Abstract
Background
Relatively few studies have been done in Tanzania to detect and classify diarrheagenic Escherichia coli (DEC) strains among children with diarrhea. This study aimed at investigating DEC among children in Dar es Salaam aged less than five years hospitalized due to acute/persistent diarrhea.
Methods
DEC were isolated from stool samples collected from two hundred and eighty children with acute/persistent diarrhea at Muhimbili National Hospital and Ilala and Mwananyamala Municipal Hospitals in Dar es Salaam. A multiplex PCR system method was used to detect a species specific gene for E.coli and ten different virulence genes for detection of five pathogroups of DEC namely enteroaggregative- (EAEC), enteropathogenic- (EPEC), enterotoxigenic- (ETEC), enteroinvasive- (EIEC) and enterohemorghagic- Escherichia coli (EHEC).
Results
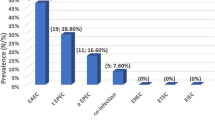
Sixty-four patients (22.9%) harbored DEC. Forty-one of them (14.6%) were categorized as EAEC. Most of the EAEC (82.9%) were classified as typical EAEC possessing the aggR gene, and 92.6% carried the aat gene. Isolates from thirteen patients were EPEC (4.6%) and most of these (92.3%) were typical EPEC with both eae and bfpA genes. Ten isolates were identified as ETEC (3.6%) with only the heat stable toxin; either st1a or st1b but not both. Age wise, EAEC and EPEC were significantly more prevalent among the age group 0–6 months (p < 0.05). Genes for EHEC (stx 1 and stx 2) and EIEC (ial) were not detected in this study group.
Conclusion
The results show a high proportion of DEC among Tanzanian children with diarrhea, with typical EAEC and typical EPEC predominating. The use of primers for both variants of ST1 (st1a and st1b) increased the sensitivity for detection of ETEC strains.
Similar content being viewed by others
Background
Diarrhea is one of the leading causes of morbidity and mortality among children under five years in the developing world [1]. During the period from 1950 to 1970s it was estimated that 4.6 million children died annually from diarrhea in developing world [2, 3]. Mortality due to diarrhea declined to approximately 3.3 million annually in the 1980s [1, 3]. Currently diarrhea has been reported to account for 1.6–2.5 million deaths annually [3, 4]. Despite the decline in mortality, diarrhea still remains one of the principal causes of morbidity in the developing world, with each child experiencing an average of three episodes of diarrhea per year[4]. In these countries, diarrheal diseases are the second most common illness of children after acute respiratory illness[5]. The causes of diarrhea include a wide range of viruses, bacteria, and parasites[6]. Among the bacterial causes diarrheagenic Escherichia coli (DEC) is the most important etiologic agent of childhood diarrhea and represents a major public health problem in developing countries[7]. Identification of DEC strains requires that these organisms be differentiated from non-pathogenic members that constitute normal intestinal flora. Molecular identification and classification of DEC is based on the presence of different chromosomal or plasmid-encoded virulence genes, which are absent in the commensal E.coli. Further features that supplement such categorization include the effects produced by the proteins encoded by these virulence genes; the pattern of their interaction with intestinal epithelial cells and tissue culture monolayers [7]. DEC strains can be divided into six main categories on the basis of distinct molecular, clinical and pathological features[7]: enteroaggregative E. coli (EAEC), enterohemorrhagic (Shiga-toxin producing E. coli (EHEC/STEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC) and diffusely adherent E.coli (DAEC). Thus, identification of different types of DEC includes biochemical reactions, serotyping, phenotypic assays based on virulence characteristics and molecular detection methods[7]. Among these, detection of specific virulence genes by polymerase chain reaction (PCR) is frequently used because this method gives rapid, reliable results with a high sensitivity and a high specificity[8, 9]. The epidemiological significance of each DEC category in childhood diarrhea varies with geographical area. Few studies have been done in Tanzania to detect DEC strains among children with diarrhea [5, 10]. One recent study has been done in Ifakara, Tanzania, which is 500 km from Dar es Salaam [5]. In Dar es Salaam only one study was conducted ten years ago to detect DEC using the DNA probe methods [10]. It is known that DNA probes tend to have a lower sensitivity and specificity than the PCR-based method [11]. Furthermore, the study included children with chronic diarrhea only, even though acute diarrhea is more predominant among children in Tanzania [5]. The aim of this study is to report epidemiological data of the different categories of DEC, 10 years after the previous one, in children with both acute and persistent diarrhea, aged less than five years, in Dar es Salaam, Tanzania and using a more reliable PCR-based method.
Methods
Study design, population and settings
This was a prospective cross-sectional study that was conducted in Dar es Salaam, Tanzania between December 2005 and February 2006. Participants were children ≤ 5 years, who during the study period, were admitted due to diarrhea at Muhimbili National Referral Hospital (MNH) and Amana, Mwananyamala, and Temeke Municipal Hospitals in Dar es Salaam, Tanzania. Enrolment was subject to obtaining an informed verbal consent from parent or guardian who accompanied the child.
Interviews
A standard structured questionnaire was used to obtain information of the children from the parents/guardians. Information that was sought included age, sex, duration and description of the stool, as watery, mucoid, or bloody. Diarrhea was defined, according WHO guidelines [12], as the occurrence of three or more, loose, liquid, or watery stools within 24 hours. The guidelines stipulate three forms of diarrhea namely: i) acute watery diarrhea defined as diarrhea that begun acutely and lasted less than or equal to 13 days, ii) dysentery defined as mucoid bloody stool associated with anorexia, abdominal cramps, and tenesmus and iii) persistent diarrhea defined as diarrhea with a duration of 14 or more days. Information was also sought regarding the use of antibiotics prior to hospitalization.
Weight measurements
Infants under two years of age were weighed using a 25 kg Salter hanging scales (CMS Weighing equipment, High Holborn, London, United Kingdom). Children over two years were weighed on scales calibrated before each session. Weight of children was recorded to the nearest kilogram.
Determination of nutritional status
Weight-for age Z-scores were calculated using EPI Info (USD, Inc., Stone Mountain, GA). According to WHO criteria Children were considered to be undernourished if the Z-scores were less than -2SD[13].
Collection and transportation of stool
Stool specimens from enrolled children were collected using wide mouthed sterile plastic containers and transported immediately to the microbiology laboratory for analysis within two hours of collection.
Bacteriological procedures
Samples were plated on MacConckey Agar (MCA), Xylose lysine Deoxycholate (XLD), Salmonella Shigella Agar (SSA), Thiosulphate Citrate Bile-salt Sucrose Agar (TCBS) (Remel Microbiology Products, Lenexa, KS) and incubated aerobically at 37°C overnight for the isolation of E.coli, V.cholerae, Shigella species, and Salmonella species. Red colonies on MCA which were Gram-negative rods and oxidase negative were provisionally identified as E.coli by a positive indole test. Since there is a possibility of picking up a commensal non-diarrheagenic colony of E.coli, three to five colonies of the bacterium from the primary streak of each fecal sample were sub-cultured on a nutrient agar slant for later analysis for DEC.
Detection of virulence factors of diarrheagenic E.coli
All species provisionally identified as E.coli were further examined for the presence of an E.coli specific uidA gene and for genes coding for virulence factors of ETEC, EPEC, EHEC, EIEC and EAEC using a multiplex PCR system method as described previously [9, 14] but with an annealing temperature of 55°C. A sweep of growth on nutrient agar slants was used for the PCR.
To extract DNA a sweep of growth on a nutrient agar slant were boiled in 500 μL of sterile distilled water for 10 minutes. Then centrifugation was done at 13000 rpm for 5 minutes to pellet the cell debris. 1.0 μL of the supernatant was used as template for the PCR amplification. Positive and negative controls were used with each PCR set up. The positive controls were either reference strains (uidA gene; E.coli ATCC 43889, eae and bfpA genes (EPEC); ATCC 43887,elt and st1a genes, (ETEC); ATCC 35401, and stx 1 and stx 2 (EHEC); ATCC 43889) or strains known to posses the target genes st1b gene (ETEC), ial gene (EIEC) and aat and aggR genes (EAEC), verified by sequencing of the amplified genes. Sterile distilled water was used as a negative control.
For the detection of ETEC, EPEC, EIEC, and EHEC initially three assays, n1, n2 and n3 were used as published earlier with some modifications [9]. Assay n1 utilized primer pairs for genes coding for heat stable toxin ST (stIa) and heat labile toxin LT (elt) of ETEC, and (uidA) for the E.coli β-glucuronidase. Assay n2 detected the presence of the eae (structural gene for intimin found in EPEC) and bfpA (structural gene for the bundle-forming pilus) of EPEC, and assay n3 screened for stx 1 and stx 2 of shiga toxin producing EHEC and ial (invasion-associated locus of the invasion plasmid) found in EIEC/Shigella.
Primers of a variant gene st1b coding for the heat stable toxin of ETEC were subsequently included in a separate tube in assay n4 together with uidA gene. Two plasmid genes of EAEC namely aat and aggR were detected simultaneously in assay n5. Table 1 shows the primer nucleotide sequence used and the predicted length of the amplicon for each primer pair.
PCR was carried out with 1.0 μL of the template added to 24 μL mix containing 10 picomoles of each primer and HotStarTaq Master Mix (QIAGEN, Norway, containing HotStarTaq DNA polymerase, PCR Buffer (with 3 mM MgCl2), and 400 μM of each dNTP). Cycling conditions were initial Hotstar Taq DNA polymerase enzyme activation 95°C for 15 minutes, followed by 30 cycles of denaturation at 94°C for 1 minute, annealing at 55°C for 1 minute, extension at 72°C for 1 minute and final extension at 72°C for 10 minutes.
The amplicons were checked by gel electrophoresis and their size determined by using 100 bp DNA molecular weight marker XIV (Roche Diagnostics). The gel electrophoresis was set at 125 V and run for 1 hour and 15 minutes. The products were visualized using a UV transilluminator.
Interpretation of results
Only the presence of the amplification product with correct sized was interpreted as a positive test (table 1). The presence of uidA gene was regarded as confirmation of E.coli identification and served as an internal PCR control.
Ethical considerations
Ethical and research clearance was obtained from the Higher Degree Research and Publication Committee of the Muhimbili University College of Health Sciences in Dar es Salaam, Tanzania. Permission to conduct the study was sought from the respective hospital authorities and the Dar es Salaam City Medical Officer. Informed verbal consent was obtained from parents/guardians of the child before enrolment into the study. The following information was given to ensure that the parents/guardians have the information needed to make an informed choice: a complete description of the aims of the study, and the potential benefits and risks, if any. Study personnel provided any other requested additional information. Children were treated by their attending clinicians according to integrated management of childhood infections (IMCI) guidelines [12] where rehydration therapy is the mainstay of treatment. Antibiotics were given to suspected cases of cholera, dysentery and persistent diarrhea, in accordance with the antimicrobial sensitivity results of bacterial pathogens, namely, Salmonella, Shigella and Vibrio cholerae. All patients' information and test results were confidentially kept.
Data analysis
The Statistical Package for the Social Sciences (SPSS 10.0) was used for statistical analysis. Assuming the data follows a normal distribution, comparison of proportions and statistical significance were tested by using the Chi-square test. A p value less than 0.05 was considered statistically significant.
Results
During the study period from December 2005 to February 2006 a total of 280 children with diarrhea were included in this study. These children were aged between 0 and 60 months, with most of them (90.4%) being below 24 months (Mean 12.9 months). DEC were detected in 64 patients (22.9%).
Of the DEC, EAEC were the most prevalent, accounting for 14.6% of all cases of diarrhea. Of a total of 41 isolates of EAEC, 34 (82.9% of EAEC) were classified as typical EAEC, harboring the aggR gene, of which 31 isolates had aat gene in addition. A total of 38 (92.7%) were aat gene positive (Table 2). Table 3 shows the association between different categories of DEC with social demographic data, nutritional status, breast-feeding behavior and type of diarrhea. Thirty-five (85.4%) of the 41 children with EAEC presented with acute watery diarrhea, while five (12.2%) and one (2.4%) presented with persistent diarrhea and dysentery respectively. There were a total of 13 EPEC (4.6% of diarrhea cases), and 12 (92.3%) were typical EPEC with both eae and bfpA genes and one strain was atypical with only the eae gene. Among children harboring EPEC, ten (76.9%) had acute watery and three (23.1%) had persistent diarrhea. ETEC were found in 10 patients (3.6%of all the diarrhea cases). None of the ETEC strains had labile toxin. All ETEC strains had either stable toxins stIa or st1b but not both. Six (60.0%), two (20.0%) and two (20.0%) of the children with ETEC had acute watery diarrhea, persistent diarrhea and dysentery, respectively. Nine patients had mixed infections; three of them harbored ETEC and EAEC, two harbored EPEC and EAEC three patients harbored ETEC and Shigella spp, while one harbored EAEC and Shigella spp, (Table 2).
There were no EIEC or EHEC strains detected in this study group. EAEC and EPEC were significantly more prevalent among the age group 0–6 month's (p < 0.05). Of these children below six months of age, 88.2% were not on exclusive breast-feeding. Seventy nine children were malnourished and of these, 20.3% and 3.0% harbored EAEC and ETEC strains of DEC respectively.
Discussion
Our results show a high proportion of EAEC accounting for 64.1% of DEC. This study was conducted during the dry season of the year and these findings are in agreement with a previous study among children of Ifakara Tanzania in which, EAEC accounted 63% of the DEC during dry season [5] and with other studies in other developing countries [15–18]. Collectively, these studies seem to suggest the predominance of EAEC among DEC in causing diarrhea in children in developing countries. It is worthy noting that the proportion EAEC among children aged less than six months is significantly higher than in older children (p < 0.05), which is in agreement with the finding of Gonzalez et al [19]. However, we noted age differences when our results are compared with findings of other countries, with a higher prevalence of EAEC in infants aged less than six months [15, 17]. In these studies most of the children younger than six months were exclusively breast fed. Correspondingly the fact that most children in the present study (88.2%) were not exclusively breast fed may explain the discrepancies.
In the present study, the plasmid-borne genes aggR and aat were used to detect EAEC. The pathogenic mechanisms of EAEC infection are only partially understood. The varying presence of the different virulence factors indicates heterogeneity of the EAEC isolates [15]. It has been hypothesized that the combination of these genes increases strain virulence. The aggR gene is a transcriptional activator gene required for the expression of aggregative adherence fimbria I gene (aagA) [20, 21] and was subsequently also shown to be required for aggregative adherence fimbria II gene (aafA) expression [22]. More recently, aggR has been shown to be required for the expression of the antiaggregation protein (dispersin) gene aap (previously called aspU) [16] and an anti-aggregation protein transporter gene aatA (previously called CVD432 or AA probe) [23] genes. Therefore, it is likely that the aggR gene serves as a marker for truly virulent EAEC strains, which causes expression of a package of virulence genes. Nataro has recently suggested the term "typical EAEC" to refer to strains expressing the aggR regulon [24]. Our data shows a high prevalence of typical EAEC than atypical among diarrheic children of Dar es Salaam which is in agreement with the findings of Sarantuya et al [16]. Other epidemiological studies have suggested greater pathogenicity of aat-positive strains than of aat-negative strains [25].
In the present study EPEC was the second most common DEC isolated and most of these were typical EPEC with both eae and bfpA genes. Typical EPEC is well recognized as a cause of gastroenteritis in infants[7]. In our study we detected only one strain of atypical EPEC in a child with diarrhea in contrast with the finding of other study in Ifakara Tanzania who found a much higher percentage of atypical EPEC [5]. The discrepancy can be, at least partly, attributed to geographical differences. The role of atypical EPEC in childhood diarrhea still remains controversial [26, 27].
ETEC in the present study accounted for 15.6% of the DEC, which is high compared with the finding of Cegielski et al (11.9%,1996) in Dar es Salaam Tanzania [10]. However, our findings were lower than those reported from Ifakara, Tanzania (20% and 51.6% during dry and rainy seasons, respectively [5]. These differences could possibly be related with seasonality as well as methodological issues. It is known that ETEC strains cause diarrhea through the action of the enterotoxins LT (labile toxin) and ST (stable toxin) and that there are two distinct classes of STs that differ in structure and mechanism of action STI and STII [25]. There are two variants of STI designated st1a (STp porcine) and st1b (STh human), both variants can be found in human ETEC strains [7, 28]. Unlike most studies where ETEC strains were detected by looking for only one variant of ST [5, 29], in our study, ETEC was detected by looking for both variants of STI. Detection of both variants of STI increases sensitivity detecting ETEC. This argument is supported by our findings showing that detection of ETEC would have had a decreased sensitivity of 50% if only either of the gene variants of ST1 had been screened for. Furthermore, it was noted that all the ETEC strains harbored only the heat-stable (ST) enterotoxin genes but not the heat-labile enterotoxin (LT) genes. Our findings showing a higher prevalence of ST producing than LT producing ETEC are in keeping with the observations of Vargas et al [5] in Ifakara, Tanzania and [5] and of other studies conducted elsewhere [29, 30]. Collectively, these studies seem to suggest a greater association between ST-producing strains and diarrhea than LT producing ETEC.
Finally, we did not find EHEC and EIEC strains, which is in agreement with the previous study in Tanzania salaam [5, 10], indicating their limited role in childhood diarrhea in Tanzania.
Conclusion
Our results show a high proportion of DEC, where typical EAEC and typical EPEC predominate among Tanzanian children with diarrhea. We also show that the use of primers (st1a and st1b) for both variants of ST1 increases the sensitivity for detection of ETEC strains.
References
Bern C, Martines J, de Zoysa I, Glass RI: The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull World Health Organ. 1992, 70 (6): 705-714.
Snyder JD, Merson MH: The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull World Health Organ. 1982, 60 (4): 605-613.
Parashar U, Umesh D, Bresee JS, Joseph S, Glass RI, Roger I: The global burden of diarrhoeal disease in children. Bulletin of the World Health Organization. 2003, 81 (4): 236-
Kosek M, Bern C, Guerrant RL: The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003, 81 (3): 197-204.
Vargas M, Gascon J, Casals C, Schellenberg D, Urassa H, Kahigwa E, Ruiz J, Vila J: Etiology of diarrhea in children less than five years of age in Ifakara, Tanzania. Am J Trop Med Hyg. 2004, 70 (5): 536-539.
Guerrant RL, Hughes JM, Lima NL, Crane J: Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Rev Infect Dis. 1990, 12 (Suppl 1): S41-50.
Nataro JP, Kaper JB: Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998, 11 (1): 142-201.
Stacy-Phipps S, Mecca JJ, Weiss JB: Multiplex PCR assay and simple preparation method for stool specimens detect enterotoxigenic Escherichia coli DNA during course of infection. J Clin Microbiol. 1995, 33 (5): 1054-1059.
Rappelli P, Maddau G, Mannu F, Colombo MM, Fiori PL, Cappuccinelli P: Development of a set of multiplex PCR assays for the simultaneous identification of enterotoxigenic, enteropathogenic, enterohemorrhagic and enteroinvasive Escherichia coli. New Microbiol. 2001, 24 (1): 77-83.
Cegielski JP, Msengi AE, Dukes CS, Levine MM: Pathogenic Escherichia coli in children with and without chronic diarrhea in Tanzania. J Infect Dis. 1996, 174 (3): 675-677.
Scaletsky ICA, Fabbricotti SH, Aranda KR, Morais MB, Fagundes-Neto U: Comparison of DNA hybridization and PCR assays for detection of putative pathogenic enteroadherent Escherichia coli. J Clin Microbiol. 2002, 40: 1254-1258. 10.1128/JCM.40.4.1254-1258.2002.
IMCI Integrated Mangagement of Childhood Illness. Model Chapter for Textbooks. Document no WHO/FCH/CAH/00.40. 2001, Geneva: World Health Organization
Waterlow JC, Buzina R, Keller W, Lane TM, Nichman MZ, Taner JM: The presentation and use of height and weight data for comparing nutritional status of groups of children under age of 10 years. Bull Wld Hlth Org. 1977, 55 (4): 489-498.
Cerna JF, Nataro JP, Estrada-Garcia T: Multiplex PCR for detection of three plasmid-borne genes of enteroaggregative Escherichia coli strains. J Clin Microbiol. 2003, 41 (5): 2138-2140. 10.1128/JCM.41.5.2138-2140.2003.
Okeke IN, Lamikanra A, Czeczulin J, Dubovsky F, Kaper JB, Nataro JP: Heterogeneous virulence of enteroaggregative Escherichia coli strains isolated from children in Southwest Nigeria. J Infect Dis. 2000, 181 (1): 252-260. 10.1086/315204.
Sarantuya J, Nishi J, Wakimoto N, Erdene S, Nataro JP, Sheikh J, Iwashita M, Manago K, Tokuda K, Yoshinaga M, et al: Typical enteroaggregative Escherichia coli is the most prevalent pathotype among E. coli strains causing diarrhea in Mongolian children. J Clin Microbiol. 2004, 42 (1): 133-139. 10.1128/JCM.42.1.133-139.2004.
Presterl E, Zwick RH, Reichmann S, Aichelburg A, Winkler S, Kremsner PG, Graninger W: Frequency and virulence properties of diarrheagenic Escherichia coli in children with diarrhea in Gabon. Am J Trop Med Hyg. 2003, 69 (4): 406-410.
Savarino SJ: Diarrhoeal disease: current concepts and future challenges. Enteroadherent Escherichia coli: a heterogeneous group of E. coli implicated as diarrhoeal pathogens. Trans R Soc Trop Med Hyg. 1993, 87 (Suppl 3): 49-53.
Gonzalez R, Diaz C, Marino M, Cloralt R, Pequeneze M, Perez-Schael I: Age-specific prevalence of Escherichia coli with localized and aggregative adherence in venezuela infants with acute diarrhea. J Clin Microbiol. 1997, 35: 1103-7.
Nataro JP, Deng Y, Maneval DR, German AL, Martin WC, Levine MM: Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect Immun. 1992, 60 (6): 2297-2304.
Nataro JP, Yikang D, Yingkang D, Walker K: AggR, a transcriptional activator of aggregative adherence fimbria I expression in enteroaggregative Escherichia coli. J Bacteriol. 1994, 176 (15): 4691-4699.
Elias WP, Czeczulin JR, Henderson IR, Trabulsi LR, Nataro JP: Organization of biogenesis genes for aggregative adherence fimbria II defines a virulence gene cluster in enteroaggregative Escherichia coli. J Bacteriol. 1999, 181 (6): 1779-1785.
Nishi J, Sheikh J, Mizuguchi K, Luisi B, Burland V, Boutin A, Rose DJ, Blattner FR, Nataro JP: The export of coat protein from enteroaggregative Escherichia coli by a specific ATP-binding cassette transporter system. J Biol Chem. 2003, 278 (46): 45680-45689. 10.1074/jbc.M306413200.
Harrington SM, Dudley EG, Nataro JP: Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol Lett. 2006, 254 (1): 12-18. 10.1111/j.1574-6968.2005.00005.x.
Elias WP, Uber AP, Tomita SK, Trabulsi LR, Gomes TA: Combinations of putative virulence markers in typical and variant enteroaggregative Escherichia coli strains from children with and without diarrhoea. Epidemiol Infect. 2002, 129 (1): 49-55. 10.1017/S0950268802007136.
Afset JE, Bergh K, Bevanger L: High prevalence of atypical enteropathogenic Escherichia coli (EPEC) in Norwegian children with diarrhoea. J Med Microbiol. 2003, 52 (11): 1015-1019. 10.1099/jmm.0.05287-0.
Afset JE, Bevanger L, Romundstad P, Bergh K: Association of atypical enteropathogenic Escherichia coli (EPEC) with prolonged diarrhoea. J Med Microbiol. 2004, 53 (11): 1137-1144. 10.1099/jmm.0.45719-0.
Moseley SL, Hardy JW, Hug MI, Echeverria P, Falkow S: Isolation and nucleotide sequence determination of a gene encoding a heat-stable enterotoxin of Escherichia coli. Infect Immun. 1983, 39 (3): 1167-1174.
Okeke IN, Lamikanra A, Steinruck H, Kaper JB: Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J Clin Microbiol. 2000, 38 (1): 7-12.
Gunzburg ST, Chang BJ, Burke V, Gracey M: Virulence factors of enteric Escherichia coli in young Aboriginal children in north-west Australia. Epidemiol Infect. 1992, 109 (2): 283-289.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/7/92/prepub
Acknowledgements
We would like to thank the administration of the Muhimbili National Hospital, Ilala, Mwananyamala and Temeke Municipal Hospitals for giving necessary administrative support. We would also like to thank the parents/guardians of the children participated in this study, without whom this study would have not been possible. We acknowledge the technical support accorded to this study by members of the Departments of Microbiology and Immunology of the Muhimbili University College of Health Sciences (MUCHS) in Dares Salaam, Tanzania and Haukeland University Hospital in Bergen, Norway. We are grateful to the Haukeland University Hospital, Norwegian Public Health Institute and Staten Serum Institute, Denmark, for providing the positive controls strains. We would also like to acknowledge Jan Egil Afset at St. Olavs Hospital, Trondheim, Norway, for useful information and advice on EAEC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
SJM was the principal investigator, who conceived and designed study and was responsible for collection of specimens and clinical information as well as data analysis. Laboratory investigations were performed by SJM, the molecular biological part under the guidance of HM. SYM, MIM, NL and HM assisted in the development of the research proposal, data analysis and preparation of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Moyo, S.J., Maselle, S.Y., Matee, M.I. et al. Identification of diarrheagenic Escherichia coli isolated from infants and children in Dar es Salaam, Tanzania. BMC Infect Dis 7, 92 (2007). https://doi.org/10.1186/1471-2334-7-92
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-7-92