Abstract
Background
Severe Clostridium difficile toxin-induced enteritis is characterized by exuberant intestinal tissue inflammation, epithelial disruption and diarrhea. Adenosine, through its action on the adenosine A2A receptor, prevents neutrophillic adhesion and oxidative burst and inhibits inflammatory cytokine production. Alanyl-glutamine enhances intestinal mucosal repair and decreases apoptosis of enterocytes. This study investigates the protection from enteritis by combination therapy with ATL 370, an adenosine A2A receptor agonist, and alanyl-glutamine in a rabbit and murine intestinal loop models of C. difficile toxin A-induced epithelial injury.
Methods
Toxin A with or without alanyl-glutamine was administered intraluminally to rabbit ileal or murine cecal loops. Animals were also given either PBS or ATL 370 parenterally. Ileal tissues were examined for secretion, histopathology, apoptosis, Cxcl1/KC and IL-10.
Results
ATL 370 decreased ileal secretion and histopathologic changes in loops treated with Toxin A. These effects were reversed by the A2A receptor antagonist, SCH 58261, in a dose-dependent manner. The combination of ATL 370 and alanyl-glutamine significantly further decreased ileal secretion, mucosal injury and apoptosis more than loops treated with either drug alone. ATL 370 and alanyl-glutamine also decreased intestinal tissue KC and IL-10.
Conclusions
Combination therapy with an adenosine A2A receptor agonist and alanyl-glutamine is effective in reversing C. difficile toxin A-induced epithelial injury, inflammation, secretion and apoptosis in animals and has therapeutic potential for the management of C. difficile infection.
Similar content being viewed by others
Background
Clostridium difficile is the most common known cause of nosocomial diarrhea. Recently, the incidence and the frequency of morbidity and mortality from C. difficile infection (CDI) have increased [1, 2]. These have been attributed to the BI/NAP1/III/027 strain which is fluoroquinolone resistant, carries an 18 bp deletion in the tcdC gene (a possible negative regulator of toxin gene production), produces binary toxin and displays hypertoxigenic properties [3]. Current treatment consists of antibiotics that suppress normal flora. While transiently effective, these treatments result in a 20-30% relapse rate, prolonged shedding and reacquisition of C. difficile [4]. Moreover, CDI secondary to the emerging strain has already been reported to be less responsive to metronidazole and is associated with more relapses compared with the historical strains [5]. There are now more patients requiring colectomies and ICU care. New non-antimicrobial approaches to treatment are urgently needed for CDI.
Several clinical studies have shown that glutamine and its stable derivative, alanyl-glutamine, improve gastrointestinal mucosal structure and function following injury from chemotherapy, radiotherapy or prolonged parenteral nutrition [6]. In addition to its role as an important building block for protein synthesis, glutamine also is a regulator of intracellular kinases [7], redox status [6], cell proliferation [8], apoptosis [9] and heat shock protein expression [10]. C. difficile toxin A induces activation of caspases 6, 8, 9 and 3 in intestinal epithelial cells [11]. Glutamine and alanylglutamine have been shown to inhibit caspase 8 and decrease toxin A-induced apoptosis [12]. Activation of adenosine A2A receptors attenuates inflammation in many tissues. A2A receptor activation in neutrophils decreases production of reactive oxygen species. In macrophages A2A receptor activation decreases secretion of inflammatory cytokines (e.g. IL-2, IL-12, IL-6, TNFα) and increases IL-10 [13, 14]. In mouse ileal tissues, ATL 313, another adenosine A2A receptor agonist, reduced C. difficile toxin A-induced mucosal injury, neutrophillic infiltration and cell death [15].
In this study we examined the effect of combining alanyl-glutamine and ATL 370 on secretion and mucosal structural damage in C. difficile toxin A-induced enteritis in rabbits and cecitis in mice. We also evaluated apoptosis by immunohistologic staining in the rabbit ileum and the secretion of inflammatory (keratinocyte-derived chemokine, KC) and anti-inflammatory (IL-10) cytokines in the mouse cecum.
Methods
Rabbit ileal loop model
Animal protocols were approved by the University of Virginia Animal Care and Use Committee. New Zealand White rabbits weighing approximately 2 kg were fasted overnight. The rabbits were anesthetized with ketamine (60-80 mg/kg) and xylazine (5-10 mg/kg), administered intramuscularly or by inhalational isoflurane. A midline abdominal incision was made to expose the small bowel. After the ileum was flushed with 5-10 ml of PBS, 8-10 loops were created by ligation using double ties and a 1-cm interval was left between the loops. Control loops were injected with either PBS (1 ml/loop) alone or C. difficile toxin A (TechLab, Blacksburg VA) (10-20 μg/loop dissolved in 1 ml of PBS). Treated animals received alanyl-glutamine (Sigma-Aldrich) at a final concentration of 3, 10, 30 or 100 mM injected into the intestinal lumen just prior to toxin A injection. The ligated loops were then returned to the abdomen and the abdominal wall sutured closed. Treated animals also received either the adenosine A2A receptor agonist, ATL 370 (Dogwood Therapeutics), at 0, 1.5 ng, 15 ng or 150 ng dissolved in 250 uL PBS by intravenous route immediately after administration of toxin A (time 0) and 2 h after the addition of toxin A. A separate experiment was performed using equimolar concentrations (3.5 ng, 34.5 ng, or 345 ng) of adenosine A2A receptor antagonist, SCH 58261 (Tocris Bioscience), given intravenously 5 min after the administration of ATL 370 (at time 0 and 2 h later) to investigate the specificity of the agonist effect.
Mouse cecal loop model
To confirm results from the rabbit experiments and determine the effects of treatment on cytokine production, mouse cecal loop experiments were also performed. C57BL/6 male mice, weighing 23-25 g each, were fasted overnight. The mice were anesthetized with ketamine (60-80 mg/kg) and xylazine (5-10 mg/kg), administered intramuscularly. A midline abdominal incision was made to expose the cecum. After flushing with PBS, a ligature was placed each at the ileal and colonic openings of the cecum, while avoiding ligation of the vascular supply. Control loops were injected with either PBS (200 μL/loop) alone or C. difficile toxin A (20 μg/loop dissolved in 200 μL of PBS). Treated animals received alanyl-glutamine at a final concentration of 100 mM injected into the cecal lumen just prior to toxin A injection. The ligated cecal loops were then returned to the abdomen and the abdominal wall sutured closed. Treated animals also received either the adenosine A2A receptor agonist, ATL 370, at a dose of 3 ng/g by subcutaneous route, immediately after administration of toxin A (time 0).
Secretion and histopathology
After 4-5 h of incubation, the animals were euthanized and the abdomen incised. The intestines were exposed and the lengths of each ligated ileal loop were measured. Intestinal fluid was extracted from the loop and quantified. The volume:length ratio (V:L) was calculated in milliliters per centimeter per loop. The gross description (clear, serous, purulent, or bloody) of the collected fluid was also noted. One set of tissues was fixed with 10% formalin and stained with hematoxylin-eosin for histopathology. Each slide was read blindly by at least 2 investigators (CAW, SWP, GC). A grading scale for histopathology of intestinal tissues, previously formulated and published [16–18], was used to grade each slide according to mucosal disruption, cellularity, and vascular congestion. The final histopathologic score is the mean of the scores of all graders. Samples of intestinal tissue from control and treated loops were placed in liquid nitrogen for subsequent protein and RNA extraction.
In situapoptotic staining
Paraffin-embedded tissues were analyzed for apoptotic cells using ApopTag® Peroxidase In Situ Apoptosis Detection Kit. All steps were performed according to the manufacturer's instructions. Crystal Violet free Methyl Green was used as the counterstain. Images were obtained by Olympus BX41TF with Digital Camera and MIcrosuite Pathology provided by the University of Virginia Biorepository and Tissue Research Facility. Apoptosis was quantified by counting peroxidase-stained cells in 3 randomly chosen high power fields (hpf) per tissue section quadrant. The total number of cells was then divided by 12 (3 hpfs × 4 quadrants) and the reading reported as number of apoptotic cells per hpf.
KC, IL-10 enzyme-linked immunosorbent assays (ELISA)
Detection and quantification of KC and IL-10 proteins present in mouse cecal samples were determined by using Mouse CXCL1/KC and IL-10 Quantikine ELISA kits (R&D Systems), respectively. Cecal tissues, harvested from the mouse cecal loop model described above, were minced, bead beat for 1 min twice (with 2 min interval on ice in between bead beating) and lysed in NP-40 lysis buffer for 20 min on ice, followed by spinning at 14,000 rpm at 4°C for 30 min. Protein concentration in a 1:10 dilution of the supernatant was measured using bicinchoninic acid assay (BCA) following the manufacturer's instructions (Pierce Thermo-Fisher Kit) [19]. ELISA on the mouse cecal lysates was performed according to manufacturer's instructions [20, 21]. Both sample sets were analyzed in triplicate. Of note, antibodies to rabbit IL-10 and IL-8 are not readily available and homologous to human sequences by only 80% and 78%, respectively. Thus, we opted to use mouse tissues to detect cytokine production.
KC and IL-10 gene expression assay
RNA was extracted from mouse cecal tissues using Qiagen RNeasy mini Kit according to manufacturer's instructions. RNA concentration was quantified and checked for purity (A260:280 ratio) by standard spectrophotometry (Biophotometer, Eppendorf, Hamburg, Germany). Synthesis of cDNA by Reverse Transcriptase PCR was performed using SuperScript III First-Strand Synthesis System SuperMix (Invitrogen) with the use of oligo (dT) as primers. cDNA was used in quantitative PCR for measuring KC and IL-10 expression compared to hypoxanthine guanine phosphoribosyl (HPRT) expression. The primers used were: murine HPRT (forward: 5'-TCC CTG GTT AAG CAG TAC AGC CCC-3; reverse: 5'-CCA ACA AAG TCT GGC CTG TAT CCA A-3') (GenBank no. NM_013556.2), IL-10 (forward: 5'-GGC GCT GTC ATC GAT TTC TCC CC-3'; reverse: 5'-GGC CCT GTA GAC ACC TTG GTC TTGG-3') (GenBank no. NM_010548.2) and Cxcl1/KC (forward: 5'-CGC ACG TGT TGA CGC TTC CCT-3'; reverse: 5'-GTC CCG AGC GAG ACG AGA CCA-3') (GenBank no. NM_008176.3). The reaction was performed in a Bio-Rad iCycler iQ multicolor PCR Detection System using iCycler software (version 3.0). The relative gene expression was determined using the 2-ΔΔCt (Livak) method using HRPT as the housekeeping gene [22].
Statistical analysis
Continuous variables, such as V:L, histopathologic score, protein levels, were expressed as means ± standard error of the mean (SEM). For statistical comparison of the magnitude of secretion, the amount of inflammation, and protein levels, a one-way analysis of variance (ANOVA) with Bonferroni post-hoc test to compare multiple selected pairs was performed. To compare 2 groups of treatment only, a 2-tailed Student's unpaired t test with Welch's correction was applied.
Results
Adenosine A2A receptor agonist, ATL 370, improved C. difficiletoxin A-induced secretion and epithelial injury in a dose-dependent manner and these effects were reversed by the receptor antagonist, SCH 58261
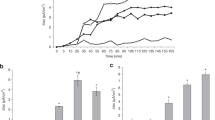
Increasing doses of IV ATL 370 produced decreased fluid volumes in the rabbit intestinal lumen (Figure 1A). Histopathology scores did not further improve beyond ATL 370 15 ng/rabbit IV (Figure 1B). However, increasing doses of SCH 58261 yielded a dose-dependent reversal of the ATL370 effect in both secretion and histopathology score suggesting adenosine A2A receptor involvement in these responses (Figure 2). SCH 58261 increased secretion and histopathology score over baseline, suggesting some activation of A2A receptors by endogenous adenosine.
Effects of ATL 370 alone on C. difficile toxin A-induced secretion and epithelial injury. Increasing doses of intravenous ATL 370 (adenosine A2A receptor agonist; ng) decreased toxin A (10 μg)-induced intraluminal fluid accumulation (A) and histopathology scores (B) in rabbit ileal loops. Panel 1A: a- P < 0.0001 (one-way ANOVA), and b(PBS vs. Toxin A)- P < 0.001, c(Toxin A vs ATL150)- P < 0.01 (Bonferroni's). Panel 1B: a- P < 0.0001 (one-way ANOVA), b(PBS vs. Toxin A) - P < 0.001 (Bonferroni's), c (Toxin A vs ATL15)- P = 0.04 (Unpaired t-test with Welch's). Results were data pooled from 8 to 28 loops from 2 to 5 rabbits per group from 5 separate experiments.
Effects of SCH-58261 treatment on ATL 370-treated C. difficile toxin A-challenged loops. Increasing doses of adenosine A2A receptor blocker, SCH 58261 (given IV), reversed the effects of ATL 370 (1.5 ng IV) on secretion (A) and epithelial injury (B) in a dose-dependent manner in rabbit ileal loops. 3.5 ng of SCH 58261 is equimolar to 1.5 ng of ATL370. Panel 2A: a- P < 0.0001 (one-way ANOVA), and b(PBS vs. Toxin A)- P < 0.001, c(Toxin A vs ATL + SCH)- P < 0.01 (Bonferroni's). Panel 2B: a- P < 0.0001 (one-way ANOVA), b(PBS vs. Toxin A)- P < 0.001 (Bonferroni's). Results were data pooled from 4 to 28 loops from 1 to 6 rabbits per group from 5 separate experiments.
ATL 370 and alanyl-glutamine synergistically decreased toxin A-induced ileal secretion and epithelial injury
As shown in Figure 3A, rabbit intestinal loops challenged with toxin A displayed a 98% increase in hemorrhagic secretion (vol/length) compared with loops treated with PBS only. Toxin A-challenged rabbits receiving either a low IV dose of ATL 370 (1.5 ng) or intraluminal alanyl-glutamine treatment (100 mM) displayed reduced secretion, each by 27%, however, this difference did not reach statistical significance. However, in rabbits treated with both ATL 370 (1.5 ng IV) and alanyl-glutamine (10-100 mM/loop), further dose-dependent decreases in secretion were noted. The V:L decreased by 53%, 67%, 77% and 92% in loops treated with ATL 370 and alanyl-glutamine at 3, 10, 30 and 100 mM, respectively. As shown in Figure 3B, by histopathology, toxin A-stimulated intestinal tissues had significantly elevated histopathology score compared to PBS-stimulated tissues alone. ATL 370 (1.5 ng) and alanyl-glutamine (100 mM) individually decreased histopathology scores by 21% and 19%, respectively. Similar to what was observed with secretion, combination treatment resulted to dose-dependent improvement in histopathology (score improved by 38%, 49%, 51% and 62% in loops treated with ATL 370 and alanyl-glutamine at 3, 10, 30 and 100 mM, respectively). Representative images of toxin-challenged ileal tissues with or without treatment are shown in Figure 4.
Effects of low dose ATL 370 and alanyl-glutamine on C. difficile toxin A-induced secretion and epithelial injury. Varying doses of alanyl-glutamine (AQ; mM) in the lumen when given in addition to intravenous ATL 370 (1.5 ng) showed decrease in toxin A (10 ug)-induced intraluminal fluid accumulation (A) and epithelial injury (B) in rabbit ileal loops. Panel 3A: a- P = 0.08 (one-way ANOVA), and b (PBS vs. Toxin A)- P = 0.0002, c (Toxin A vs ATL/AQ10)- P = 0.02, d (Toxin A vs ATL/AQ30)- P = 0.01, e (Toxin A vs ATL/AQ100)- P = 0.0004 (Unpaired t-test with Welch's). Panel 3B: a- P < 0.0001 (one-way ANOVA), and b(PBS vs. Toxin A)- P < 0.001, c (Toxin A vs ATL/AQ10)- P < 0.01, d (Toxin A vs ATL/AQ30)- P < 0.01, e (Toxin A vs ATL/AQ100)- P < 0.001 (Bonferroni's). Results were data pooled from 4 to 16 loops from 2 rabbits per group from 2 separate experiments.
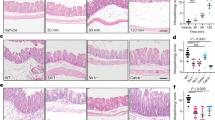
Effects of ATL 370 and alanyl-glutamine on C. difficile toxin A-induced ileal histopathology. Toxin A (10 μg, B) caused mucosal disruption, increased cellularity and vascular congestion compared with PBS (A) in rabbit ileal tissues. Treatment with intravenous ATL 370 (1.5 ng, D) or intraluminal alanyl-glutamine (100 mM, C) reduced some epithelial injury. Marked preservation of mucosal architecture was noted in tissues treated with the combination of ATL and alanyl-glutamine (E)
ATL 370 and alanyl-glutamine decreased KC and IL-10 in toxin A-stimulated mouse cecal tissues
In mouse ligated cecal tissues, KC levels were increased by 81% in toxin A-treated loops compared with PBS-treated loops (96 ± 6 vs 53 ± 2 μg/mg, p < 0.0001). Treatment with either AQ or ATL 370 decreased KC in intoxicated cecal tissues by 73% from the toxin A-treated loops level (Figure 5A). IL-10 secretion was more than 2-fold increased in toxin A-treated loops compared to PBS alone (591 ± 94 vs 228 ± 34 pg/mg tissue, P < 0.01) (Figure 5B). Alanyl-glutamine or ATL 370 alone did not reduce IL-10 secretion but when combined together caused a 65% reduction of the cytokine level (p < 0.01). KC and IL-10 mRNA expressions followed a similar trend as the ELISAs (Figure 5C and 5D, respectively).
Effects of ATL 370 and alanyl-glutamine on cytokine expression. After four hours of incubation, alanyl-glutamine (AQ; 100 mM in loops) or ATL 370 (3 ng/g s.c.) significantly decreased C. difficile toxin A-induced elevation of KC secretion in mouse cecal tissues (A). Combination treatment with ATL 370 and AQ significantly decreased KC secretion further. Likewise, IL-10 secretion was increased by toxin A although not significantly reduced by either AQ or ATL 370 alone (B). Only combination treatment with ATL 370 and alanyl-glutamine significantly decreased IL-10 secretion (B). C and D show corresponding KC and IL-10 mRNA expressions expressed as fold-change from PBS control. Number of mice per group: Panels A&B- 5-6; Panels C&D- 3-5. Panel 5A: a- P < 0.0001 (one-way ANOVA), and b (Toxin A vs AQ)- P < 0.01, c (Toxin A vs AQ)- P < 0.001, d (Toxin A vs ATL)- P < 0.01, e (Toxin A vs ATL/AQ)- P < 0.001 (Bonferroni's). Panel 5B: a- P < 0.0001 (one-way ANOVA), and b (Toxin A vs PBS)- P < 0.01, c (Toxin A vs ATL/AQ)- P < 0.01 (Bonferroni's). Panel 5C: a- P = 0.04 (one-way ANOVA), and b (Toxin A vs ATL/AQ)- P < 0.05 (Bonferroni's). Panel 5D: a- P = 0.056 (one-way ANOVA)
ATL 370 and alanyl-glutamine decreased toxin A-induced apoptosis in the intestinal tissues
Toxin A treated rabbit intestinal tissues (Figure 6A) showed marked apoptotic activity compared with PBS control tissues (Figure 6B). Due to mucosal sloughing, most of the apoptotic cells were located in the lumen of toxin A-treated tissues. Treatment with alanyl-glutamine (Figure 6C) or ATL 370 (Figure 6D) alone resulted in increased mucosal preservation. Apoptotic cells were noted both in the lumen and within the intact mucosa and submucosa. Alanyl-glutamine treatment exhibited better anti-apoptotic activity than ATL 370. Combination of alanyl-glutamine and ATL 370 resulted in further decrease in the number of apoptotic cells and increased preservation of epithelial architecture (Figure 6E).
Effects of ATL 370 and alanyl-glutamine on C. difficile toxin A-induced apoptosis. Toxin A (10 μg, B) caused significant apoptosis in the rabbit ileal tissues compared with PBS (A). Treatment with ATL 370 (1.5 ng/rabbit IV, D) or alanyl-glutamine (AQ;100 mM in loops, C) caused some reduction of apoptotic cells but only significant difference was noted with treatment with AQ. A marked decrease in apoptotic cells was noted in tissues treated with the combination of ATL and alanyl-glutamine (E). a- P < 0.0001 (one-way ANOVA), and b (PBS vs. Toxin A)- P < 0.001, c (Toxin A vs ATL/AQ10)- P < 0.01, d (Toxin A vs ATL/AQ100)- P < 0.001 (Bonferroni's). Apoptotic cells were counted in 12 high power fields (3 hpfs/quadrant) per slide from 3 to 4 ileal specimens per group.
Discussion
C. difficile toxin A has repeatedly been shown in numerous in vitro and in vivo studies to elicit an exuberant inflammatory response causing recruitment of inflammatory cells (neutrophils, macrophages and mast cells), secretion of inflammatory cytokines (IL-8, IL1B, TNFα), cell death, mucosal damage and intestinal secretion [23–25]. Although both C. difficile toxins A and B glucosylate Rho GTPases [25] and are important in pathogenesis during infection [26, 27], most animal models of intoxication--rabbits, mouse, rats and hamsters, are more responsive to the enterotoxic effects of toxin A than toxin B [23, 28]. This study has shown that adenosine A2A receptor agonist, ATL 370, ameliorates C. difficile toxin A-induced intestinal epithelial disruption, inflammation and secretion in the ligated loop model of enteritis in rabbits. These effects were augmented by co-administration of the dipeptide, alanyl-glutamine. Significant decreases in IL-8 and IL-10 in mouse cecal tissues were also mostly noted with the co-administration of ATL370 and alanyl-glutamine.
Adenosine is a naturally occurring purine nucleoside that is produced in many tissues in response to inflammatory or hypoxic stimuli. There are 4 known G-protein coupled adenosine receptors- A1, A2A, A2B and A3. Adenosine produces anti-inflammatory activity via A2A receptors found in immune cells including neutrophils, macrophages, dendritic cells and platelets. In the presence of tissue injury or hypoxia, generation of adenosine is increased and adenosine receptors are upregulated [29]. Activation of the A2A receptor with adenosine or a specific A2A receptor agonists inhibits recruitment of inflammatory cells and expression of inflammatory cytokines [30]. Adenosine inhibits expression of adhesion molecules (E-selectin and vascular cell adhesion molecule-1, VCAM-1) and secretion of IL-6 and IL-8 in human endothelial cells [31]. Indeed, it has previously been reported that intraluminal administration of A2A agonist, ATL 313, in C. difficile toxin A-challenged murine ileal loops decreased neutrophil infiltration as measured by myeloperoxidase activity and tissue TNFα secretion [15]. C. difficile toxin A-induced intestinal mucosal injury, intraluminal secretion and apoptosis were all improved in ileal tissues treated with ATL 313. Our study using two different animal models (rabbit ileal and murine cecal loops) and a different adenosine A2A receptor agonist (ATL 370) corroborated these previous findings. Interestingly, the high doses of ATL 370 (150 ng) used in this study did not offer additional improvement in histopathologic scores indicating that lower doses may be sufficient for an optimal effect. The observed effects of ATL 370 on intestinal secretion were dose-dependent and reversed by highly specific A2A antagonist, SCH 58261, also in a dose-dependent manner suggesting the specificity of the observations to the adenosine A2A receptor. In addition, our study showed that the anti-inflammatory, anti-secretory and anti-apoptotic effects from treatment with A2A agonist may be enhanced by the addition of alanyl-glutamine.
The exact mechanism as to how glutamine and alanyl-glutamine (a highly soluble and stable derivative of glutamine) protect enterocytes from injury and modulate inflammatory response is unclear. Alanyl-glutamine is hydrolyzed to alanine and glutamine in the gut. Glutamine is the major oxidative energy source for enterocytes, being the building block for purine and pyrimidine nucleotide synthesis and precursor of glutathione in the gut [32]. Under pathologic or catabolically stressful conditions, i.e. infection or injury, glutamine is observed to be depleted and thus, it is considered as a conditionally essential amino acid [33]. Moreover, glutamine deprivation has been known to induce apoptosis in intestinal epithelial cells [34]. The protective effect of glutamine against mucosal injury and inflammation is thought to be partly mediated by heat shock proteins [10, 35]. More recently, glutamine has also been considered to be an agonist of peroxisome proliferator-activated receptor gamma, which belongs to the family of nuclear receptor proteins which regulates expression of inflammatory cytokines [36]. It has also been previously shown that C. difficile toxin A induces apoptosis by activation of caspases 3, 6, 8, and 9 and Bid, mitochondrial damage and release of cytochrome c in T84 cells [11]. Glutamine and alanyl-glutamine inhibited activation of caspase 8 and reduced apoptosis in T84 cells and improved intestinal secretion and mucosal disruption in rabbit ileal loops challenged with C. difficile toxin A [12]. These findings are consistent with what we have observed where the presence of alanyl-glutamine enhanced reduction of apoptosis, inflammation, secretion and epithelial injury in ATL 370-treated, C. difficile toxin A-stimulated ileal tissues.
The beneficial effect of combination therapy for toxin A-induced enteritis is less explored in the literature. In this study we attempted to control both epithelial disruption and inflammatory response with an adenosine A2A receptor, ATL 370, and alanyl-glutamine. ATL 370 was delivered parenterally and glutamine intraluminally. Given the short incubation time (4-5 h) of the experiments, it is conceivable that systemically administered A2A agonist may have acted mostly on the immune cells, specifically neutrophils, and endothelial cells, which are known to express more of the A2A receptors than on the enterocytes, which express more of A2B receptors [37–39]. Indeed, other A2A agonists have been shown to decrease inflammatory responses and improved survival in mouse models of endotoxemia and sepsis [40, 41]. Several experimental studies have shown that alanyl-glutamine is at least as effective as glutamine in improving electrolyte absorption and preserving mucosal integrity against enterotoxin or chemical insult [42–46]. Alanyl-glutamine enters enterocytes through PepT1 proton-coupled peptide transporters and release L-glutamine by the action of cytosolic peptidases [47, 48]. Although glutamine may affect leukocyte-dependent inflammatory events, such as migration and release of inflammatory cytokines, in this particular study, glutamine may have caused more of the protective effect on mucosal architecture and prevention of enterocyte apoptosis than prevented recruitment of inflammatory cells. However, release of cytokines from disrupted epithelial cells contributes to recruitment of immune cells and inflammatory mediators from the later also contribute significantly to mucosal injury [49]. These joint epithelial and immune responses to a biologic insult such as an enterotoxin, possibly explains the additive amelioration of mucosal injury, secretion, and apoptosis or even the synergistic effects on the expression of cytokines with combined treatment in this study. Whether these observations will hold true in the mouse model of C. difficile infection remain to be seen but these preliminary findings raise the potential use of combination therapy with enteral alanyl-glutamine and systemic low-dose A2A adenosine agonist in the management of C. difficile-induced diarrhea.
Conclusions
In conclusion, activation of A2A adenosine in combination with alanyl-glutamine reduced toxin-induced intestinal secretion, epithelial disruption and inflammation in the ileal loop model of C. difficile enteritis. A2A adenosine agonists and alanyl-glutamine may have therapeutic potential for the management of C. difficile infection.
References
Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, Posey K, Roberts T, Croyle K, Krystofiak S, Patel-Brown S, et al: A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005, 26: 273-280. 10.1086/502539.
Pepin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, Pepin K, Chouinard D: Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004, 171: 466-472. 10.1503/cmaj.1041104.
McDonald LC, Killgore GE, Thompson A, Owens RC, Kazakova SV, Sambol SP, Johnson S, Gerding DN: An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005, 353: 2433-2441. 10.1056/NEJMoa051590.
Johnson S, Homann SR, Bettin KM, Quick JN, Clabots CR, Peterson LR, Gerding DN: Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann Intern Med. 1992, 117: 297-302.
McFarland LV: Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol. 2008, 5: 40-48. 10.1038/ncpgasthep1029.
Carneiro-Filho BA, Bushen OY, Brito GA, Lima AA, Guerrant RL: Glutamine Analogues As Adjunctive Therapy for Infectious Diarrhea. Curr Infect Dis Rep. 2003, 5: 114-119. 10.1007/s11908-003-0046-2.
Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC: Glutamine-dependent changes in gene expression and protein activity. Cell Biochem Funct. 2005, 23: 77-84. 10.1002/cbf.1165.
Rhoads JM, Argenzio RA, Chen W, Rippe RA, Westwick JK, Cox AD, Berschneider HM, Brenner DA: L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997, 272: G943-G953.
Chang WK, Yang KD, Chuang H, Jan JT, Shaio MF: Glutamine protects activated human T cells from apoptosis by up-regulating glutathione and Bcl-2 levels. Clin Immunol. 2002, 104: 151-160. 10.1006/clim.2002.5257.
Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB: Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol. 1997, 272: G879-G884.
Brito GA, Fujji J, Carneiro-Filho BA, Lima AA, Obrig T, Guerrant RL: Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J Infect Dis. 2002, 186: 1438-1447. 10.1086/344729.
Carneiro BA, Fujii J, Brito GA, Alcantara C, Oria RB, Lima AA, Obrig T, Guerrant RL: Caspase and bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro. Infect Immun. 2006, 74: 81-87. 10.1128/IAI.74.1.81-87.2006.
Cronstein BN: Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994, 76: 5-13.
Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J: Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. Br J Pharmacol. 2001, 132: 1017-1026. 10.1038/sj.bjp.0703893.
Cavalcante IC, Castro MV, Barreto AR, Sullivan GW, Vale M, Almeida PR, Linden J, Rieger JM, Cunha FQ, Guerrant RL, et al: Effect of novel A2A adenosine receptor agonist ATL 313 on Clostridium difficile toxin A-induced murine ileal enteritis. Infect Immun. 2006, 74: 2606-2612. 10.1128/IAI.74.5.2606-2612.2006.
Alcantara C, Stenson WF, Steiner TS, Guerrant RL: Role of inducible cyclooxygenase and prostaglandins in Clostridium difficile toxin A-induced secretion and inflammation in an animal model. J Infect Dis. 2001, 184: 648-652. 10.1086/322799.
Alcantara CS, Jin XH, Brito GA, Carneiro-Filho BA, Barrett LJ, Carey RM, Guerrant RL: Angiotensin II subtype 1 receptor blockade inhibits Clostridium difficile toxin A-induced intestinal secretion in a rabbit model. J Infect Dis. 2005, 191: 2090-2096. 10.1086/430316.
Pawlowski SW, Calabrese G, Kolling GL, Freire R, Alcantara Warren C, Liu B, Sartor B, Guerrant RL: Murine model of Clostridium difficile infection using gnotobiotic aged C57Bl/6 mice and a BI strain. J Infect Dis. 2010, 202: 1708-1712. 10.1086/657086.
Pierce BCA Protein Assay Kit. [http://www.piercenet.com/instructions/2161296.pdf]
Quantikine® Mouse CXCL1/KC Immunoassay. [http://www.rndsystems.com/pdf/mkc00b.pdf]
Quantikine® Mouse IL-10 Immunoassay. [http://www.rndsystems.com/pdf/m1000.pdf]
Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25: 402-8. 10.1006/meth.2001.1262.
Lima AA, Lyerly DM, Wilkins TD, Innes DJ, Guerrant RL: Effects of Clostridium difficile toxins A and B in rabbit small and large intestine in vivo and on cultured cells in vitro. Infect Immun. 1988, 56: 582-588.
Pothoulakis C, Lamont JT: Microbes and microbial toxins: paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol. 2001, 280: G178-G183.
Voth DE, Ballard JD: Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005, 18: 247-263. 10.1128/CMR.18.2.247-263.2005.
Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP: The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010, 467: 711-3. 10.1038/nature09397.
Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI: Toxin B is essential for virulence of Clostridium difficile. Nature. 2009, 458: 1176-9. 10.1038/nature07822.
Lyerly DM, Saum KE, MacDonald DK, Wilkins TD: Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985, 47: 349-52.
Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J: International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001, 53: 527-552.
Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, et al: Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005, 129: 26-33. 10.1053/j.gastro.2005.05.032.
Bouma MG, van den Wildenberg FA, Buurman WA: Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am J Physiol. 1996, 270: C522-C529.
Blachier F, Boutry C, Bos C, Tome D: Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr. 2009, 90: 814S-821S. 10.3945/ajcn.2009.27462S.
Lacey JM, Wilmore DW: Is glutamine a conditionally essential amino acid?. Nutr Rev. 1990, 48: 297-309.
Papaconstantinou HT, Hwang KO, Rajaraman S, Hellmich MR, Townsend CM, Ko TC: Glutamine deprivation induces apoptosis in intestinal epithelial cells. Surgery. 1998, 124: 152-159. 10.1016/S0039-6060(98)70115-1.
Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB: Glutamine reduces cytokine release, organ damage, and mortality in a rat model of endotoxemia. Shock. 2001, 16: 398-402. 10.1097/00024382-200116050-00014.
Ban K, Kozar RA: Enteral glutamine: a novel mediator of PPARgamma in the postischemic gut. J Leukoc Biol. 2008, 84: 595-599. 10.1189/jlb.1107764.
Feoktistov I, Biaggioni I: Adenosine A2B receptors. Pharmacol Rev. 1997, 49: 381-402.
Strohmeier GR, Reppert SM, Lencer WI, Madara JL: The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995, 270: 2387-2394. 10.1074/jbc.270.5.2387.
Hasko G, Pacher P: A2A receptors in inflammation and injury: lessons learned from transgenic animals. J Leukoc Biol. 2008, 83: 447-455.
Moore CC, Martin EN, Lee GH, Obrig T, Linden J, Scheld WM: An A2A adenosine receptor agonist, ATL313, reduces inflammation and improves survival in murine sepsis models. BMC Infect Dis. 2008, 8: 141-10.1186/1471-2334-8-141.
Sullivan GW, Fang G, Linden J, Scheld WM: A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004, 189: 1897-1904. 10.1086/386311.
Bai MX, Jiang ZM, Liu YW, Wang WT, Li DM, Wilmore DW: Effects of alanyl-glutamine on gut barrier function. Nutrition. 1996, 12: 793-796. 10.1016/S0899-9007(96)00217-1.
Braga-Neto MB, Warren CA, Oria RB, Monteiro MS, Maciel AA, Brito GA, Lima AA, Guerrant RL: Alanyl-glutamine and glutamine supplementation improves 5-fluorouracil-induced intestinal epithelium damage in vitro. Dig Dis Sci. 2008, 53: 2687-2696. 10.1007/s10620-008-0215-0.
Lima AA, Carvalho GH, Figueiredo AA, Gifoni AR, Soares AM, Silva EA, Guerrant RL: Effects of an alanyl-glutamine-based oral rehydration and nutrition therapy solution on electrolyte and water absorption in a rat model of secretory diarrhea induced by cholera toxin. Nutrition. 2002, 18: 458-462. 10.1016/S0899-9007(02)00775-X.
Scheppach W, Loges C, Bartram P, Christl SU, Richter F, Dusel G, Stehle P, Fuerst P, Kasper H: Effect of free glutamine and alanyl-glutamine dipeptide on mucosal proliferation of the human ileum and colon. Gastroenterology. 1994, 107: 429-434.
Carneiro-Filho BA, Oria RB, Wood RK, Brito GA, Fujii J, Obrig T, Lima AA, Guerrant RL: Alanyl-glutamine hastens morphologic recovery from 5-fluorouracil-induced mucositis in mice. Nutrition. 2004, 20: 934-941. 10.1016/j.nut.2004.06.016.
Saito H, Terada T, Shimakura J, Katsura T, Inui K: Regulatory mechanism governing the diurnal rhythm of intestinal H+/peptide cotransporter 1 (PEPT1). Am J Physiol Gastrointest Liver Physiol. 2008, 295: G395-G402. 10.1152/ajpgi.90317.2008.
Schiller CM, Huang TI, Heizer WD: Isolation and characterization of four peptide hydrolases from the cytosol of rat intestinal mucosa. Gastroenterology. 1977, 72: 93-100.
Bloom PD, Boedeker EC: Mucosal immune responses to intestinal bacterial pathogens. Semin Gastrointest Dis. 1996, 7: 151-166.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/12/13/prepub
Acknowledgements
We acknowledge John Williams for assisting in rabbit surgeries, Dr. Relana Pinkerton for statistical support, Dr. David Lyerly for supplying Toxin A, Dr. Corrie Brown for comments on the histopathological scoring system, the UVA Histopathology Core and the UVA Biorepository and Tissue Core Facility. Funding for this study was provided by the National Institutes of Health/National Institutes of Allergy and Infectious Diseases grants U01 AI075526 and U01 AI070491.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
CAW, GMC, YL and SWP have no competing interests. RAF is partially funded by Dogwood Pharmaceuticals, Inc. (formerly ATL, LLC). JR is employed by Dogwood Pharmaceuticals, Inc. PBE has collaboration on NIH grant with Dogwood Pharmaceuticals, Inc. JL is a consultant for Dogwood Pharmaceuticals, Inc. RLG co-founded AlGlutamine, LLC.
Authors' contributions
CAW conceptualized study design, performed animal experiments, collated and analyzed data, drafted manuscript. GMC participated in animal experiments, assisted in study design, performed protein and gene expression assays and immunohistochemistry. YL participated in animal experiments and assisted in mouse study design. SWP participated in rabbit experiments. RAF participated in reviewing data and manuscript. JR participated in reviewing data and manuscript. PBE critically reviewed data and manuscript. JML critically reviewed data and manuscript. RLG critically reviewed data and manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Warren, C.A., Calabrese, G.M., Li, Y. et al. Effects of adenosine A2A receptor activation and alanyl-glutamine in Clostridium difficile toxin-induced ileitis in rabbits and cecitis in mice. BMC Infect Dis 12, 13 (2012). https://doi.org/10.1186/1471-2334-12-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-12-13