations between these polymorphisms and the GD risk in Chinese population are needed to be validated by more case–control studies.
In the present meta-analysis, sensitivity analysis was performed and stability of the results was guaranteed. Publication bias was assessed by Begg’s funnel plot and Egger’s test [40]. No significant publication bias was found for the -318C/T and the CT60 polymorphism analysis, suggesting the results of these two polymorphisms were more reliable. However, we found significant publication bias for the +49A/G polymorphism. The reason might be that some reports were not published, especially for those with negative results. The results might affect the strength of the association, thus, large scale case–control studies are needed to assess the association between the +49A/G polymorphism and GD risk.
We have to mention the heterogeneity. We found significant heterogeneity for the +49A/G polymorphism. Since all participants were Chinese, the genetic background might not be taken as a factor for the heterogeneity for +49A/G polymorphism. However, some other factors, such as gender, age and location might affect the heterogeneity. In addition, we found no heterogeneity for the -318C/T and the CT60 polymorphisms, which suggested that the association for these two polymorphisms are more reliable than the +49A/G polymorphism.
It is reported that GD occurs more frequently but less severe in women than in men. In China, the different condition of disease in men and women might be similar to the situation of the world. In our study, the data was not analyzed by gender because of the lack of original information for these populations. In future, such subgroup studies are also needed to be carried out. Moreover, the cases and controls in this meta-analysis were mostly based on Han nationality, but not in the minorities. In order to get comprehensive results of the Chinese population, studies based on the minorities are also needed.
There are several limitations in this meta-analysis. First, the quantity of enrolled published studies was not very ideal, especially for the -318C/T and CT60 polymorphism. This might cause some potential publication bias, although the results of the above mentioned bias tests was not significant for these two polymorphisms. Second, data were not stratified into subgroups according to some other factors such as age, gender, location and ethnicity (Han or others), due to the lack of information in the original studies. Third, the interactions between genetic factors and environmental factors were not discussed for these three polymorphisms. Fourth, the current meta-analysis only investigated the three most widely studied polymorphisms, and some other polymorphisms with fewer reports were not included. And in future, if there were more case–control studies, new meta-analysis should be conducted. Despite of these limitations, we have minimized the bias through the whole process based on means in study identification, data selection and statistical analysis as well as in the control of publication bias and sensitivity, and got a more reliable result.
Conclusions
To our knowledge, this is the first comprehensive genetic meta-analysis performed in Chinese population for Graves’ disease and CTLA-4 gene. We found that three polymorphisms (+49A/G, -318C/T and CT60) in the CTLA-4 gene were associated with the risk of GD. Our results supported the classic view that GD is associated with heredity and revealed that genes in the pathogenesis are important for GD. These results may have implications for further medicine researches about GD for the Chinese population. In future, more large-scale case–control studies are needed to validate our results.
References
Wen-Ling L, Rong-Hsing C, Hui-Ju L, Yu-Huei L, Wen-Chi C, Yuhsin T, Lei W, Fuu-Jen T: Toll-like receptor gene polymorphisms are associated with susceptibility to graves’ ophthalmopathy in Taiwan males. BMC Med Genet. 2010, 11: 154-10.1186/1471-2350-11-154.
Han SZ, Zhang SH, Li R, Zhang WY, Li Y: The common − 318C/T polymorphism in the promoter region of CTLA4 gene is associated with reduced risk of ophthalmopathy in Chinese Graves’ patients. Int J Immunogenet. 2006, 33 (4): 281-287. 10.1111/j.1744-313X.2006.00614.x.
Chu X, Dong Y, Shen M, Sun L, Dong C, Wang Y, Wang B, Zhang K, Hua Q, Xu S: Polymorphisms in the ADRB2 gene and Graves disease: a case–control study and a meta-analysis of available evidence. BMC Med Genet. 2009, 10 (1): 26-
Dechairo BM, Zabaneh D, Collins J, Brand O, Dawson GJ, Green AP, Mackay I, Franklyn JA, Connell JM, Wass JAH: Association of the TSHR gene with Graves’ disease: the first disease specific locus. Eur J Hum Genet. 2005, 13 (11): 1223-1230. 10.1038/sj.ejhg.5201485.
Zhao SX, Pan CM, Cao HM, Han B, Shi JY, Liang J, Gao GQ, Peng YD, Su Q, Chen JL: Association of the CTLA4 gene with Graves’ disease in the Chinese Han population. PLoS One. 2010, 5 (3): e9821-10.1371/journal.pone.0009821.
Hiromatsu Y, Fukutani T, Ichimura M, Mukai T, Kaku H, Nakayama H, Miyake I, Shoji S, Koda Y, Bednarczuk T: Interleukin-13 gene polymorphisms confer the susceptibility of Japanese populations to Graves’ disease. J Clin Endocrinol Metab. 2005, 90 (1): 296-301.
Mayans S, Lackovic K, Nyholm C, Lindgren P, Ruikka K, Eliasson M, Cilio C, Holmberg D: CT60 genotype does not affect CTLA-4 isoform expression despite association to T1D and AITD in northern Sweden. BMC Med Genet. 2007, 8 (1): 3-
Kotsa K, Watson PF, Weetman AP: A CTLA‐4 gene polymorphism is associated with both Graves’ disease and autoimmune hypothyroidism. Clin Endocrinol (Oxf). 1997, 46 (5): 551-554. 10.1046/j.1365-2265.1997.1710996.x.
Zhang YG, Li XB, Zhang J, Huang J, He C, Tian C, Deng Y, Wan H, Shrestha D, Yang YY: The I/D polymorphism of angiotensin‐converting enzyme gene and asthma risk: a meta-analysis. Allergy. 2011, 66 (2): 197-205. 10.1111/j.1398-9995.2010.02438.x.
Chong KK, Chiang SW, Wong GW, Tam PO, Ng TK, Hu YJ, Yam GH, Lam DS, Pang CP: Association of CTLA-4 and IL-13 gene polymorphisms with Graves’ disease and ophthalmopathy in Chinese children. Invest Ophthalmol Vis Sci. 2008, 49 (6): 2409-2415. 10.1167/iovs.07-1433.
Du YT, Zhang Q, Li M, Zhang P, Qiu MC: Study on the Relativity of Leukopenia and Cytotoxic T Lymphocyte Associated Antigen-4 Gene Polymorphism in Graves’Disease. Tian Jin Yi Yao (Chinese). 2005, 33 (10): 624-626.
Guo ZQ, Chen XM, Wu G, Su M, Jie YL, Wu MF, Wu XM: Association in the polymorphism of cytotoxic T lymphocyte antigen-4 gene and the hereditary susceptibility to Graves’ disease and Graves’ ophthalmopathy of Han population in western region of Guangdong province. Xian Dai Mian Yi Xue (Chinese). 2010, 30 (2): 151-154.
Jiang BR: Association of polymorphsim of CTLA-4 gene exon 1 with Graves’ disease and TCM syndrome differentiation in Chinese Shandong province population. 2005, http://d.wanfangdata.com.cn/Thesis_Y724586.aspx.
Kang YZ, Yang BZ, Wang LB, Wu RF, Shi ZY: Research on the correlation of gene polymorphism of cytotoxic T lymphocyte · associated antigen 4 and Graves’disease in Ningxia. Jian Yan Yi Xue (Chinese). 2010, 25 (4): 292-295.
Shen FX, Wang DW, Quan JX, Jiang L, Zhang HL, Feng WH: Relationship between of thyroid associated ophthalmopathy and T-lymphocyte-associated antigen-4 gene A/G polymorphism at position 49 in exon 1. Wen Zhou Yi Xue Yuan Xue Bao (Chinese). 2005, 35 (5): 356-358.
Tsai ST, Huang CY, Lo FS, Chang YT, Tanizawa T, Chen CK, Wang ZC, Liu HF, Chu CC, Lin M: Association of CT60 polymorphism of the CTLA4 gene with Graves’ disease in Taiwanese children. J Pediatr Endocrinol Metab. 2008, 21 (7): 665-672.
Wang L, Yu HW, Yan SL, Zhao SH, Wang YG, Wang P: The association of cytotoxic T lymphocyte-associated antigen 4 gene polymorphism with type 1 diabetes mellitus and autoimmune thyroid disease in Chinese Han population. Zhong Hua Nei Fen Mi Dai Xie Za Zhi (Chinese). 2001, 17 (4): 228-231.
Wang PW, Chen IY, Liu RT, Hsieh CJ, Hsi E, Juo SH: Cytotoxic T lymphocyte-associated molecule-4 gene polymorphism and hyperthyroid Graves’ disease relapse after antithyroid drug withdrawal: a follow-up study. J Clin Endocrinol Metab. 2007, 92 (7): 2513-2518. 10.1210/jc.2006-2761.
Wang QH, Ren YZ: Association of polymorphism of CTLA-4 gene exon 1 with Graves’ disease in Chinese population. Zhong Hua Nei Fen Mi Dai Xie Za Zhi (Chinese). 2003, 19 (4): 297-299.
Wang SQ, Gao JD, Zhang HL: Association of CTLA-4 gene exon 1 polymorphism with Graves’ disease in Han population. Qing Hai Yi Xue Yuan Xue Bao (Chinese). 2010, 31 (4): 225-227.
Weng YC, Wu MJ, Lin WS: CT60 single nucleotide polymorphism of the CTLA-4 gene is associated with susceptibility to Graves’ disease in the Taiwanese population. Ann Clin Lab Sci. 2005, 35 (3): 259-
Yang J, Qin Q, Yan N, Zhu YF, Li C, Yang XJ, Wang X, Pandey M, Hou P, Zhang JA: CD40 C/T(−1) and CTLA-4 A/G(49) SNPs are associated with autoimmune thyroid diseases in the Chinese population. Endocrine. 2012, 41 (1): 111-115. 10.1007/s12020-011-9510-1.
Yao B, Hao LM, Yan JH, Weng JP, Li YB: Association between the CTLA-4 gene polymorphism and Graves’disease in the Southern Chinese Han population. Zhong Hua Nei Fen Mi Dai Xie Za Zhi (Chinese). 2006, 22 (4): 363-364.
Yu QL, Chen DY, Xiao ZH, Wang Y: Association of polymorphism of CTLA-4 gene exon 1 with Graves’ disease in Cantonese Han population. Jie Pou Xue Yan Jiu (Chinese). 2006, 28 (4): 278-280.
Yu ZY, Zhang JA, Maier HB, Wang Y, Xiao WX, Quan Y, Dong BN: Assoc ia tion of polymorphism of prote in tyrosine phosphatase nonreceptor222 gene with AITD. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi (Chinese). 2008, 24 (8): 804-807.
Zhang H, Yan SL, Wang L, Qi YQ: Relatio nship between CTLA-4 gene promoter region −318 site C/T polymorphism and the leukopenia caused by antithyroid drug. Mian Yi Xue Za Zhi (Chinese). 2010, 26 (12): 1070-1073.
Zhang JL, Yan SL: CTLA-4 gene 49A/G polymorphism is associated with relapse of Graves’ disease. Zhong Hua Nei Fen Mi Dai Xie Za Zhi (Chinese). 2008, 24 (2): 192-193.
Zhang Q, Yang Y, Lv X: Association of Graves’ disease and Graves’ ophthalmopathy with the polymorphisms in promoter and exon 1 of cytotoxic T lymphocyte associated antigen-4 gene. J Zhejiang University-Sci B. 2006, 7 (11): 887-891. 10.1631/jzus.2006.B0887.
Marissa PM, Elizabeth RL, Inka R, Heinrich K, Stefanie H, Holger W, Christian S, Maria S, Klaus B: The rs1990760 polymorphism within the IFIH1 locus is not associated with Graves’ disease. Hashimoto’s thyroiditis and Addison’s disease. BMC Med Genet. 2009, 10: 126-10.1186/1471-2350-10-126.
Braun J, Donner H, Siegmund T, Walfish PG, Usadel KH, Badenhoop K: CTLA‐4 promoter variants in patients with Graves’ disease and Hashimoto’s thyroiditis. Tissue Antigens. 1998, 51 (5): 563-566. 10.1111/j.1399-0039.1998.tb02993.x.
Ueda H, Howson JMM, Esposito L, Heward J, Snook GC, Rainbow DB, Hunter KMD, Smith AN, Di Genova G, Herr MH: Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003, 423 (6939): 506-511. 10.1038/nature01621.
Jacobson EM, Tomer Y: The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future. J Autoimmun. 2007, 28 (2): 85-98. 10.1016/j.jaut.2007.02.006.
Vaidya B, Oakes E, Imrie H, Dickinson AJ, Perros P, Kendall Taylor P, Pearce S: CTLA4 gene and Graves’ disease: association of Graves’ disease with the CTLA4 exon 1 and intron 1 polymorphisms, but not with the promoter polymorphism. Clin Endocrinol (Oxf). 2003, 58 (6): 732-735. 10.1046/j.1365-2265.2003.01778.x.
Esteghamati A, Khalilzadeh O, Mobarra Z, Anvari M, Tahvildari M, Amiri HM, Rashidi A, Solgi G, Parivar K, Nikbin B: Association of CTLA-4 gene polymorphism with Graves’ disease and ophthalmopathy in Iranian patients. Eur J Intern Med. 2009, 20 (4): 424-428. 10.1016/j.ejim.2008.12.005.
Chang MC, Chang YT, Tien YW, Liang PC, Jan IS, Wei SC, Wong JM: T-cell regulatory gene CTLA-4 polymorphism/haplotype association with autoimmune pancreatitis. Clin Chem. 2007, 53 (9): 1700-1705. 10.1373/clinchem.2007.085951.
Chu X, Dong C, Lei R, Sun L, Wang Z, Dong Y, Shen M, Wang Y, Wang B, Zhang K: Polymorphisms in the interleukin 3 gene show strong association with susceptibility to Graves’ disease in Chinese population. Genes Immun. 2009, 10 (3): 260-266. 10.1038/gene.2009.3.
Guo T, Yang S, Liu N, Wang S, Cui B, Ning G: Association study of interleukin‐12A gene polymorphisms with Graves’ disease in two Chinese populations. Clin Endocrinol (Oxf). 2011, 74 (1): 125-129. 10.1111/j.1365-2265.2010.03905.x.
Liu N, Lu H, Tao F, Guo T, Liu C, Cui B, Ning G: An association of interleukin-10 gene polymorphisms with Graves’ disease in two Chinese populations. Endocrine. 2011, 40 (1): 90-94. 10.1007/s12020-011-9444-7.
Pan TR, Xing SM: Association of TNF-α gene polymorphisms with Graves disease susceptibility and early course thyroid stimulating hormone receptor antibody level in Chinese Han population in Anhui region. Zhonghua Yi Xue Yi Chuan Xue Za Zhi (Chinese). 2012, 29 (3): 347-351.
Matsuda A, Kishi T, Jacob A, Aziz M, Wang P: Association between insertion/deletion polymorphism in angiotensin-converting enzyme gene and acute lung injury/acute respiratory distress syndrome: a meta-analysis. BMC Med Genet. 2012, 13 (1): 76-
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2350/14/46/prepub
Acknowledgements
This study was supported by grants 81101939 from National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LD designed the research. JH and JQY searched the publications, extracted the data and wrote the article. YGZ checked all data. JCH and ZPX was responsible for data synthesis and helped designed the study’s analytic strategy. YGZ and LD edited the manuscript. YXM, TYX and HCW revised the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Du, L., Yang, J., Huang, J. et al. The associations between the polymorphisms in the CTLA-4gene and the risk of Graves’ disease in the Chinese population. BMC Med Genet 14, 46 (2013). https://doi.org/10.1186/1471-2350-14-46
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2350-14-46