Abstract
Background
The effects of HAART may differ between children and adults because children have a developing immune system, and the long-term immunological outcome in HIV-infected children on HAART is not well-known. A major aim of our study was to determine CD4+ evolution associated with long-term VL control during 4 years of observation on HAART.
Methods
We carried out a retrospective study on a cohort of 160 vertically HIV-infected children. It was carried out from 1996 to 2004 in six large Spanish pediatric referral hospitals. We compared 33 children who had long-term VL suppression (VL ≤400 copies/ml) in the first 12 months of follow-up and maintained that level throughout follow-up (Responders-group), and 127 children with persistently detectable VL in spite of ART switches (Non-Responders-group).
Results
We observed a quick initial and significant increase in CD4+ counts from the baseline to 12 months on HAART in both groups (p < 0.01). The Non-Responders group sustained CD4+ increases and most of these children maintained high CD4+ level counts (≥25%). The Non-Responders group reached a plateau between 26% and 27% CD4+ at the first 12 months of follow-up that remained stable during the following 3 years. However, the Responders group reached a plateau between 30% and 32% CD4+ at 24, 36 and 48 months of follow-up. We found that the Responders group had higher CD4+ count values and higher percentages of children with CD4+ ≥25% than the Non-Responders group (p < 0.05) after month 12.
Conclusion
Long-term VL suppression in turn induces large beneficial effects in immunological responses. However, it is not indispensable to recover CD4+ levels.
Similar content being viewed by others
Background
The efficacy of highly active antiretroviral therapy (HAART) is shown by the fact that many patients achieve suppression of viral load (VL) below the limits of detection (uVL) along with an increase of CD4+ T-lymphocytes (CD4+) [1, 2], resulting in a good clinical outcome [3, 4]. Immediate suppression of VL is often achieved with HAART, but long-term suppression of VL is not always feasible [1]. Children receiving treatment usually have higher VL and lower virologic response rates than adults [1, 5]. Moreover, the effects of HAART may differ between children and adults because children have a developing immune system, but the long-term immunological outcome in HIV-infected children on HAART is not well-known [6]. A major aim of our study was to determine CD4+ evolution associated with long-term VL control.
Methods
Population and study design
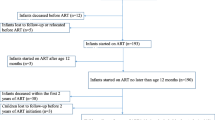
A retrospective study on a cohort of 160 vertically HIV-infected children has been carried out from 1996 to 2004 in six large Spanish pediatric referral hospitals. The inclusion criteria of HIV-1-infected children were the following: a) starting HAART with protease inhibitor (PI) or non-nucleoside analogue HIV-1 reverse transcriptase inhibitor (NNRTI); b) 4 years of follow-up after starting HAART; c) VL >5,000 copies/ml at entry to the study, d) older than 6 months of age at entry. From an initial cohort of 200 vertically HIV-1-infected children with at least 4 years on HAART, 40 children were excluded because they had VL <5,000 copies/ml (30 children), no data regarding VL (1 child), or they were less than 6 months old (9 children). Therefore, 160 out 200 HIV-infected children fulfilled the criteria of inclusion and were enrolled in the present retrospective study.
This study was approved by the Ethical Committees of all hospitals involved. Clinical classification was based on the 1994 revised guidelines of the Centers for Disease Control (CDC). The children were monitored at least every 3 months with repeated interviews, physical examinations, and blood sample collection. There was not an uniform approach regarding antiretroviral treatment. Instead, each pediatrician administered the appropriate antiretroviral therapy (ART) regimen and changed the drugs according to his/her interpretation of the child's data and following international guidelines. The adherence of antiretroviral drugs was measured by each pediatrician by examination of the dose taken by each child and through interviews with his parents or tutors.
Response to long-term HAART
A completed long-term virological response to HAART was defined when HIV-child achieved undetectable VL (≤400 copies/ml) in the first 12 months of therapy and maintained that level during at least 12 months. Thus, HIV-infected children were divided into two groups according to responses to long-term HAART:
a) Responders group
33 HIV-infected children with a full long-term virological response during at least 12 months. Detectable VL during a 12 months period, even if a small "blip", disqualified children from being included in the Responders group. Moreover, 29 of 33 had full long-term virological response during at least 24 months, and 25 of 33 had full long-term virological response during at least 36 months.
b) Non-Responders group
127 HIV-infected children with persistently VL despite HAART (VL ≥400 copies/ml during follow-up). These HIV-infected children had prolonged virological failure in spite of ART switches.
HIV-1 infection laboratory markers
T-lymphocyte subsets in peripheral blood were quantified by flow cytometry (FACScan, Becton-Dickinson Immunocytometry Systems, San Jose, CA, USA). VL was measured in 200 μl plasma samples using a quantitative assay (Amplicor monitor, Roche Diagnostic Systems, Brandenburg, NJ, USA).
Statistical analysis
We analyzed the CD4+ at 0, 12, 24, 36, and 48 months follow-up of children on HAART grouped according to response to long-term virological suppression with a General Lineal Model (GLM) Univariate (regression analysis) adjusted by baseline characteristics (age, sex, ART-naïve, CD4+, and VL). In addition, we analyzed immunological response to HAART in HIV-infected children (CD4+ >25% at 12, 24, 36, 48 months follow-up) by logistic regression.
Results
At baseline (before starting HAART), the two groups had similar age, percentage in clinical category C, levels of CD4+ and CD8+ T-cells, and VL (Table 1). However, the Non-Responders group had a higher percentage of the pre-treatment with ART.
During follow-up, one child progressed to AIDS and one child died. Moreover, the Non-Responders group presented a significantly higher number of ART switches, higher number of different drugs used in ART than children of the Responders group (p ≤ 0.05) (Table 1).
Long-term viral load suppression was achieved in only about 20% of followed children. This low figure is mostly related to poor adherence by the non-responders group (48% vs 91% in responders) (Table 1). There were not significant differences in adherence according to hospital and region involved.
Table 2 shows the first line HAART schemes. We did not find significant differences in PI used in first line HAART between groups. Moreover, there were 3320 VL assays performed on samples obtained from HIV-infected children during the 4 years of follow-up; 1054 (47.3%) of those did not reach the lower limit of detection of 400 HIV-RNA copies/mL. The VL tests performed were similar for the two groups, but the Responders group had a higher number of tests with VL ≤400 copies/ml. Moreover, the median of time between the first and last VL ≤400 copies/ml measurement was lower in the Responder group than the Non-responder group (Table 2). The Responders group had uVL after the 1st year on HAART and the Non-Responders group had a low decrease of VL during the 4 years of follow-up (Figure 1A). Furthermore, the Responders group had a higher percentage of children with CD4+ >30% than the Non-Responders group after 1st year on HAART (Figure 1B).
Evolution of mean plasma log10 VL (A) and percentage of HIV-infected children with CD4+ >30% (B) grouped by long-term VL suppression (Responders vs. Non-Responders). an: number of children in the Responders group. bn: number of children in the Non-Responders group. Differences between groups (*: p < 0.01).
Table 3 shows the mean of %CD4+ and percentage of HIV-infected children with CD4+ ≥25% during the 4 years of follow-up stratified by virological response to HAART. We observed an initial and significant fast increase in CD4+ counts from baseline to 12 months on HAART in both groups (p < 0.01). Interestingly, there were children on HAART with sustained CD4+ increases but with detectable VL. Non-Responders group was sustained CD4+ increases and most of theses HIV-infected children maintained high CD4+ level counts (≥25%). The Non-Responders group also reached a plateau between 26% and 27% CD4+ at 12 months of follow-up that remained stable during the following 3 years (Table 3). However, the Responders group reached a plateau between 30% and 32% CD4+ at 24, 36 and 48 months of follow-up. We found that the Responders group had higher CD4+ count values and a higher percentage of children with CD4+ ≥25% than the Non-Responders group (p < 0.05) after month 12 (Table 3).
Discussion
The introduction of HAART represented a major breakthrough in the therapeutics of HIV-infected patients. However, the overall effectiveness of long-term HAART on HIV-infected children has been scarcely studied. Thus, to date, few studies reflect the evolution of a large cohort of HIV-infected children throughout long-term HAART [6]. To analyze this, we recruited a large group of HIV-infected children starting HAART and tracked their progress for 4 years. Our retrospective longitudinal study provides evidence from current clinical practice that HAART always show long-term sustained uVL only in a low proportion of children (20.6%) just as it has previously been showing in published results in adults [7].
CD4+ recovery despite virological failure has been referred to as discordant responses and this phenomenon has been observed in children [8]. However, in children on HAART, levels of CD4+ recovery were not comparable for virological responders and non-responders, indicating that CD4+ recovery during virological suppression is more profound than during virological failure [8]. This may reflect the inhibitory effect of HIV on thymic function since a marked decrease or suppression in VL is necessary to allow the thymus to replenish the CD4+ T-cells. Moreover, CD4+ T-cells are productively infected by HIV, undergoing apoptosis induced by abnormal cellular activation when the VL is not controlled [9].
In children with virological failure and good immunological and clinical outcomes observations have that virological failure does not always equal clinical failure [10, 11]. Others authors have suggested that in children VL suppression may not be the best way to clinically evaluate ART success as it seem to be for HIV-infected adults [7]. Addition, HIV-infected children on HAART usually have higher VL and lower virological response rates than adults [12]. Thus, maintaining constant or improving CD4+ counts may represent alternative indices of ART success in children [13]. We found an increase of CD4+ counts in the Responders and the Non-Responders groups. This indicates that VL suppression was not indispensable to the recovery of the immune system in vertically HIV-infected children. Moreover, only one Non-Responders child died during follow-up and another evolved to AIDS. We carried out indirect measurement between responders and non-responders (hospitalization rates, weight/height growth curves, and numbers of opportunistic infections), and we found an improvement in these indices but not find differences between groups (data not shown). Therefore, virological failure does not always equal clinical failure and virological suppression does not necessarily mean proper function. However, long-term suppression of VL allowed higher values of CD4+ counts.
High VL is associated with immune system activation which is used as a predictive marker of virologic failure [14, 15]. Moreover, viral suppression is a powerful predictor of CD4+ increase [16]. About 30% of HIV-infected adults receiving HAART exhibit a sustained CD4+ increase despite therapy failure, or they have persistently low CD4+ counts despite a significant decrease in VL [17]. In this study, approximately 30% of HIV-infected children also had an increase of CD4+ to a level ≥25% at 24 (57.39%-35.52%), 36 (62.38%-35.52%), and 48 (62.88%-35.52%) months.
The capacity for CD4+ regeneration during long-term HAART has not been well defined. In our study, children reached a plateau in CD4+ cell after 2 years on HAART just as others authors have published results in adults [18, 19]. Moreover, Hunt et al. present strong evidence that CD4+ counts continue to increase up to 4 years after HAART [20]. However, the rate of CD4+ recovery in adults is slow and a steady state is not usually reached after 4 years most likely because the adult thymus is less functional than in children [21].
A limitation of our study was that we did not have virologic resistance data previous to HAART and during follow-up. Increasingly many clinicians and investigators are describing multi-drug resistant HIV among children who are extensively HAART experienced [22]. Moreover, high baseline VL and substantial but imperfect levels of adherence were associated with HIV-resistance [23] and may facilitate an earlier virological failure [22]. Therefore, adherence to medication is extremely important and may have a significant, effect unaccounted for in the interpretation of our results. All efforts were made by health personnel to improve adherence in each child. Traditionally, children's adherence to ART has been limited, even though response to therapy has been shown to be highly dependent on the patient's adherence [24, 25]. In this study, the adherence to ART was not strictly monitored, as opposed to standard clinical trials. We also found that adherence to ART was the most important variable associated with long-term VL suppression (data not shown). However, therapy adherence is almost impossible to reliably measure, and accurately reproduce in a long-term dynamic cohort [7]. This low adherence in the Non-Responders group is not related to the level of training received by the health personal who provided the medical care. Furthermore, the number of doctor visits was similar in both groups of children, but the Responders group had a higher number of tests with VL ≤400 copies/mL and a lower time with the first uVL measurement than the Non-Responders group.
Conclusion
In conclusion, long-term VL suppression induces large beneficial effects in immunological responses. However, it is not indispensable to recover CD4+ counts (levels).
References
Resino S, Bellón JM, Gurbindo D, Ramos JT, León JA, Mellado MJ, Muñoz-Fernández MA: Viral Load and CD4+ T-Cells Response to HAART in HIV-Infected Children: a Observational Study. Clin Infect Dis. 2003, 37: 1216-1225. 10.1086/378804.
Borkowsky W, Stanley K, Douglas SD, Lee S, Wiznia A, Pelton S, Yogev R, McIntosh K, Nachman S: Immunologic response to combination nucleoside analogue plus protease inhibitor therapy in stable antiretroviral therapy-experienced human immunodeficiency virus-infected children. J Infect Dis. 2000, 182: 96-103. 10.1086/315672.
Resino S, Bellón JM, Resino R, Navarro ML, Ramos JT, Mellado MJ, de Jose MI, Muñoz-Fernández MA: Extensive implementation of highly active antiretroviral therapy shows great effectiveness on the survival and surrogate markers in vertically HIV-infected children. Clin Infect Dis. 2004, 38: 1605-1612. 10.1086/420738.
Wiznia A, Stanley K, Krogstad P, Johnson G, Lee S, McNamara J, Moye J, Jackson JB, Mendez H, Aguayo R, Dieudonne A, Kovacs A, Bamji M, Abrams E, Rana S, Sever J, Nachman S: Combination nucleoside analog reverse transcriptase inhibitor(s) plus nevirapine, nelfinavir, or ritonavir in stable antiretroviral therapy- experienced HIV-infected children: week 24 results of a randomized controlled trial--PACTG 377. Pediatric AIDS Clinical Trials Group 377 Study Team. AIDS Res Hum Retroviruses. 2000, 16: 1113-1121. 10.1089/088922200414956.
van Rossum AM, Geelen SP, Hartwig NG, Wolfs TF, Weemaes CM, Scherpbier HJ, van Lochem EG, Hop WC, Schutten M, Osterhaus AD, Burger DM, de Groot R: Results of 2 years of treatment with protease-inhibitor--containing antiretroviral therapy in dutch children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2002, 34: 1008-1016. 10.1086/339443.
Soh CH, Oleske JM, Brady MT, Spector SA, Borkowsky W, Burchett SK, Foca MD, Handelsman E, Jimenez E, Dankner WM, Hughes MD: Long-term effects of protease-inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet. 2003, 362: 2045-2051. 10.1016/S0140-6736(03)15098-2.
Holmberg SD, Hamburger ME, Moorman AC, Wood KC, Palella FJJ: Factors associated with maintenance of long-term plasma human immunodeficiency virus RNA suppression. Clin Infect Dis. 2003, 37: 702-707. 10.1086/376992.
Resino S, Galan I, Perez A, Leon JA, Seoane E, Gurbindo D, Muñoz-Fernández MA: HIV-infected children with moderate-severe immune-suppression: changes in the immune system after highly active antiretroviral therapy. Clin Exp Immunol. 2004, 137: 570-577. 10.1111/j.1365-2249.2004.02583.x.
Douek DC, Picker LJ, Koup RA: T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003, 21: 265-304. 10.1146/annurev.immunol.21.120601.141053.
Chiappini E, Galli L, Zazzi M, de Martino M: Immunological recovery despite virological failure is independent of human immunodeficiency virus-type 1 resistant mutants in children receiving highly active antiretroviral therapy. J Med Virol. 2003, 70: 506-512. 10.1002/jmv.10424.
Peruzzi M, Azzari C, Galli L, Vierucci A, De Martino M: Highly active antiretroviral therapy restores in vitro mitogen and antigen-specific T-lymphocyte responses in HIV-1 perinatally infected children despite virological failure. Clin Exp Immunol. 2002, 128: 365-371. 10.1046/j.1365-2249.2002.01814.x.
Melvin AJ, Rodrigo AG, Mohan KM, Lewis PA, Manns-Arcuino L, Coombs RW, Mullins JI, Frenkel LM: HIV-1 dynamics in children. J Acquir Immune Defic Syndr Hum Retrovirol. 1999, 20: 508-513.
Deeks SG, Barbour JD, Grant RM, Martin JN: Duration and predictors of CD4 T-cell gains in patients who continue combination therapy despite detectable plasma viremia. AIDS. 2002, 16: 201-207. 10.1097/00002030-200201250-00009.
Resino S, Bellón JM, Ramos JT, Gonzalez-Rivera M, de José MI, González MI, Gurbindo D, Mellado MJ, Cabrero E, Muñoz-Fernández MA: Positive virologic outcome after lopinavir/ritonavir salvage therapy in protease inhibitor-experienced HIV-1-infected children. A prospective cohort study. J Antimicrob Chemother. 2004, 54: 921-931. 10.1093/jac/dkh431.
Paul ME, Mao C, Charurat M, Serchuck L, Foca M, Hayani K, Handelsman EL, Diaz C, McIntosh K, Shearer WT: Predictors of immunologic long-term nonprogression in HIV-infected children: Implications for initiating therapy. J Allergy Clin Immunol. 2005, 115: 848-855. 10.1016/j.jaci.2004.11.054.
Koletar SL, Williams PL, Wu J, McCutchan JA, Cohn SE, Murphy RL, Lederman HM, Currier JS: Long-term follow-up of HIV-infected individuals who have significant increases in CD4+ cell counts during antiretroviral therapy. Clin Infect Dis. 2004, 39: 1500-1506. 10.1086/424882.
Deeks SG, Barbour JD, Martin JN, Swanson MS, Grant RM: Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficiency virus infection. J Infect Dis. 2000, 181: 946-953. 10.1086/315334.
Kaufmann GR, Bloch M, Finlayson R, Zaunders J, Smith D, Cooper DA: The extent of HIV-1-related immunodeficiency and age predict the long- term CD4 T lymphocyte response to potent antiretroviral therapy. Aids. 2002, 16: 359-367. 10.1097/00002030-200202150-00007.
Tarwater PM, Margolick JB, Jin J, Phair JP, Detels R, Rinaldo C, Giorgi J, Munoz A: Increase and plateau of CD4 T-cell counts in the 3(1/2) years after initiation of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2001, 27: 168-175.
Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI, Kitahata MM, Krone M, Neilands TB, Brand RJ, Lederman MM, Martin JN: Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. Aids. 2003, 17: 1907-1915. 10.1097/00002030-200309050-00009.
Franco JM, Leon-Leal JA, Leal M, Cano-Rodriguez A, Pineda JA, Macias J, Rubio A, Rey C, Sanchez B, Lissen E: CD4+ and CD8+ T lymphocyte regeneration after anti-retroviral therapy in HIV-1-infected children and adult patients. Clin Exp Immunol. 2000, 119: 493-498. 10.1046/j.1365-2249.2000.01152.x.
Jimenez JL, Resino S, Martinez-Colom A, Bellon JM, Munoz-Fernandez MA: Mutations at codons 54 and 82 of HIV protease predict virological response of HIV-infected children on salvage lopinavir/ritonavir therapy. J Antimicrob Chemother. 2005.
Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, Brumme CJ, Brumme ZL, Mo T, Alexander CS, Montaner JS: Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005, 191: 339-347. 10.1086/427192.
Watson DC, Farley JJ: Efficacy of and adherence to highly active antiretroviral therapy in children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1999, 18: 682-689. 10.1097/00006454-199908000-00006.
Reddington C, Cohen J, Baldillo A, Toye M, Smith D, Kneut C, Demaria A, Bertolli J, Hsu HW: Adherence to medication regimens among children with human immunodeficiency virus infection. Pediatr Infect Dis J. 2000, 19: 1148-1153.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/6/10/prepub
Acknowledgements
Financial Support: Fundación para la Investigación y la Prevención del SIDA en España (FIPSE) (grant 12456/03) Fundación para la Investigación Sanitaria (FIS) del Ministerio de Sanidad y Consumo (PI040883, PI052479, PI052472, PI052411), Plan Nacional de Salud (SAF 2003-09209, SAF-2004-06778), Red Temática Cooperativa de investigación en SIDA (RIS G03/173) of FIS, and Red Temática Cooperativa de investigación en Genética (RIG C03/07) of FIS.
Salvador Resino and Jose María Bellón are supported by a grant of FIS (grant CP04/00090, 01/A016 respectively).
Participating hospitals and sites
MADRID:
Hospital Universitario "12 Octubre": J.T. Ramos, P. Carreño, J. Ruiz, J. Clemente.
Hospital General Universitario "Gregorio Marañón": S. Resino, A. Alvaro-Meca, R. Resino, J.M. Bellón, M.D. Gurbindo, M.L. Navarro, M.A. Muñoz-Fernández.
Hospital Universitario "La Paz": M.I. Isabel de José.
Hospital Universitario "Carlos III": P. Martín-Fontelos, M.J. Mellado, J. Villota.
SEVILLE:
Hospital Universitario "Virgen del Rocio": J.A. León Leal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
- Salvador Resino had primary responsibility for protocol development, patient screening, enrollment, outcome assessment, preliminary data analysis, and contributed to the writing of the manuscript.
- Rosa Resino had primary responsibility for the collecting and recording data, and contributed to the writing of the manuscript.
- José María Bellón participated in analytic framework for the study, and contributed to the writing of the manuscript.
- Pediatricians: Juan Antonio León, Pablo Matín Fontelos, José Tomás Ramos, Ma Dolores Gurbindo Gutiérrez, Ma Isabel de José, and Luis Ciria were responsible for patient screening, and contributed to the writing of the manuscript.
- Ma Angeles Muñoz-Fernández supervised the design and execution of the study, the final data analyses, and the writing of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Resino, S., Resino, R., Leon, J.A. et al. Impact of long-term viral suppression in CD4+ recovery of HIV-children on Highly Active Antiretroviral Therapy. BMC Infect Dis 6, 10 (2006). https://doi.org/10.1186/1471-2334-6-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-6-10