Abstract
Background
Although research productivity in the field of frailty has risen exponentially in recent years, there remains a lack of consensus regarding the measurement of this syndrome. This overview offers three services: first, we provide a comprehensive catalogue of current frailty measures; second, we evaluate their reliability and validity; third, we report on their popularity of use.
Methods
In order to identify relevant publications, we searched MEDLINE (from its inception in 1948 to May 2011); scrutinized the reference sections of the retrieved articles; and consulted our own files. An indicator of the frequency of use of each frailty instrument was based on the number of times it had been utilized by investigators other than the originators.
Results
Of the initially retrieved 2,166 papers, 27 original articles described separate frailty scales. The number (range: 1 to 38) and type of items (range of domains: physical functioning, disability, disease, sensory impairment, cognition, nutrition, mood, and social support) included in the frailty instruments varied widely. Reliability and validity had been examined in only 26% (7/27) of the instruments. The predictive validity of these scales for mortality varied: for instance, hazard ratios/odds ratios (95% confidence interval) for mortality risk for frail relative to non-frail people ranged from 1.21 (0.78; 1.87) to 6.03 (3.00; 12.08) for the Phenotype of Frailty and 1.57 (1.41; 1.74) to 10.53 (7.06; 15.70) for the Frailty Index. Among the 150 papers which we found to have used at least one of the 27 frailty instruments, 69% (n = 104) reported on the Phenotype of Frailty, 12% (n = 18) on the Frailty Index, and 19% (n = 28) on one of the remaining 25 instruments.
Conclusions
Although there are numerous frailty scales currently in use, reliability and validity have rarely been examined. The most evaluated and frequently used measure is the Phenotype of Frailty.
Similar content being viewed by others
Background
The global population of elderly people aged 60 years or more was 600 million in 2000; it is expected to rise to around 2 billion by 2050 [1]. With an aging population, researchers are increasingly interested in frailty [2, 3], a syndrome characterized by age-related declines in functional reserves across an array of physiologic systems. Frail older adults experience an increased risk of a number of adverse health outcomes such as comorbidity, disability, dependency, institutionalization, falls, fractures, hospitalization, and mortality [4–21]. Identification of frail adults is important as trial evidence suggest that frailty status might be reversible with the implementation of exercise programs or hormone treatment [22–25].
A series of frailty measures have emerged in recent years. The aim of this overview is three-fold: 1) provide a comprehensive catalogue of existing frailty measures; 2) review evidence on the validity and reliability of these measures; and 3) quantify the popularity of each frailty measure by investigators other than the originators.
Methods
Search strategy
We took three approaches. First, we searched the electronic database MEDLINE (1948 to May 2011) through the OvidSP interface for all articles using the keyword “frailty” (using the term “frail” yielded an unmanageably large literature with little relevance to the present aims). This strategy allowed us to identify articles where this keyword appeared at least once in the title, abstract, or subject heading. Second, the reference sections of the retrieved articles were scrutinized for additional relevant papers by manual searches. Third, we searched our own records which included interrogation of our own relational databases (e.g. Reference Manager, Endnote). This overview followed the guidelines for the Meta-analysis of Observational Studies in Epidemiology (MOOSE) [26].
Selection criteria
We included studies with participants aged 50 years and older at baseline examination in which the authors purport to have measured frailty. Further inclusion criteria were: 1) articles written in English, French, or Spanish; and 2) articles describing the reliability and validity of a frailty instrument.
Assessment of the reliability and validity of frailty measures
The reliability and validity were assessed using suggested guidelines [27, 28]. Reliability, which determines if a scale measures an entity (here frailty) in a reproducible way, was investigated through the following definitions: internal consistency (the average of the correlations among all items in the measure), intra-rater reliability (the agreement between observations made by the same rater on two different occasions), inter-rater reliability (the agreement between different raters), and test-retest reliability (the agreement between observations on the participants on two occasions separated by an interval of time). Validity – whether the scale in question measures what it purports to – was assessed by criterion and construct validity. Criterion validity refers to how well the instrument predicts an outcome. When frailty and the outcome data are collected simultaneously, the criterion validity is referred to as the concurrent validity. When the outcome data are prospectively collected, it is called predictive validity. Finally, in this context, construct validity refers to the extent to which a frailty measure correlates with factors that are, based on the extant literature, known to have an association (e.g. age, comorbidity, disability, physical capabilities or performances) [27, 28].
Use of frailty measurements by researchers
To evaluate the level of utilization of a given frailty instrument by researchers, we counted, among the selected articles, the number of publications which had been authored by researchers other than the originators in the periods ≤ 2000, 2001-2005, and ≥2006. In addition to this, we used the Scopus citation database [29] of peer-reviewed literature to analyze the number of citations in original research articles, excluding those cited by the creators of a given frailty instrument, for each frailty scales up to October 2011. In order to have an indication about the level of predictive validity of the identified frailty instruments, estimates – hazard ratios (or relative risks) and odds ratios – for the association between a frailty score and an adverse health outcome, in particular mortality, were examined.
Results
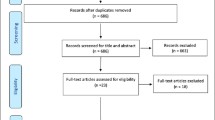
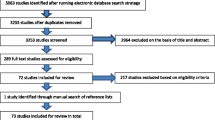
The initial keyword search using “frailty” identified 2,166 articles (Figure 1). Based on the content of the title and the abstract, 1,509 articles were excluded for the following reasons: article not published in English, French, or Spanish; article untraceable; studied population not of interest (animals, non-elderly population); statistical methods paper; or topic of the articles was not focused on measurement of frailty but its mechanism, predictors, prevention, intervention, and management/treatment. A further 209 papers were excluded because they were reviews rather than empirical papers. Of the remaining 448 articles, 27 [30–56] described the construction or psychometric properties of measures of frailty, and were included in this review. Among them, five instruments initially created to assess disability [57], vulnerability [58], and physical capabilities or performances [59–61] were used subsequently to assess frailty [36, 39, 41, 42, 44]. For these five instruments, their reliability, validity, and use were studied as a measure of frailty. A further 150 articles either applying or testing the validity of these 27 frailty measurements were included in our synthesis.
Classifications: self-report, objective, and mixed frailty measures
All 27 identified frailty measurements were grouped into three categories (Additional file 1: Table S1): subjective (self-reported items only), objective (inclusion of only directly measured components), or subjective and objective combined (mixed). Eleven of the 27 instruments included only subjective components which were either reported by a participant (self-evaluation) in nine out of 11 cases [30–34, 36, 38–40], or reported by a clinician or a researcher (hetero-evaluation) [35, 37]. Of the 27 frailty instruments, five included only objective components [41–45]. Finally, the remaining 11 instruments included both subjective and objective (mixed) components [46–56].
General description of frailty measurements
Of the 27 frailty assessments, 19 were developed in population-based samples [30–32, 34–37, 40–44, 46–48, 50, 51, 53, 55], 7 among hospitalized patients [33, 39, 45, 49, 52, 54, 56], and 1 without specification [38]. Half of the frailty scales (n=14) were created by research groups in the USA [30, 31, 36, 39, 41, 42, 43, 44, 46, 47, 48, 49, 53, 56], five in Canada [32, 34, 37, 52, 54], three in the Netherlands [33, 40, 51], two in Italy [38, 45], and one each in Australia [55], France [50], and Sweden [35]. Five of the 27 frailty instruments were adapted from those developed initially to assess functional status [57], vulnerability [58], or physical performances [59–61]. These were used to measure frailty for the first time by Cacciatore and colleagues [36], Kanauchi and colleagues [39], Brown and colleagues [41], Gill and colleagues [42], and Bandinelli and colleagues [44], respectively. Furthermore, recently tested tools assessing frailty such as Static/Dynamic Frailty Index [51], Study of Osteoporotic Fractures Index [53], FRAIL scale [55], and Comprehensive Assessment of Frailty [56] were based on the Fried’s frailty scale [47] and/or the Mitnitski’s Frailty Index [34].
All identified frailty measures were composed of at least two items, except that of Gerdhem and colleagues [35] where a general assessment of health is made within a 15-second observation by the investigator. Of the subjective and mixed frailty measures, most contained disability and/or comorbidity components. Instruments without disability or comorbidity information were: the 1994 Frailty Measure [31], Subjective Frailty Score [35], Tilburg Frailty Indicator [40] all objective measures (Modified Physical Performance Test [41], Physical Frailty Score [42], Klein’s frailty index [43], Short Physical Performance Battery [44], and Opasich’s frailty scale [45]), Speechley & Tinetti’s frailty scale [46], Fried’s frailty scale [47], Score-Risk Correspondence for dependency [50], Study of Osteoporotic Fractures Index [53], and Brief Frailty Index [54]. Further descriptions of characteristics of population and type of components included in each instrument are also provided in (Additional file 1: Table S1).
Assessment of the reliability and validity of frailty measures
Additional file 2: Table S2 presents reliability and validity data taken from the original articles and other related articles on the frailty measurements. Three approaches were used for reliability assessment: internal consistency, inter-rater, and test-retest reliability. Concurrent and predictive validity were mainly assessed using outcomes such as mortality, institutionalization, activities of daily living (ADL) disability, hospitalization, and quality of life. Only 7 out of 27 instruments (26%) were found to have had both reliability and validity ascertained [33, 35, 37, 40, 43, 49, 52].
Of all, 19 instruments had either their reliability or validity assessed. Among them, 4 instruments were tested for validity only once in the original sample/cohort of participants [32, 36, 55, 56], and the Phenotype of Frailty by Fried and colleagues [47] and the Frailty Index by Mitnitski and colleagues [34] had their concurrent or predictive validity assessed in more than 3 samples/cohorts (17 and 13 samples/cohorts, respectively). One instrument out of 27, the Short Physical Performance Battery, previously used to assess physical functioning [61], had neither reliability nor validity information in measuring frailty [44].
Information on the predictive validity was available for 16 instruments. In 69% (n=11/16), the predictive validity was quantified by relating the frailty measure to mortality. With average follow-ups varying from 1 month to 12 years, hazard ratios or relative risks (from Cox regression) or odds ratios (from logistic regression) for mortality risk for frail people relative to those with no record of the condition ranged from 1.21 (95% confidence interval (CI): 0.78; 1.87) to 6.03 (95% CI: 3.00; 12.08) for the Phenotype of Frailty [47] and 1.57 (95% CI: 1.41; 1.74) to 10.53 (95% CI: 7.06; 15.70) for the Frailty Index [34]. The Phenotype of Frailty has been rarely used in a continuous fashion. One exception is Kulminski et al who found an increased mortality risk of 2% (RR=1.02; 95% CI: 1.02; 1.03) for a one unit of increase in this scale. For the Frailty Index, the estimates ranged from 1.008 (95% CI: 1.005; 1.011) to 10.53 (95% CI: 7.06; 15.70). The estimates – hazard ratios (or relative risks) and odds ratios – examining the association between a frailty score and mortality do not allow to affirm which score is the best in the prediction of mortality for several reasons: 1) relative risks and odds ratios are calculated differently [62]; 2) estimates were assessed in different populations, therefore with different baseline risks; 3) follow-ups and adjustment for confounding factors were heterogeneous. In spite of these limits, the estimates in Additional file 2: Table S2 give a qualitative appreciation on the magnitude of the association between a frailty score and mortality.
Use of frailty instruments
Additional file 3: Table S3 presents the number of publications in which a frailty measure had been used by investigators other than those who created it. In 69% of publications, a frailty scale developed by Fried and colleagues [47] was utilized; 12% used the Frailty Index developed by Mitnitski and colleagues [34]; 4% the Edmonton Frail Scale [52]; and ≤ 2% used the remaining instruments. This analysis also shows that half the frailty instruments (n=14) have not been employed at all by other researchers [30, 35, 36, 38, 43–45, 48–51, 54–56]. Figure 2 displays the number of original research articles based on the Scopus citation database, which referenced one of the 27 frailty instruments: the 3 most cited papers were that of Fried and colleagues, 2001 [47] (n=676), Speechley and colleagues, 1991 [46] (n=167), and Gill and colleagues, 2002 [42] (n=150). The citation rank for Mitnitski and colleagues’ paper, 2002 [34] was ninth (n=52).
Discussion
In this overview, we aimed at providing a comprehensive catalogue of frailty measures, reviewing evidence on their validity and reliability, and quantifying the use of each measure by investigators other than the originators. We identified 27 frailty scales used in 150 studies to date. We made a series of observations. First, although frailty, disability, and comorbidity are inter-related, they are distinct clinical entities [63, 64]. Integrating disability or comorbidity items into a frailty scale may be debatable as they are not equivalent concepts. However, half the frailty instruments (n=14) include either disability or comorbidity components [30, 32–34, 36–39, 48, 49, 51, 52, 55],[56]. Second, at least five measures [36, 39, 41, 42, 44] of frailty were originally created to measure vulnerability, functional status, and physical performances, suggesting a lack of terminological rigor. Third, we observed that four recent scales [51, 53, 55, 56] are based on existing measures, in particular the Fried scale. Finally, confusion between frailty scales can be generated because sometimes a specific instrument is named differently in different studies (the Fried scale [47] being labelled as Fried Frailty Index [65] on occasion). Elsewhere, several instruments are identically named but have different item content: for instance, the term “frailty index” was used by different researchers [34, 43, 54]. This was also the case with “frail scale” [52, 66].
Assessment of the reliability and validity of frailty measures
The Standards for Educational and Psychological Testing [67], a guideline which describes the best practice in the development of complex measures such as frailty, recommends the reporting of the basic principles of test construction such as reliability and validity. However, this information was available only for a few instruments: CSHA Clinical Frailty Scale [32] and Edmonton Frail Scale [52]. They had acceptable reliability (Kappa coefficient ≥ 0.7) and good concurrent and predictive validity. Two instruments were widely tested for their validity but not reliability: the Frailty Index [34] and the Fried’s scale [47]. Reliability and validity are the most important indicators when selecting one measure over another. However, even among 7 frailty measurements with such information [33, 35, 37, 40, 43, 49, 52], none of them appear to be recognized as a “gold standard”. Comparing the performances of different frailty scales in predicting an objective health outcome such as mortality was complicated by the use of different confounding factors across studies.
In several studies, investigators have examined the inter-relationships between different measures of frailty. Thus, the Fried’s scale has been compared with the Frailty Index [10, 68, 69] and the Study of Osteoporotic Fracture index [15, 53] using different methods: correlation analyses [69], comparison of strength of cross-sectional [68] and prospective associations [10, 15], and use of the c-index statistic [53]. The Fried’s scale is moderately well correlated with the Frailty Index [69], and shows a stronger association with age and sex (important criteria of construct validity [28]) [68] but a weaker association with mortality [10]. The Fried’s scale and the Study of Osteoporotic Fracture index have a similar strength of association with falls, disability, hospitalization [15] and death [53]. As Streiner and Norman [27] highlighted, we found that it was sometimes difficult to disentangle whether an assessment belongs to concurrent validity or construct validity. Therefore, certain classifications in either category might be arguable.
Use of the frailty instruments
We attempted to assess the use of a frailty instrument by counting the number of publications that had adopted the instrument other than the original creators. The two instruments which have had their external validity most extensively evaluated against adverse health outcomes were those developed by Fried group (Phenotype of Frailty) and Mitnitski group (Frailty Index). These are based on two different conceptual frameworks. The Fried group has suggested that frailty represents a phenotype which reflects underlying age-related changes in multiple systems. By contrast, the Mitniski group advances that frailty is the accumulation of multiple deficits, with the degree of frailty denoted by the number of such deficits. This highlights that although some investigators recognize that frailty, comorbidity, and disability are distinct entities [28, 47, 70], for others they are overlapping. Most reviews or editorials on frailty have implicitly presented the Phenotype of Frailty as standard [63, 71–81] whereas for others the standard is the Frailty Index [82, 83]. Recommendations from other researchers are more nuanced. For Sternberg and colleagues [84], the choice depends on the definition and outcomes that best suit the investigators or clinicians responsible for the screening. The European, Canadian and American Geriatric Advisory Panel [66] recommend using a hybrid measure, the “FRAIL” scale, comprising components from both the Phenotype of Frailty and the Frailty Index.
The Fried’s scale [47] has been the most extensively tested for its validity and is the most widely used instrument in frailty research [65, 78, 85–134]. Randomized controlled trials have also used the scale to screen elderly participants [24, 25, 135–140], or as an outcome of interventions [22, 23, 139]. The Fried’s scale is widely used, allowing comparisons to be made between studies.
The main limitation of our assessment of use of these instruments is that it penalizes the more recently published frailty instruments. However, the Fried’s scale is not the oldest measure in the field and several more recent frailty instruments are either derived or similar to that measure, suggesting that qualities other than duration of availability explain the popularity of this instrument. Another limitation lies in the lack of elimination of articles that may have resulted from the original authors’ circle of influence. For example, some of the articles which report on the use of the Fried’s scale may have been produced from former co-workers who had previously utilized the CHS data – the dataset in which the Fried’s scale was first validated.
In spite of its wide use, the Fried’s scale has some drawbacks common to other frailty instruments. Chiefly, different scales utilize different classification of the individual components. For example, in the Cardiovascular Health Study (CHS), participants were considered positive for weight loss if they reported having lost more than 10 pounds unintentionally in the last year or they objectively lost 5% or more in comparison with the previous year’s body weight [47]. In Women’s Health Aging Study-I, however, a cut-off of 10% in comparison with the self-reported weight at age 60 years [4] was utilized. These important variations in the operationalization of frailty measurement render comparisons of findings between studies as problematic.
In addition to the manual counting procedure to estimate the use of the frailty instruments, we also examined the number of citations in original research articles (excluding those cited by the creators of a given frailty instrument) for the 27 papers describing the frailty instruments. Even though the rank of citations was different for some of the frailty instruments than that of the manual counting, the paper on the Fried’s scale was still the most highly cited. Although the number of citations can be easily accessed, this electronic database search cannot replace the manual counting method as the papers citing the original articles do not necessarily use the tool in question.
Among previously published reviews [66, 83, 84, 141–145] on frailty measures, only one [83] assessed them in terms of reliability and validity. Compared with the De Vries and colleagues’ paper [83], our review presents additional strengths. First, to evaluate reliability and validity of a given instrument, we have extracted data from other studies, reflecting its level of external validation. Second, to our knowledge, no article has been published on the extent to which frailty measures have been used by other researchers. This finding might reflect the level preference of researchers for a given frailty measurement in the absence of a consensually recognized tool. Moreover, we identified 18 other frailty instruments [30, 32, 35–38, 40–46, 48, 52, 54–56], 5 of them created in 2010 and after. Another limitation of our review may lie in the use of a unique keyword “frailty” to identify relevant publications on frailty measurements. One may find such a strategy restrictive, leading to miss some screening tools helping to identify frail elderly. In fact, we included similar frailty instruments than those comprised in the recent reviews [83, 84].
Conclusions
This review provides a comprehensive overview of existing frailty measurements. We identified 27 measures of frailty but none of them have been recognized as a gold standard. Difficulties in accepting one measure as a reference may lie in the following reasons: the existence of frailty as a clinical entity is quite new; the definition of frailty is still debatable, therefore, it is difficult to create a composite measure that would meet all criteria. Components to include in the frailty instruments need to be further discussed to reach a consensus, in particular on whether to include disability and/or disease data. The most widely used frailty measurements by investigators [34, 47], such as the frailty scales developed by Fried and colleagues and Mitnitski and colleagues need to be further assessed, including attempts to improve them, before being recognized as a gold standard.
Abbreviations
- ADL:
-
Activities of daily living
- CHS:
-
Cardiovascular health study
- CSHA:
-
Canadian study of health and aging.
References
World Health Organization: Ageing and life course. 2011, http://www.who.int/ageing/age_friendly_cities/en/index.html,
Karunananthan S, Wolfson C, Bergman H, Beland F, Hogan DB: A multidisciplinary systematic literature review on frailty: overview of the methodology used by the Canadian Initiative on Frailty and Aging. BMC Med Res Methodol. 2009, 9: 68-10.1186/1471-2288-9-68.
Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al: Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006, 54: 991-1001. 10.1111/j.1532-5415.2006.00745.x.
Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP: Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005, 118: 1225-1231. 10.1016/j.amjmed.2005.01.062.
Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al: Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005, 53: 1321-1330. 10.1111/j.1532-5415.2005.53405.x.
Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al: Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006, 61: 262-266. 10.1093/gerona/61.3.262.
Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, et al: Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007, 55: 1216-1223. 10.1111/j.1532-5415.2007.01259.x.
Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, et al: Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007, 62: 744-751. 10.1093/gerona/62.7.744.
Avila-Funes JA, Helmer C, Amieva H, Barberger-Gateau P, Le Goff M, Ritchie K, et al: Frailty among community-dwelling elderly people in France: the three-city study. J Gerontol A Biol Sci Med Sci. 2008, 63: 1089-1096. 10.1093/gerona/63.10.1089.
Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI: Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008, 56: 898-903. 10.1111/j.1532-5415.2008.01656.x.
Sarkisian CA, Gruenewald TL, Boscardin WJ, Seeman TE: Preliminary evidence for subdimensions of geriatric frailty: the MacArthur study of successful aging. J Am Geriatr Soc. 2008, 56: 2292-2297. 10.1111/j.1532-5415.2008.02041.x.
Al Snih S, Graham JE, Ray LA, Samper-Ternent R, Markides KS, Ottenbacher KJ: Frailty and incidence of activities of daily living disability among older Mexican Americans. J Rehabil Med. 2009, 41: 892-897. 10.2340/16501977-0424.
Buchman AS, Wilson RS, Bienias JL, Bennett DA: Change in frailty and risk of death in older persons. Exp Aging Res. 2009, 35: 61-82. 10.1080/03610730802545051.
Graham JE, Al Snih S, Berges IM, Ray LA, Markides KS, Ottenbacher KJ: Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology. 2009, 55: 644-651. 10.1159/000235653.
Kiely DK, Cupples LA, Lipsitz LA: Validation and comparison of two frailty indexes: the MOBILIZE Boston Study. J Am Geriatr Soc. 2009, 57: 1532-1539. 10.1111/j.1532-5415.2009.02394.x.
Ottenbacher KJ, Graham JE, Al Snih S, Raji M, Samper-Ternent R, Ostir GV, et al: Mexican Americans and frailty: findings from the Hispanic established populations epidemiologic studies of the elderly. Am J Public Health. 2009, 99: 673-679. 10.2105/AJPH.2008.143958.
Gill TM, Gahbauer EA, Han L, Allore HG: Trajectories of disability in the last year of life. N Engl J Med. 2010, 362: 1173-1180. 10.1056/NEJMoa0909087.
Rochat S, Cumming RG, Blyth F, Creasey H, Handelsman D, Le Couteur DG, et al: Frailty and use of health and community services by community-dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing. 2010, 39: 228-233. 10.1093/ageing/afp257.
Seematter-Bagnoud L, Santos-Eggimann B, Rochat S, Martin E, Karmaniola A, Aminian K, et al: Vulnerability in high-functioning persons aged 65 to 70 years: the importance of the fear factor. Aging Clin Exp Res. 2010, 22: 212-218.
Wong CH, Weiss D, Sourial N, Karunananthan S, Quail JM, Wolfson C, et al: Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: a cross-sectional study. Aging Clin Exp Res. 2010, 22: 54-62.
Bouillon K, Sabia S, Jokela M, Gale CR, Singh-Manoux A, Shipley MJ, et al: Validating a widely used measure of frailty: are all sub-components necessary? Evidence from the Whitehall II cohort study. Age (forthcoming). 10.1007/s11357-012-9446-2.
Faber MJ, Bosscher RJ, Chin APM, van Wieringen PC: Effects of exercise programs on falls and mobility in frail and pre-frail older adults: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006, 87: 885-896. 10.1016/j.apmr.2006.04.005.
Peterson MJ, Sloane R, Cohen HJ, Crowley GM, Pieper CF, Morey MC: Effect of telephone exercise counseling on frailty in older veterans: project LIFE. Am J Mens Health. 2007, 1: 326-334. 10.1177/1557988307306153.
Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA: Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010, 58: 1707-1714. 10.1111/j.1532-5415.2010.03019.x.
Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, et al: Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010, 95: 639-650. 10.1210/jc.2009-1251.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al: Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000, 283: 2008-2012. 10.1001/jama.283.15.2008.
Streiner DL, Norman GR: Health measurement scales: a practical guide to their development and use. 2008, Oxford, UK: Oxford University Press, 4
Rockwood K: What would make a definition of frailty successful?. Age Ageing. 2005, 34: 432-434. 10.1093/ageing/afi146.
Elsevier. Scopus. 2011, http://www.scopus.com,
Dayhoff NE, Suhrheinrich J, Wigglesworth J, Topp R, Moore S: Balance and muscle strength as predictors of frailty among older adults. J Gerontol Nurs. 1998, 24: 18-27.
Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA: Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998, 53: S9-16.
Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB: A brief clinical instrument to classify frailty in elderly people. Lancet. 1999, 353: 205-206. 10.1016/S0140-6736(98)04402-X.
Steverink N, Slaets JPJ, Schuurmans H, van Lis M: Measuring frailty: developing and testing the GFI (Groningen Frailty Indicator) [abstract]. Gerontologist. 2001, 41: 236-237.
Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K: Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002, 2: 1-10.1186/1471-2318-2-1.
Gerdhem P, Ringsberg KA, Magnusson H, Obrant KJ, Akesson K: Bone mass cannot be predicted by estimations of frailty in elderly ambulatory women. Gerontology. 2003, 49: 168-172. 10.1159/000069169.
Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D’Ambrosio D, et al: Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005, 35: 723-730. 10.1111/j.1365-2362.2005.01572.x.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al: A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005, 173: 489-495.
Amici A, Baratta A, Linguanti A, Giudice G, Servello A, Scalise C, et al: The Marigliano-Cacciafesta polypathological scale: a tool for assessing fragility. Arch Gerontol Geriatr. 2008, 46: 327-334. 10.1016/j.archger.2007.05.007.
Kanauchi M, Kubo A, Kanauchi K, Saito Y: Frailty, health-related quality of life and mental well-being in older adults with cardiometabolic risk factors. Int J Clin Pract. 2008, 62: 1447-1451. 10.1111/j.1742-1241.2008.01830.x.
Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM: The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc. 2010, 11: 344-355. 10.1016/j.jamda.2009.11.003.
Brown M, Sinacore DR, Binder EF, Kohrt WM: Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000, 55: M350-M355. 10.1093/gerona/55.6.M350.
Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A: A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002, 347: 1068-1074. 10.1056/NEJMoa020423.
Klein BE, Klein R, Knudtson MD, Lee KE: Relationship of measures of frailty to visual function: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2003, 101: 191-196.
Bandinelli S, Lauretani F, Boscherini V, Gandi F, Pozzi M, Corsi AM, et al: A randomized, controlled trial of disability prevention in frail older patients screened in primary care: the FRASI study. Design and baseline evaluation. Aging Clin Exp Res. 2006, 18: 359-366.
Opasich C, Patrignani A, Mazza A, Gualco A, Cobelli F, Pinna GD: An elderly-centered, personalized, physiotherapy program early after cardiac surgery. Eur J Cardiov Prev R. 2010, 17: 582-587. 10.1097/HJR.0b013e3283394977.
Speechley M, Tinetti M: Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc. 1991, 39: 46-52.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al: Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001, 56: M146-M156. 10.1093/gerona/56.3.M146.
Binder EF, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, et al: Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002, 50: 1921-1928. 10.1046/j.1532-5415.2002.50601.x.
Studenski S, Hayes RP, Leibowitz RQ, Bode R, Lavery L, Walston J, et al: Clinical Global Impression of Change in Physical Frailty: development of a measure based on clinical judgment. J Am Geriatr Soc. 2004, 52: 1560-1566. 10.1111/j.1532-5415.2004.52423.x.
Carriere I, Colvez A, Favier F, Jeandel C, Blain H, EPIDOS study group: Hierarchical components of physical frailty predicted incidence of dependency in a cohort of elderly women. J Clin Epidemiol. 2005, 58: 1180-1187. 10.1016/j.jclinepi.2005.02.018.
Puts MT, Lips P, Deeg DJ: Static and dynamic measures of frailty predicted decline in performance-based and self-reported physical functioning. J Clin Epidemiol. 2005, 58: 1188-1198. 10.1016/j.jclinepi.2005.03.008.
Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K: Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006, 35: 526-529. 10.1093/ageing/afl041.
Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, et al: Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008, 168: 382-389. 10.1001/archinternmed.2007.113.
Freiheit EA, Hogan DB, Eliasziw M, Meekes MF, Ghali WA, Partlo LA, et al: Development of a frailty index for patients with coronary artery disease. J Am Geriatr Soc. 2010, 58: 1526-1531. 10.1111/j.1532-5415.2010.02961.x.
Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SA, et al: Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab. 2010, 95: 3165-3172. 10.1210/jc.2009-2754.
Sundermann S, Dademasch A, Praetorius J, Kempfert J, Dewey T, Falk V, et al: Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011, 39: 33-37. 10.1016/j.ejcts.2010.04.013.
Lachs MS, Feinstein AR, Cooney LMJ, Drickamer MA, Marottoli RA, Pannill FC, et al: A simple procedure for general screening for functional disability in elderly patients. Ann Intern Med. 1990, 112: 699-706. 10.7326/0003-4819-112-9-699.
Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, et al: The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001, 49: 1691-1699. 10.1046/j.1532-5415.2001.49281.x.
Reuben DB, Siu AL: An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990, 38: 1105-1112.
Gill TM, Richardson ED, Tinetti ME: Evaluating the risk of dependence in activities of daily living among community-living older adults with mild to moderate cognitive impairment. J Gerontol A Biol Sci Med Sci. 1995, 50: M235-M241.
Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB: Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995, 332: 556-561. 10.1056/NEJM199503023320902.
Davies HT, Crombie IK, Tavakoli M: When can odds ratios mislead?. BMJ. 1998, 316: 989-991. 10.1136/bmj.316.7136.989.
Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al: Frailty: an emerging research and clinical paradigm–issues and controversies. J Gerontol A Biol Sci Med Sci. 2007, 62: 731-737. 10.1093/gerona/62.7.731.
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G: Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004, 59: 255-263. 10.1093/gerona/59.3.M255.
Chang CI, Chan DC, Kuo KN, Hsiung CA, Chen CY: Vitamin D insufficiency and frailty syndrome in older adults living in a Northern Taiwan community. Arch Gerontol Geriatr. 2010, 50 (Suppl 1): S17-S21.
van Abellan Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B: The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008, 12: 29-37. 10.1007/BF02982161.
American Educational Research Association: American Psychological Association, National Council on Measurement in Education: Standards for educational and psychological testing. 1999, Washington DC: American Educational Research Association
Cigolle CT, Ofstedal MB, Tian Z, Blaum CS: Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009, 57: 830-839. 10.1111/j.1532-5415.2009.02225.x.
Rockwood K, Andrew M, Mitnitski A: A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007, 62: 738-743. 10.1093/gerona/62.7.738.
Campbell AJ, Buchner DM: Unstable disability and the fluctuations of frailty. Age Ageing. 1997, 26: 315-318. 10.1093/ageing/26.4.315.
Ahmed N, Mandel R, Fain MJ: Frailty: an emerging geriatric syndrome. Am J Med. 2007, 120: 748-753. 10.1016/j.amjmed.2006.10.018.
Boockvar KS, Meier DE: Palliative care for frail older adults: "there are things I can't do anymore that I wish I could…". JAMA. 2006, 296: 2245-2253. 10.1001/jama.296.18.2245.
Brown NA, Zenilman ME: The impact of frailty in the elderly on the outcome of surgery in the aged. Adv Surg. 2010, 44: 229-249. 10.1016/j.yasu.2010.05.014.
Bylow K, Mohile SG, Stadler WM, Dale W: Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer?: a conceptual review. Cancer. 2007, 110: 2604-2613. 10.1002/cncr.23084.
Duque G: Taking musculoskeletal aging out of the bench: do we finally understand frailty?. Mol Aspects Med. 2005, 26: 141-143. 10.1016/j.mam.2005.01.001.
Evans WJ, Paolisso G, Abbatecola AM, Corsonello A, Bustacchini S, Strollo F, et al: Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010, 11: 527-536. 10.1007/s10522-010-9297-0.
Ferrucci L, Maggio M, Ceda GP, Beghi C, Valenti G, De Cicco G: Acute postoperative frailty. J Am Coll Surg. 2006, 203: 134-135. 10.1016/j.jamcollsurg.2006.03.005.
Hubbard RE, Woodhouse KW: Frailty, inflammation and the elderly. Biogerontology. 2010, 11: 635-641. 10.1007/s10522-010-9292-5.
Lally F, Crome P: Understanding frailty. Postgrad Med J. 2007, 83: 16-20. 10.1136/pgmj.2006.048587.
Phan HM, Alpert JS, Fain M: Frailty, inflammation, and cardiovascular disease: evidence of a connection. Am J Geriatr Cardiol. 2008, 17: 101-107.
Rolland Y, van Abellan Kan G, Benetos A, Blain H, Bonnefoy M, Chassagne P, et al: Frailty, osteoporosis and hip fracture: causes, consequences and therapeutic perspectives. J Nutr Health Aging. 2008, 12: 335-346.
Fisher AL: Just what defines frailty?. J Am Geriatr Soc. 2005, 53: 2229-2230. 10.1111/j.1532-5415.2005.00510.x.
de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW: Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011, 10: 104-114. 10.1016/j.arr.2010.09.001.
Sternberg SA, Wershof SA, Karunananthan S, Bergman H, Clarfield AM: The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011, 59: 2129-2138. 10.1111/j.1532-5415.2011.03597.x.
Bartali B, Frongillo EA, Bandinelli S, Lauretani F, Semba RD, Fried LP, et al: Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006, 61: 589-593. 10.1093/gerona/61.6.589.
Beasley JM, LaCroix AZ, Neuhouser ML, Huang Y, Tinker L, Woods N, et al: Protein intake and incident frailty in the Women's Health Initiative observational study. J Am Geriatr Soc. 2010, 58: 1063-1071. 10.1111/j.1532-5415.2010.02866.x.
Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP: The association between obesity and the frailty syndrome in older women: the Women's Health and Aging Studies. J Am Geriatr Soc. 2005, 53: 927-934. 10.1111/j.1532-5415.2005.53300.x.
Ble A, Cherubini A, Volpato S, Bartali B, Walston JD, Windham BG, et al: Lower plasma vitamin E levels are associated with the frailty syndrome: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006, 61: 278-283. 10.1093/gerona/61.3.278.
Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM: The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008, 56: 454-461. 10.1111/j.1532-5415.2007.01601.x.
Boxer RS, Wang Z, Walsh SJ, Hager D, Kenny AM: The utility of the 6-minute walk test as a measure of frailty in older adults with heart failure. Am J Geriatr Cardiol. 2008, 17: 7-12. 10.1111/j.1076-7460.2007.06457.x.
Buchman AS, Boyle PA, Wilson RS, Gu L, Bienias JL, Bennett DA: Pulmonary function, muscle strength and mortality in old age. Mech Ageing Dev. 2008, 129: 625-631. 10.1016/j.mad.2008.07.003.
Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, et al: Sex Hormones and Frailty in Older Men: The Osteoporotic Fractures in Men (MrOS) Study. J Clin Endocrinol Metab. 2009, 94: 3806-3815. 10.1210/jc.2009-0417.
Cesari M, Leeuwenburgh C, Lauretani F, Onder G, Bandinelli S, Maraldi C, et al: Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006, 83: 1142-1148.
Chaves PH, Varadhan R, Lipsitz LA, Stein PK, Windham BG, Tian J, et al: Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J Am Geriatr Soc. 2008, 56: 1698-1703. 10.1111/j.1532-5415.2008.01858.x.
Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al: HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007, 62: 1279-1286. 10.1093/gerona/62.11.1279.
Desquilbet L, Margolick JB, Fried LP, Phair JP, Jamieson BD, Holloway M, et al: Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr. 2009, 50: 299-306. 10.1097/QAI.0b013e3181945eb0.
Endeshaw YW, Unruh ML, Kutner M, Newman AB, Bliwise DL: Sleep-disordered breathing and frailty in the Cardiovascular Health Study Cohort. Am J Epidemiol. 2009, 170: 193-202. 10.1093/aje/kwp108.
Ensrud KE, Blackwell TL, Redline S, Ancoli-Israel S, Paudel ML, Cawthon PM, et al: Sleep disturbances and frailty status in older community-dwelling men. J Am Geriatr Soc. 2009, 57: 2085-2093. 10.1111/j.1532-5415.2009.02490.x.
Ensrud KE, Ewing SK, Fredman L, Hochberg MC, Cauley JA, Hillier TA, et al: Circulating 25-hydroxyvitamin D levels and frailty status in older women. J Clin Endocrinol Metab. 2010, 95: 5266-5273. 10.1210/jc.2010-2317.
Ensrud KE, Blackwell TL, Cauley JA, Cummings SR, Barrett-Connor E, Dam TT, et al: Circulating 25-hydroxyvitamin D levels and frailty in older men: the osteoporotic fractures in men study. J Am Geriatr Soc. 2011, 59: 101-106. 10.1111/j.1532-5415.2010.03201.x.
Frisoli A, Chaves PH, Ingham SJ, Fried LP: Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women's Health and Aging Study (WHAS) II. Bone. 2011, 48: 952-957. 10.1016/j.bone.2010.12.025.
Hubbard RE, O'Mahony MS, Calver BL, Woodhouse KW: Nutrition, inflammation, and leptin levels in aging and frailty. J Am Geriatr Soc. 2008, 56: 279-284. 10.1111/j.1532-5415.2007.01548.x.
Hubbard RE, O'Mahony MS, Savva GM, Calver BL, Woodhouse KW: Inflammation and frailty measures in older people. J Cell Mol Med. 2009, 13: 3103-3109. 10.1111/j.1582-4934.2009.00733.x.
Kang HG, Costa MD, Priplata AA, Starobinets OV, Goldberger AL, Peng CK, et al: Frailty and the degradation of complex balance dynamics during a dual-task protocol. J Gerontol A Biol Sci Med Sci. 2009, 64: 1304-1311.
Le Couteur DG, Blyth FM, Creasey HM, Handelsman DJ, Naganathan V, Sambrook PN, et al: The association of alanine transaminase with aging, frailty, and mortality. J Gerontol A Biol Sci Med Sci. 2010, 65: 712-717.
Leng SX, Xue QL, Tian J, Walston JD, Fried LP: Inflammation and frailty in older women. J Am Geriatr Soc. 2007, 55: 864-871. 10.1111/j.1532-5415.2007.01186.x.
Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP: White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2009, 64: 499-502.
Matteini AM, Walston JD, Fallin MD, Bandeen-Roche K, Kao WH, Semba RD, et al: Markers of B-vitamin deficiency and frailty in older women. J Nutr Health Aging. 2008, 12: 303-308. 10.1007/BF02982659.
Mohr BA, Bhasin S, Kupelian V, Araujo AB, O'Donnell AB, McKinlay JB: Testosterone, sex hormone-binding globulin, and frailty in older men. J Am Geriatr Soc. 2007, 55: 548-555. 10.1111/j.1532-5415.2007.01121.x.
Montero-Odasso M, Muir SW, Hall M, Doherty TJ, Kloseck M, Beauchet O, et al: Gait Variability Is Associated With Frailty in Community-dwelling Older Adults. J Gerontol A Biol Sci Med Sci. 2011, 66: 568-576.
Moore AZ, Biggs ML, Matteini A, O'Connor A, McGuire S, Beamer BA, et al: Polymorphisms in the mitochondrial DNA control region and frailty in older adults. PLoS ONE. 2010, 5: e11069-10.1371/journal.pone.0011069.
Qu T, Yang H, Walston JD, Fedarko NS, Leng SX: Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009, 46: 319-324. 10.1016/j.cyto.2009.02.015.
Raji MA, Al Snih S, Ostir GV, Markides KS, Ottenbacher KJ: Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010, 65: 1228-1234.
Romero-Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW: Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J Am Geriatr Soc. 2011, 59: 655-665. 10.1111/j.1532-5415.2011.03352.x.
Ronning B, Wyller TB, Seljeflot I, Jordhoy MS, Skovlund E, Nesbakken A, et al: Frailty measures, inflammatory biomarkers and post-operative complications in older surgical patients. Age Ageing. 2010, 39: 758-761. 10.1093/ageing/afq123.
Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD: Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005, 53: 747-754. 10.1111/j.1532-5415.2005.53250.x.
Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP: T cell subsets and mortality in older community-dwelling women. Exp Gerontol. 2005, 40: 81-87. 10.1016/j.exger.2004.09.006.
Semba RD, Bartali B, Zhou J, Blaum C, Ko CW, Fried LP: Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci. 2006, 61: 594-599. 10.1093/gerona/61.6.594.
Serra-Prat M, Palomera E, Clave P, Puig-Domingo M: Effect of age and frailty on ghrelin and cholecystokinin responses to a meal test. Am J Clin Nutr. 2009, 89: 1410-1417. 10.3945/ajcn.2008.27076.
Serviddio G, Romano AD, Greco A, Rollo T, Bellanti F, Altomare E, et al: Frailty syndrome is associated with altered circulating redox balance and increased markers of oxidative stress. Int J Immunopathol Pharmacol. 2009, 22: 819-827.
Shardell M, Hicks GE, Miller RR, Kritchevsky S, Andersen D, Bandinelli S, et al: Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci. 2009, 64: 69-75.
Terzian AS, Holman S, Nathwani N, Robison E, Weber K, Young M, et al: Factors Associated with Preclinical Disability and Frailty among HIV-Infected and HIV-Uninfected Women in the Era of cART. J Womens Health (Larchmt ). 2009, 18: 1965-1974. 10.1089/jwh.2008.1090.
Theou O, Jones GR, Vandervoort AA, Jakobi JM: Daily muscle activity and quiescence in non-frail, pre-frail, and frail older women. Exp Gerontol. 2010, 45: 909-917. 10.1016/j.exger.2010.08.008.
Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP: Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008, 63: 190-195. 10.1093/gerona/63.2.190.
Varadhan R, Chaves PH, Lipsitz LA, Stein PK, Tian J, Windham BG, et al: Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J Gerontol A Biol Sci Med Sci. 2009, 64: 682-687.
Vaz Fragoso CA, Gahbauer EA, Van Ness PH, Gill TM: Sleep-wake disturbances and frailty in community-living older persons. J Am Geriatr Soc. 2009, 57: 2094-2100. 10.1111/j.1532-5415.2009.02522.x.
Voznesensky M, Walsh S, Dauser D, Brindisi J, Kenny AM: The association between dehydroepiandosterone and frailty in older men and women. Age Ageing. 2009, 38: 401-406. 10.1093/ageing/afp015.
Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al: Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002, 162: 2333-2341. 10.1001/archinte.162.20.2333.
Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, et al: Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010, 171: 1144-1152. 10.1093/aje/kwq062.
Wang GC, Talor MV, Rose NR, Cappola AR, Chiou RB, Weiss C, et al: Thyroid autoantibodies are associated with a reduced prevalence of frailty in community-dwelling older women. J Clin Endocrinol Metab. 2010, 95: 1161-1168. 10.1210/jc.2009-1991.
Wilhelm-Leen ER, Hall YN, Deboer IH, Chertow GM: Vitamin D deficiency and frailty in older Americans. J Intern Med. 2010, 268: 171-180. 10.1111/j.1365-2796.2010.02248.x.
Wu CH, Chen CY, Wu YC, Weng LJ, Baai-Shyun H: Diabetes mellitus and functional impairment in Taiwanese older men and women. Arch Gerontol Geriatr. 2010, 50 (Suppl 1): S6-10.
Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K: Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010, 65: 377-381.
Wu IC, Shiesh SC, Kuo PH, Lin XZ: High oxidative stress is correlated with frailty in elderly chinese. J Am Geriatr Soc. 2009, 57: 1666-1671. 10.1111/j.1532-5415.2009.02392.x.
Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA, Kenny AM: Effects of dehydroepiandrosterone (DHEA) on cardiovascular risk factors in older women with frailty characteristics. Age Ageing. 2010, 39: 451-458. 10.1093/ageing/afq043.
Drey M, Pfeifer K, Sieber CC, Bauer JM: The Fried frailty criteria as inclusion criteria for a randomized controlled trial: personal experience and literature review. Gerontology. 2011, 57: 11-18. 10.1159/000313433.
Fairhall N, Aggar C, Kurrle SE, Sherrington C, Lord S, Lockwood K, et al: Frailty Intervention Trial (FIT). BMC Geriatr. 2008, 8: 27-10.1186/1471-2318-8-27.
Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, et al: Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010, 58: 1134-1143. 10.1111/j.1532-5415.2010.02865.x.
Li CM, Chen CY, Li CY, Wang WD, Wu SC: The effectiveness of a comprehensive geriatric assessment intervention program for frailty in community-dwelling older people: a randomized, controlled trial. Arch Gerontol Geriatr. 2010, 50 (Suppl 1): S39-S42.
Lustosa LP, Coelho FM, Silva JP, Pereira DS, Parentoni AN, Dias JM, et al: The effects of a muscle resistance program on the functional capacity, knee extensor muscle strength and plasma levels of IL-6 and TNF-alpha in pre-frail elderly women: a randomized crossover clinical trial - a study protocol. Trials. 2010, 11: 82-10.1186/1745-6215-11-82.
Pel-Littel RE, Schuurmans MJ, Emmelot-Vonk MH, Verhaar HJ: Frailty: defining and measuring of a concept. J Nutr Health Aging. 2009, 13: 390-394. 10.1007/s12603-009-0051-8.
van Kan GA A, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B: The assessment of frailty in older adults. Clin Geriatr Med. 2010, 26: 275-286. 10.1016/j.cger.2010.02.002.
Pijpers E, Ferreira I, Stehouwer CD, Nieuwenhuijzen Kruseman AC: The frailty dilemma. Review of the predictive accuracy of major frailty scores. Eur J Intern Med. 2012, 23: 118-123. 10.1016/j.ejim.2011.09.003.
Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, et al: Aging, frailty and age-related diseases. Biogerontology. 2010, 11: 547-563. 10.1007/s10522-010-9287-2.
Hogan DB, MacKnight C, Bergman H: Steering Committee Canadian Initiative on Frailty and Aging.: Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003, 15: 1-29.
Morris JN, Sherwood S, Mor V: An assessment tool for use in identifying functionally vulnerable persons in the community. Gerontologist. 1984, 24: 373-379. 10.1093/geront/24.4.373.
Matthews M, Lucas A, Boland R, Hirth V, Odenheimer G, Wieland D, et al: Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res. 2004, 16: 34-40.
Metzelthin SF, Daniels R, van Rossum E, de Witte L, van den Heuvel WJ, Kempen GI: The psychometric properties of three self-report screening instruments for identifying frail older people in the community. BMC Public Health. 2010, 10: 176-10.1186/1471-2458-10-176.
Goggins WB, Woo J, Sham A, Ho SC: Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci. 2005, 60: 1046-1051. 10.1093/gerona/60.8.1046.
Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L, Ukraintseva SV: Cumulative index of elderly disorders and its dynamic contribution to mortality and longevity. Rejuvenation Res. 2007, 10: 75-86. 10.1089/rej.2006.0500.
Hastings SN, Purser JL, Johnson KS, Sloane RJ, Whitson HE: Frailty predicts some but not all adverse outcomes in older adults discharged from the emergency department. J Am Geriatr Soc. 2008, 56: 1651-1657. 10.1111/j.1532-5415.2008.01840.x.
Dupre ME, Gu D, Warner DF, Yi Z: Frailty and type of death among older adults in China: prospective cohort study. BMJ. 2009, 338: b1175-10.1136/bmj.b1175.
Gu D, Dupre ME, Sautter J, Zhu H, Liu Y, Yi Z: Frailty and mortality among Chinese at advanced ages. J Gerontol B Psychol Sci Soc Sci. 2009, 64: 279-289.
Garcia-Gonzalez JJ, Garcia-Pena C, Franco-Marina F, Gutierrez-Robledo LM: A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr. 2009, 9: 47-10.1186/1471-2318-9-47.
Armstrong JJ, Stolee P, Hirdes JP, Poss JW: Examining three frailty conceptualizations in their ability to predict negative outcomes for home-care clients. Age Ageing. 2010, 39: 755-758. 10.1093/ageing/afq121.
Mitnitski A, Song X, Skoog I, Broe GA, Cox JL, Grunfeld E, et al: Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005, 53: 2184-2189. 10.1111/j.1532-5415.2005.00506.x.
Rockwood K, Mitnitski A, Song X, Steen B, Skoog I: Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006, 54: 975-979. 10.1111/j.1532-5415.2006.00738.x.
Lucicesare A, Hubbard RE, Fallah N, Forti P, Searle SD, Mitnitski A, et al: Comparison of two frailty measures in the Conselice Study of Brain Ageing. J Nutr Health Aging. 2010, 14: 278-281. 10.1007/s12603-010-0061-6.
Song X, Mitnitski A, Rockwood K: Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010, 58: 681-687. 10.1111/j.1532-5415.2010.02764.x.
Chan DC, Tsou HH, Chen CY, Chen CY: Validation of the Chinese-Canadian study of health and aging clinical frailty scale (CSHA-CFS) telephone version. Arch Gerontol Geriatr. 2010, 50: e74-e80. 10.1016/j.archger.2009.06.004.
Ma SL, Oyler J, Glavin S, Alavi A, Vokes T: Self-reported frailty is associated with low calcaneal bone mineral density in a multiracial population of community-dwelling elderly. Osteoporos Int. 2009, 20: 1837-1846. 10.1007/s00198-009-0884-3.
Mohile SG, Xian Y, Dale W, Fisher SG, Rodin M, Morrow GR, et al: Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009, 101: 1206-1215. 10.1093/jnci/djp239.
Gallucci M, Ongaro F, Amici GP, Regini C: Frailty, disability and survival in the elderly over the age of seventy: Evidence from "The Treviso Longeva (TRELONG) Study". Arch Gerontol Geriatr. 2009, 48: 281-283. 10.1016/j.archger.2008.02.005.
Gill TM, Allore H, Holford TR, Guo Z: The development of insidious disability in activities of daily living among community-living older persons. Am J Med. 2004, 117: 484-491. 10.1016/j.amjmed.2004.05.018.
Folsom AR, Boland LL, Cushman M, Heckbert SR, Rosamond WD, Walston JD: Frailty and risk of venous thromboembolism in older adults. J Gerontol A Biol Sci Med Sci. 2007, 62: 79-82. 10.1093/gerona/62.1.79.
Chang CI, Chan DC, Kuo KN, Hsiung CA, Chen CY: Prevalence and correlates of geriatric frailty in a northern taiwan community. Journal of the Formosan Medical Association. 2011, 110: 247-257. 10.1016/S0929-6646(11)60037-5.
Masel MC, Graham JE, Reistetter TA, Markides KS, Ottenbacher KJ: Frailty and health related quality of life in older Mexican Americans. Health Qual Life Outcomes. 2009, 7: 70-10.1186/1477-7525-7-70.
Berges IM, Graham JE, Ostir GV, Markides KS, Ottenbacher KJ: Sex differences in mortality among older frail Mexican Americans. J Womens Health (Larchmt). 2009, 18: 1647-1651. 10.1089/jwh.2008.1083.
Chen CY, Wu SC, Chen LJ, Lue BH: The prevalence of subjective frailty and factors associated with frailty in Taiwan. Arch Gerontol Geriatr. 2010, 50 (Suppl 1): S43-S47.
Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al: Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010, 210: 901-908. 10.1016/j.jamcollsurg.2010.01.028.
Samper-Ternent R, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ: Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc. 2008, 56: 1845-1852. 10.1111/j.1532-5415.2008.01947.x.
Fabricio-Wehbe SC, Schiaveto FV, Vendrusculo TR, Haas VJ, Dantas RA, Rodrigues RA: Cross-cultural adaptation and validity of the 'Edmonton Frail Scale - EFS' in a Brazilian elderly sample. Rev Lat Am Enfermagem. 2009, 17: 1043-1049. 10.1590/S0104-11692009000600018.
Bilotta C, Bowling A, Case A, Nicolini P, Mauri S, Castelli M, et al: Dimensions and correlates of quality of life according to frailty status: a cross-sectional study on community-dwelling older adults referred to an outpatient geriatric service in Italy. Health Qual Life Outcomes. 2010, 8: 56-10.1186/1477-7525-8-56.
Kellen E, Bulens P, Deckx L, Schouten H, Van Dijk M, Verdonck I, et al: Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol. 2010, 75: 243-248. 10.1016/j.critrevonc.2009.12.002.
Metzelthin SF, van Rossum E, de Witte LP, Hendriks MR, Kempen GI: The reduction of disability in community-dwelling frail older people: design of a two-arm cluster randomized controlled trial. BMC Public Health. 2010, 10: 511-10.1186/1471-2458-10-511.
Kulminski A, Yashin A, Ukraintseva S, Akushevich I, Arbeev K, Land K, et al: Accumulation of health disorders as a systemic measure of aging: Findings from the NLTCS data. Mech Ageing Dev. 2006, 127: 840-848. 10.1016/j.mad.2006.08.005.
Kulminski AM, Ukraintseva SV, Akushevich IV, Arbeev KG, Yashin AI: Cumulative index of health deficiencies as a characteristic of long life. J Am Geriatr Soc. 2007, 55: 935-940. 10.1111/j.1532-5415.2007.01155.x.
Kulminski A, Yashin A, Arbeev K, Akushevich I, Ukraintseva S, Land K, et al: Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: results from analyses of the National Long Term Care Survey. Mech Ageing Dev. 2007, 128: 250-258. 10.1016/j.mad.2006.12.004.
Ridda I, Lindley R, MacIntyre RC: The challenges of clinical trials in the exclusion zone: the case of the frail elderly. Australas J Ageing. 2008, 27: 61-66. 10.1111/j.1741-6612.2008.00288.x.
Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, et al: Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009, 27: 1628-1636. 10.1016/j.vaccine.2008.11.098.
Woo J, Goggins W, Sham A, Ho SC: Social determinants of frailty. Gerontology. 2005, 51: 402-408. 10.1159/000088705.
Woo J, Chan R, Leung J, Wong M: Relative contributions of geographic, socioeconomic, and lifestyle factors to quality of life, frailty, and mortality in elderly. PLoS ONE. 2010, 5: e8775-10.1371/journal.pone.0008775.
Yang Y, Lee LC: Dynamics and heterogeneity in the process of human frailty and aging: evidence from the U.S. older adult population. J Gerontol B Psychol Sci Soc Sci. 2010, 65B: 246-255. 10.1093/geronb/gbp102.
Wong RY, Miller WC: Adverse outcomes following hospitalization in acutely ill older patients. BMC Geriatr. 2008, 8: 10-10.1186/1471-2318-8-10.
Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, et al: Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008, 71: 210-216. 10.1212/01.wnl.0000317094.86209.cb.
Delbaere K, Crombez G, Vanderstraeten G, Willems T, Cambier D: Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age Ageing. 2004, 33: 368-373. 10.1093/ageing/afh106.
Peterson MJ, Giuliani C, Morey MC, Pieper CF, Evenson KR, Mercer V, et al: Physical activity as a preventative factor for frailty: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2009, 64: 61-68.
Beauchet O, Dubost V, Gonthier R, Kressig RW: Dual-task-related gait changes in transitionally frail older adults: the type of the walking-associated cognitive task matters. Gerontology. 2005, 51: 48-52. 10.1159/000081435.
Kressig RW, Wolf SL, Sattin RW, O'Grady M, Greenspan A, Curns A, et al: Associations of demographic, functional, and behavioral characteristics with activity-related fear of falling among older adults transitioning to frailty. J Am Geriatr Soc. 2001, 49: 1456-1462. 10.1046/j.1532-5415.2001.4911237.x.
Kressig RW, Gregor RJ, Oliver A, Waddell D, Smith W, O'Grady M, et al: Temporal and spatial features of gait in older adults transitioning to frailty. Gait Posture. 2004, 20: 30-35. 10.1016/S0966-6362(03)00089-4.
Abou-Raya S, Abou-Raya A: Osteoporosis and congestive heart failure (CHF) in the elderly patient: double disease burden. Arch Gerontol Geriatr. 2009, 49: 250-254. 10.1016/j.archger.2008.09.004.
Ahmed NN, Sherman SJ, Vanwyck D: Frailty in Parkinson's disease and its clinical implications. Parkinsonism Relat Disord. 2008, 14: 334-337. 10.1016/j.parkreldis.2007.10.004.
Alcala MV, Puime AO, Santos MT, Barral AG, Montalvo JI, Zunzunegui MV: Prevalence of frailty in an elderly Spanish urban population. Relationship with comorbidity and disability]. [Spanish. Aten Primaria. 2010, 42: 520-527. 10.1016/j.aprim.2009.09.024.
Alvarado BE, Zunzunegui MV, Beland F, Bamvita JM: Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008, 63: 1399-1406. 10.1093/gerona/63.12.1399.
Avila-Funes JA, Amieva H, Barberger-Gateau P, Le GM, Raoux N, Ritchie K, et al: Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009, 57: 453-461. 10.1111/j.1532-5415.2008.02136.x.
Blyth FM, Rochat S, Cumming RG, Creasey H, Handelsman DJ, Le Couteur DG, et al: Pain, frailty and comorbidity on older men: the CHAMP study. Pain. 2008, 140: 224-230. 10.1016/j.pain.2008.08.011.
Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, Kenny A: The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congestive Heart Failure. 2010, 16: 208-213. 10.1111/j.1751-7133.2010.00151.x.
Boyle PA, Buchman AS, Bennett DA: Purpose in life is associated with a reduced risk of incident disability among community-dwelling older persons. Am J Geriatr Psychiatry. 2010, 18: 1093-1102. 10.1097/JGP.0b013e3181d6c259.
Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA: Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med. 2007, 69: 483-489. 10.1097/psy.0b013e318068de1d.
Drey M, Wehr H, Wehr G, Uter W, Lang F, Rupprecht R, et al: The frailty syndrome in general practitioner care: a pilot study. Zeitschrift fur Gerontologie und Geriatrie. 2011, 44: 48-54. 10.1007/s00391-010-0136-3.
Espinoza SE, Hazuda HP: Frailty in older Mexican-American and European-American adults: is there an ethnic disparity?. J Am Geriatr Soc. 2008, 56: 1744-1749. 10.1111/j.1532-5415.2008.01845.x.
Espinoza SE, Jung I, Hazuda H: Lower frailty incidence in older Mexican Americans than in older European Americans: the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc. 2010, 58: 2142-2148. 10.1111/j.1532-5415.2010.03153.x.
Ewe SH, Ajmone MN, Pepi M, Delgado V, Tamborini G, Muratori M, et al: Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. American Heart Journal. 2010, 160: 1113-1120. 10.1016/j.ahj.2010.09.003.
Fairhall N, Sherrington C, Kurrle SE, Lord SR, Cameron ID: ICF participation restriction is common in frail, community-dwelling older people: an observational cross-sectional study. Physiotherapy. 2011, 97: 26-32. 10.1016/j.physio.2010.06.008.
Gill TM, Gahbauer EA, Allore HG, Han L: Transitions between frailty states among community-living older persons. Arch Intern Med. 2006, 166: 418-423.
Gray SL, LaCroix AZ, Aragaki AK, McDermott M, Cochrane BB, Kooperberg CL, et al: Angiotensin-converting enzyme inhibitor use and incident frailty in women aged 65 and older: prospective findings from the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2009, 57: 297-303. 10.1111/j.1532-5415.2008.02121.x.
Hubbard RE, O'Mahony MS, Calver BL, Woodhouse KW: Plasma esterases and inflammation in ageing and frailty. Eur J Clin Pharmacol. 2008, 64: 895-900. 10.1007/s00228-008-0499-1.
Jurschik Gimenez P, Escobar Bravo MA, Nuin Orrio C, Botigue Satorra T: [Frailty criteria in the elderly: a pilot study]. [Spanish]. Aten Primaria. 2011, 43: 190-196. 10.1016/j.aprim.2010.03.020.
Kenny AM, Waynik IY, Smith J, Fortinsky R, Kleppinger A, McGee D: Association between level of frailty and bone mineral density in community-dwelling men. J Clin Densitom. 2006, 9: 309-314. 10.1016/j.jocd.2006.03.010.
Kim MJ, Yabushita N, Kim MK, Nemoto M, Seino S, Tanaka K: Mobility performance tests for discriminating high risk of frailty in community-dwelling older women. Arch Gerontol Geriatr. 2010, 51: 192-198. 10.1016/j.archger.2009.10.007.
Le Couteur DG, Benson VL, McMahon AC, Blyth F, Handelsman DJ, Seibel MJ, et al: Determinants of serum-induced SIRT1 expression in older men: the CHAMP study. J Gerontol A Biol Sci Med Sci. 2011, 66: 3-8.
Leung JM, Tsai TL, Sands LP: Brief report: preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesthesia & Analgesia. 2011, 112: 1199-1201. 10.1213/ANE.0b013e31820c7c06.
Masel MC, Ostir GV, Ottenbacher KJ: Frailty, mortality, and health-related quality of life in older Mexican Americans. J Am Geriatr Soc. 2010, 58: 2149-2153. 10.1111/j.1532-5415.2010.03146.x.
Masel MC, Howrey B, Peek MK: The effect of acculturation on frailty among older mexican americans. J Aging Health. 2011, 23: 704-713. 10.1177/0898264310391786.
Matsuda PN, Shumway-Cook A, Ciol MA: The effects of a home-based exercise program on physical function in frail older adults. J Geriatric Physical Therapy. 2010, 33: 78-84.
Mitchell SJ, Kirkpatrick CM, Le Couteur DG, Naganathan V, Sambrook PN, Seibel MJ, et al: Estimation of lean body weight in older community-dwelling men. Br J Clin Pharmacol. 2010, 69: 118-127. 10.1111/j.1365-2125.2009.03586.x.
Onen NF, Agbebi A, Shacham E, Stamm KE: Frailty among HIV-infected persons in an urban outpatient care setting. J Infect. 2009, 59: 346-352. 10.1016/j.jinf.2009.08.008.
Onen NF, Shacham E, Stamm KE, Turner Overton E: Sexual Behaviors and Results of Bacterial Sexually Transmitted Infections Testing Among Frail HIV-Infected Individuals. J Int Assoc Physicians AIDS Care (Chic Ill). 2010, 9: 30-33. 10.1177/1545109709356946.
Ottenbacher KJ, Ostir GV, Peek MK, Al Snih S, Raji MA, Markides KS: Frailty in older Mexican Americans. J Am Geriatr Soc. 2005, 53: 1524-1531. 10.1111/j.1532-5415.2005.53511.x.
Penninx BW, Beekman AT, Bandinelli S, Corsi AM, Bremmer M, Hoogendijk WJ, et al: Late-life depressive symptoms are associated with both hyperactivity and hypoactivity of the hypothalamo-pituitary-adrenal axis. Am J Geriatr Psychiatry. 2007, 15: 522-529. 10.1097/JGP.0b013e318033ed80.
Perez-Lopez FR, Chedraui P, Fernandez-Alonso AM: Vitamin D and aging: Beyond calcium and bone metabolism. Maturitas. 2011, 69: 27-36. 10.1016/j.maturitas.2011.02.014.
Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP: Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006, 54: 1674-1681. 10.1111/j.1532-5415.2006.00914.x.
Rockwood K, Abeysundera MJ, Mitnitski A: How should we grade frailty in nursing home patients?. J Am Med Dir Assoc. 2007, 8: 595-603. 10.1016/j.jamda.2007.07.012.
Rockwood K, Nassar B, Mitnitski A: Apolipoprotein E-polymorphism, frailty and mortality in older adults. J Cell Mol Med. 2008, 12: 2754-2761. 10.1111/j.1582-4934.2008.00270.x.
Rothman MD, Leo-Summers L, Gill TM: Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008, 56: 2211-116. 10.1111/j.1532-5415.2008.02008.x.
Rousseau JH, Kleppinger A, Kenny AM: Self-reported dietary intake of omega-3 fatty acids and association with bone and lower extremity function. J Am Geriatr Soc. 2009, 57: 1781-1788. 10.1111/j.1532-5415.2008.01870.x.
Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J: Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009, 64: 675-681.
Syddall H, Roberts HC, Evandrou M, Cooper C, Bergman H, Aihie Sayer A: Prevalence and correlates of frailty among community-dwelling older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2010, 39: 197-203. 10.1093/ageing/afp204.
Travison TG, Shackelton R, Araujo AB, Morley JE, Williams RE, Clark RV, et al: Frailty, serum androgens, and the CAG repeat polymorphism: results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2010, 95: 2746-2754. 10.1210/jc.2009-0919.
Vaz Fragoso CA, Gahbauer EA, Van Ness PH, Gill TM: Reporting peak expiratory flow in older persons. J Gerontol A Biol Sci Med Sci. 2007, 62: 1147-1151. 10.1093/gerona/62.10.1147.
Verghese J, Xue X: Identifying frailty in high functioning older adults with normal mobility. Age Ageing. 2010, 39: 382-385. 10.1093/ageing/afp226.
Weaver GD, Kuo YF, Raji MA, Al Snih S, Ray L, Torres E, et al: Pain and disability in older Mexican-American adults. J Am Geriatr Soc. 2009, 57: 992-999. 10.1111/j.1532-5415.2009.02263.x.
Wilhelm-Leen ER, Hall YN, Tamura K, Chertow GM: Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009, 122: 664-671. 10.1016/j.amjmed.2009.01.026.
Bylow K, Hemmerich J, Mohile SG, Stadler WM, Sajid S, Dale W: Obese Frailty, Physical Performance Deficits, and Falls in Older Men with Biochemical Recurrence of Prostate Cancer on Androgen Deprivation Therapy: A Case–control Study. Urology. 2011, 77: 934-940. 10.1016/j.urology.2010.11.024.
Kenny AM, Boxer R, Walsh S, Hager WD, Raisz LG: Femoral bone mineral density in patients with heart failure. Osteoporos Int. 2006, 17: 1420-1427. 10.1007/s00198-006-0148-4.
Romero-Ortuno R, Walsh CD, Lawlor BA, Kenny RA: A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr. 2010, 10: 57-10.1186/1471-2318-10-57.
Bourgault-Fagnou MD, Hadjistavropoulos HD: Understanding health anxiety among community dwelling seniors with varying degrees of frailty. Aging Ment Health. 2009, 13: 226-237. 10.1080/13607860802380664.
Perera V, Bajorek BV, Matthews S, Hilmer SN: The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009, 38: 156-162.
Bilotta C, Case A, Nicolini P, Mauri S, Castelli M, Vergani C: Social vulnerability, mental health and correlates of frailty in older outpatients living alone in the community in Italy. Aging Ment Health. 2010, 14: 1024-1036. 10.1080/13607863.2010.508772.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2318/13/64/prepub
Acknowledgements
All authors contributed to conception and design, and to analysis and interpretation of data; or, wrote the first draft of the article and revised it critically for important intellectual content. All approve the final version to be published. Mika Kivimaki is supported by the Medical Research Council, UK, the US National Institutes of Health (R01 HL036310 & R01 AG034454), and the Academy of Finland. David Batty is a Wellcome Trust Fellow.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
All authors contributed to writing this review and have approved the final version for publication.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bouillon, K., Kivimaki, M., Hamer, M. et al. Measures of frailty in population-based studies: an overview. BMC Geriatr 13, 64 (2013). https://doi.org/10.1186/1471-2318-13-64
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2318-13-64