Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is defined as a spectrum of conditions ranging from hepatocellular steatosis to steatohepatitis and fibrosis, progressing to cirrhosis, which occur in the absence of excessive alcohol use. Several animal models capture aspects of NAFLD but are limited either in their representation of the disease stages or use for development of therapeutics due to the extended periods of time required to develop full histological features.
Methods
Here, we report the development of a novel rat model for NAFLD that addresses some of these limitations. We used a fast food diet (FFD) and a CCl4 micro dose (0.5 ml/kg B.wt) for 8 weeks in Wistar rats. Serological analyses, gene expression profiling and liver histology studies were conducted to investigate the development of steatosis, steatohepatitis and fibrosis in the FFD-CCl4 model when compared to the individual effects of a FFD or a micro dose of CCl4 in rats.
Results
The serum biochemical profile of the FFD-CCl4 model showed an increase in liver injury and fibrosis. This was also accompanied by a significant increase in liver triglycerides (TG), inflammation and oxidative stress. Importantly, we observed extensive fibrosis confirmed by: i) increased gene expression of fibrosis markers and, ii) moderate to severe collagen deposition seen as perisinusoidal and bridging fibrosis using H&E, Trichome and Sirius Red staining.
Conclusions
In summary, we find that the FFD-CCl4 rat model developed NAFLD histological features including, steatosis, inflammation and fibrosis in 8 weeks showing promise as a model that can be used to develop NAFLD therapeutics and liver anti-fibrotics.
Similar content being viewed by others
Background
Non-alcoholic fatty liver disease (NAFLD) is defined by a spectrum of conditions that occur in the absence of excessive alcohol use and range from hepatocellular steatosis to steatohepatitis (NASH) and fibrosis, progressing to cirrhosis [1, 2]. The current prevalence of NAFLD in the western world is ~20–30% of the population [3, 4] and is expected to increase to >40% by 2030 [5], indicating the growing risk for individuals leading a sedentary lifestyle on a high-fat, high-carbohydrate, calorie-rich diet.
Research efforts in NAFLD have been aimed at understanding disease development, progression and pathophysiology simultaneously facilitating drug discovery. To this end, various animal models of NAFLD have been developed involving genetic and diet manipulation, treatment with toxins as well as combination models [6–19]. Genetic models of NAFLD include AOX null mice [6, 7], MAT1A null mice [8], liver-specific NRF1 knockouts [9], liver-specific PTEN knockouts [10] and leptin-deficient obese mice [11, 12], which develop NAFLD due to a disruption in the antioxidant mechanism, fatty acid metabolism or triglyceride synthesis/secretion. Diet modulations leading to NAFLD include the methionine and choline deficient diet-fed mice [13] and high fat diet (HFD) mice [14, 15] wherein, NAFLD severity varies with diet composition, duration of feeding, species, strain, and gender of animals. Well-known chemical-induced models of NAFLD include carbon tetrachloride (CCl4) and thioacetamide-treated mice [16, 17]. The main advantages of the well-established CCl4 model include convenience and establishment of both mouse and rat models with evidence of fibrosis/cirrhosis across routes of CCl4 administration. However, multiple reports have revealed considerable disadvantages in the intraperitoneal, subcutaneous, inhalation and oral routes of CCl4 administration, including chronic peritonitis, necrosis at injection site with inconsistent fibrosis, respiratory arrest and higher mortality with inconsistent fibrosis, respectively [19].
Recently, Charlton et al. reported the development of a fast food diet mouse model (FFD) of NASH recapitulating features of the metabolic syndrome and NASH with progressive fibrosis [20]. The FFD comprised of high saturated fats, cholesterol and fructose, and mimicked the metabolic profile in NAFLD including obesity and insulin resistance, along with features of NASH such as increased inflammation, fibrosis, ER stress and lipoapoptosis. The model showed significant physiological similarity to human NASH but took 24 weeks to develop all the histological features [20].
Here, we report the development of a novel combination rat model for NAFLD established by modifying the FFD [20] and administering a micro dose (0.5 ml/kg bwt) of CCl4 recapitulating steatosis, steatohepatitis and fibrosis in an accelerated manner (8 weeks). The serum biochemical profile of the FFD-CCl4 model showed an increase in liver injury and fibrosis when compared to FFD alone, CCl4 alone or the chow diet control animals along with significant increase in liver TG, inflammation and oxidative stress in the FFD-CCl4 model. Importantly, gene expression markers of fibrosis were significantly elevated in the FFD-CCl4 model when compared to FFD or CCl4 alone or the chow diet control animals, which was further confirmed by histological staining using H&E as well as assessment of collagen deposition using Trichome and Sirius red techniques. In summary, we find that the FFD-CCl4 rat model, in 8 weeks, developed NAFLD histological features including, steatosis, inflammation and fibrosis.
Methods
Compliance with ethical requirements
The study protocol, animal maintenance, and experimental procedures were approved by the Institutional Animal Ethics Committee (IAEC) of Connexios Life Sciences. All institutional and national guidelines for the care and use of laboratory animals were followed. This article does not contain any studies with human subjects.
Development of animal model
Ten week old Wistar rats (Charles River Labs, USA) were used. Animals were housed in groups of three in polypropylene cages and maintained at 23 ± 1°C at 60 ± 10% humidity and 12 hour cycles of light and dark with free access to feed and water (ad libitum). Animals were randomly assigned to four groups, consisting 10 animals/group per sex. Group G1 comprised of animals on chow diet, G2 animals were fed a chow diet and received a CCl4 micro dose of 0.5 ml/kg B.wt, G3 animals were fed a FFD alone and G4 animals were fed a FFD and received a CCl4 micro dose of 0.5 ml/kg B.wt. The FFD consisted of 2 g cholesterol and 0.5 g cholic acid mixed with normal chow diet made up to 100 g to increase calorie content in comparison to the chow diet. Corn oil (5 ml/kg b.wt) was administered through oral gavage once daily to all animals in G3 and G4 whereas G1 and G2 animals were given drinking water. Further, 15 g fructose was mixed in 100 ml drinking water for all G3 and G4 animals. Group G2 and G4 were administered CCl4 (assay purity: >98%) at 0.5 ml/kg B.wt by oral gavage after dissolving in corn oil once weekly for the first two weeks and then on alternate weeks thereafter (i.e., 4th, 6th and 8th week). Food consumption and body weight were measured weekly for all animals during the experimental period.
The experimental period was 8 weeks and the last CCl4 dose was administered 48 h prior to sacrificing the animals. All animals were fasted overnight and body weight was measured. Blood was collected from the orbital sinus under isoflurane anesthesia; serum was separated and subjected for clinical chemistry studies. Animals were sacrificed and necropsied, the liver was excised immediately, weighed and taken to estimate liver triglyceride (TG), glutathione (GSH) and thiobarbituric acid reactive substances (TBARS) and gene expression profile using RT-PCR. The rest was preserved, for histology, in 10% phosphate-buffered formalin.
Clinical chemistry and biomarker analysis
Serum levels of Aspartate transaminase (AST), Alanine transaminase (ALT), Alkaline phosphatase (ALP), triglyceride (TG), total bilirubin, Gamma-glutamyl transpeptidase (GGT) were measured in automated bio- analyzer EM360 (Transasia Bio-medicals Ltd) using ERBA Mannheim kits (Transasia Bio-medicals Ltd, India). Serum procollagen type III levels were measured as per manufacturer's instructions using the PIIINP ELISA kit manufactured by USCN Life Science Inc.
Assessment of liver triglyceride, glutathione and TBARS
100 mg of liver sample was collected in 1 ml PBS (pH 7.4) and lysed using a tissue lyser (25 Hz for 5 min). Liver TG was extracted as per Folch’s method. Briefly, 0.3 ml of 10% liver homogenate was extracted in 1.5 ml of chloroform: methanol (2:1) and the organic layer was dried in a vacuum dryer. The residue was re-suspended in absolute isopropyl alcohol and TG levels were estimated using DiaSys Diagnostic Systems GmbH kit. Levels of total glutathione and TBARS in the liver, known indicators of oxidative stress, were measured as described earlier [21, 22].
Assessment of gene expression profile in liver
Real-time PCR was used to evaluate expression of COL1A1, TIMP1, ACTA2, and TGFβ as markers of liver fibrosis, TNFa and osteopontin were studied for inflammation and FABP4 for fatty acid trafficking. All primers were procured from Integrated DNA Technologies, Germany. Total RNA was extracted from liver using Trizol (Sigma, St. Louis, MO, USA) followed by chloroform extraction and isopropyl alcohol precipitation. cDNA was synthesized by reverse transcription (ABI, CA, USA) and amplified using MESA Green PCR Master Mix (Eurogenetic, Belgium). The Primers sequence for assessment of gene expression profiles are mentioned in Table 1.
Pathology, staging of fibrosis
Formalin-fixed liver samples were paraffin-embedded, sectioned at 5 μm and stained using hematoxylin and eosin (H&E) to examine morphology. Masson’s Trichome and Sirius Red staining techniques were used to assess fibrosis. All slides were examined under light microscopy at low (X10), high (X40) magnification and also at X20. Histological staging were conducted by modification of earlier methods [23, 24]. Grading and scoring for fatty change, hepatocellular ballooning and inflammation was conducted, by a pathologist, Dr. Harish Chandrasekharan, who carried out blind-fold evaluation to the study, as described in (Additional file 1: Table S1 and Additional file 2: Table S2).
Statistics
Data are presented as mean ± SEM. Comparisons among groups were performed with one-way ANOVA followed by Dunnett's multiple comparison post-hoc test to identify significant differences between groups, p < 0.05 was considered significant.
Results
Animals on FFD with a micro dose of CCl4(0.5 ml/Kg B.wt, po) showed liver injury and fibrosis in 8 weeks
Livers from all animals on FFD with/without CCl4 appeared pale, enlarged and showed a significant increase in liver weight (p < 0.001) (Table 2). There were no changes in liver weight and gross morphology in animals that were administered CCl4 on chow diet. Across all groups, animals did not show any signs of toxicity or overt behavioral changes. All animals on the FFD with/ without CCl4 animals gained >10% weight compared to the chow diet-fed animals without any change in weekly food consumption (Additional file 3: Table S3 and Additional file 4: Table S4).
To estimate liver injury, we compared the serum biochemical profiles of the FFD-CCl4 animals to the FFD, CCl4 and chow diet controls. ALT, AST, GGT and ALP (known serum markers of liver injury) and procollagen type III (known serum marker of fibrosis) were significantly elevated in the FFD-CCl4 animals compared to the chow diet controls (Table 3). Serum triglyceride levels were reduced significantly in FFD with/ without CCl4. Apart from the increase in serum triglycerides, animals on a chow diet administered a CCl4 micro dose did not show a significant change in the serum biochemical profile when compared to the chow diet controls. However, we did not observe any changes in blood glucose levels under both fasting and fed condition across all the groups (data not shown). Taken together, these data indicate that the FFD-CCL4 rats show an increase in markers of liver injury and fibrosis.
In agreement with the liver injury reflected in the serum biochemical profile, liver TG content was significantly elevated in FFD and FFD-CCl4 animals (Table 2). Reactive oxygen species (ROS)-mediated oxidative stress markers were measured to access liver damage. We observed a significant (p < 0.01) depletion in liver glutathione levels in FFD-CCL4 animals compared to FFD and chow diet controls. In line with this, liver TBARS was elevated in both FFD and FFD-CCl4 animals (Table 2). Animals on the chow diet did not show a significant change in oxidative stress markers with/without CCl4. Thus, along with an increase in liver injury, the FFD-CCl4 animals also showed an overall increase in liver steatosis and oxidative stress.
Pro-fibrotic and pro-inflammatory pathways are activated in the FFD-CCl4animals
To follow up on elevated liver injury and fibrosis markers observed in the FFD-CCl4 sera, we studied gene expression of key genes in inflammation, fibrosis and fatty acid trafficking known to be involved in NAFLD. The gene expression levels of FABP4 (protein associated with fatty acid uptake, transport and metabolism, implicated in development of NASH) were significantly increased in the FFD-CCl4 animals compared to the FFD, CCl4 and chow diet controls (Table 2). Similarly, osteopontin, a marker for immune cell activation was significantly increased in the FFD and FFD-CCl4 animals compared to the control animals. In agreement with this, TGFβ, a well-established marker of fibrosis and inflammation, was expressed at significantly higher levels in FFD-CCl4 animals compared to the FFD, CCl4 and chow diet controls. Other markers of extracellular matrix (ECM) deposition including COL1A1 and TIMP1 also showed a significant increase in the FFD-CCl4 animals compared to the FFD and chow diet controls (Table 4). ACTA2 (ECM deposition marker smooth muscle actin) did not show significant gene expression changes in FFD, CCl4 or the FFD-CCl4 animals (Table 4). TNFα (proinflammatory cytokine implicated in fibrogenesis) increased both in the FFD and FFD-CCl4 animals. Animals that were administered a CCl4 micro dose on the chow diet did not show a significant change in the expression of key genes in inflammation, fibrosis and fatty acid trafficking when compared to the chow diet controls. Similarly, except for the increase in TNFα, the FFD animals also did not show a change in the gene expression profile when compared to the chow diet controls.
Together, these data show that the FFD-CCl4 animals show pro-fibrotic and pro-inflammatory changes in gene expression which are not shown by FFD or the CCl4-treated animals.
The FFD-CCl4mice show an increase in steatosis, hepatocellular ballooning and fibrosis
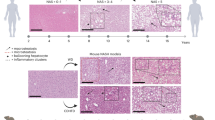
Histological examination of liver sections from FFD-CCl4 animals showed a statistically significant increase in micro vesicular and macro vesicular steatosis, hepatocellular ballooning and fibrosis compared to the FFD, CCl4 and chow diet controls (Table 5 and Figure 1). As per the scoring system shown in (Additional file 2: Table S2), we observed moderate infiltration of mononuclear inflammatory cells with predominance of macrophages in both the FFD and FFD-CCl4 animals along with both periportal and perivenular steatosis and hepatocellular ballooning which are features of steatohepatitis (Figure 2). To evaluate collagen distribution, Masson trichome and picrosirius red staining were carried out (Table 5 and Figure 1). However, liver sections from FFD and FFD-CCl4 animals showed increased collagen deposition with the latter showing moderate-severe collagen deposition detectable as perisinusoidal fibrosis and bridging fibrosis (Figure 1). Importantly, this was true for all the animals on the FFD-CCl4 regime. Animals on a chow diet with/without CCl4 did not show a significant increase in macro vesicular steatosis, hepatocellular ballooning and infiltration of inflammatory cells (Figure 2).
Hematoxylin-Eosin (H&E, 1st and 2nd rows), Masson’s trichrome (3rd and 4th rows) and Sirius red (5th and 6th rows) – stained sections of liver tissues from chow diet, chow diet + 0.5 ml/Kg B.wt CCl 4, fast food diet (FFD), FFD + 0.5 ml/Kg B.wt CCl 4 fed animals on 8th week. There was no steatosis, hepatocellular ballooning or fibrosis in animals fed with chow diet. Mild micro-vesicular fatty changes and mild hepatocellular ballooning, without fibrosis observed in animals fed with chow diet + 0.5 ml/Kg B.wt CCl4. Fast food fed animals without CCl4 showing moderate micro-vesicular and macro-vesicular fatty changes, hepatocellular ballooning and minimal perisinusoidal fibrosis. FFD + 0.5 ml/Kg B.wt CCl4 fed animals showing severe micro-vesicular and macro-vesicular fatty changes with significant hepatocellular ballooning and prominent perisinusoidal, pericellular region with extensive distribution and bridging fibrosis. All liver sections were evaluated at X10 (low-magnification) and X40 (high-magnification).
Row1: Hematoxylin-Eosin (H&E) stained sections of liver tissues from chow diet, chow diet + 0.5 ml/Kg B.wt CCl 4, fast food diet (FFD), FFD + 0.5 ml/Kg B.wt CCl 4 fed animals on 8th week. Liver section showing moderate infiltration of inflammatory cells (shown by black arrows) in fast food fed animals with and without CCl4 along with moderate micro-vesicular and macro-vesicular fatty changes and hepatocellular ballooning. Mild inflammation was observed in animals fed with chow diet + 0.5 ml/Kg B.wt CCl4. Row2: Hematoxylin-Eosin (H&E) stained sections of liver tissues. Liver sections showing increased hepatocellular ballooning in fast food fed animals with and without CCl4 along with moderate micro-vesicular and macro-vesicular fatty changes. All liver sections are represented at X40 (high-magnification).
Hepatocellular ballooning, which has been challenging to establish in animal models of NAFLD, was observed in the FFD-CCl4 and the FFD animals but not the CCl4-treated animals (Figure 2). Together these data indicate that the FFD-CCL4 model recapitulates the steatosis, inflammatory and fibrotic lesions associated with NAFLD.
Discussion
NAFLD is a spectrum of disorders ranging from fatty liver (steatosis), NASH and fibrosis resulting in cirrhosis [25, 26]. Several animal models have been developed to study NAFLD pathogenesis and screen for therapeutics. These animal models vary by way of nature of pathology and evolution of fibrosis [6–19]. Animal models commonly used to study NAFLD include the high fat diet-induced, FFD mouse and CCl4-induced liver injury models [16, 17, 27–29]. The FFD mouse model develops features of human NAFLD. However, it takes about 6 months to establish histological features [20]. Acute/ chronic exposure to CCl4 has shown elevated serum liver enzymes, steatosis, centrilobular necrosis, increased liver weight and fibrosis/cirrhosis. However the CCL4-induced fibrosis model is very severe and associated with peritonitis, necrosis and lack of consistent development of fibrosis [19]. Chronic CCl4 treatment (>2 weeks), on the other hand, is known to invoke adaptive mechanisms, reducing vulnerability to oxidative stress and hepatocellular damage with restorative macrophages showing potential to reverse fibrosis upon CCl4 withdrawal [28, 30, 31].
In the current study, we present a rat model of NAFLD developed over 8 weeks on a modified FFD with a CCL4 micro dose (0.5 ml CCl4/kg bwt) that captures steatosis, inflammation and fibrosis stages of NAFLD. We used CCl4 micro dosing to induce oxidative stress and inflammation without causing overt hepatotoxic effects. In course of our studies, we had tested a micro dose of 1 ml/kg bwt CCl4 (unpublished data). However, this resulted in mortality leading us to reduce the dose to 0.5 ml/kg bwt. In this study, we modified the published FFD composition [20] by increasing the amount of fructose used in drinking water to mimic a metabolic overload [32–34] and accelerate disease progression. As expected, all FFD-CCl4 animals showed an increase in liver injury, fibrosis and oxidative stress confirmed by changes in serum AST, ALT, GGT, ALP; Procollagen III, and liver GSH and TBARS [35–37], respectively. This was consistent with previous models of CCl4-induced fibrosis and the FFD mouse model [20, 31, 38, 39].
Serum triglyceride levels were reduced significantly in FFD with/ without CCl4. This decrease is a distinctive feature of CCl4 which rapidly increases the triglyceride accumulation in the liver due to a failure in their secretory mechanisms [31, 39] and also increased uptake of triglycerides into the liver. We observed, in 8 weeks, an increase in liver TG, fatty acid trafficking, inflammation and fibrosis, which was in consistence with earlier findings where the increase in gene expression of FABP4 (marker of fatty acid trafficking), osteopontin (marker of inflammation), COL1A1 and TIMP1 (markers of fibrosis) [40, 41], which have been reported and duration for appearance of liver fibrosis was 24 weeks. In the current study, the liver histological lesions showed all the features of NAFLD in animals treated with FFD-CCl4. Development of steatosis is largely due to increased rate of import or synthesis of fatty acids by hepatocytes that exceeds the rate of export or catabolism [42–44]. Steatosis thus developed has an inflammatory response which may be precipitated by a variety of stimuli such as oxidative stress and pro-inflammatory cytokine mediated hepatocyte injury progressing to NASH [43–46].
Considering how this model is distinct from previous models of NAFLD is important and we find that the FFD-CCl4 rat model recreates in 8 weeks most histological lesions seen in the FFD mouse [20, 47, 48] with the exception of a change in serum glycemic/lipid profiles. This model has not shown features of metabolic syndrome thus this model does not exactly mimic human NAFLD. The FFD mouse established by Charlton et al. [20], showed features of metabolic syndrome with hepatocellular ballooning and progressive fibrosis which makes it a good model that mimics human NAFLD but it takes more than 6 months. In the FFD-CCl4 model, although we were unable to detect insulin resistance or hyprinsulinemia, we observed steatosis, inflammation and fibrosis associated with NAFLD in 8 weeks.
A significant aspect of this model is that it is able to replicate hepatocellular ballooning and fibrosis in 8 weeks. Developing a rapid fibrosis model in short duration require a variety of stimuli, thus animals fed a high-fat diet for more than 24 weeks are associated with their susceptibility to diet-induced obesity that develop steatohepatitis. In the current model, FFD along with micro dose CCl4 provoke an array of responses that results in hepatocellular ballooning, inflammation, and fibrosis. Hepatocellular ballooning has been difficult to establish but was shown recently in the FFD mouse by Charlton et al. [20]. In our study, Masson trichome and picrosirius red staining techniques revealed increased collagen deposition in the form of pericellular and bridging fibrosis. The necroinflammatory foci showed mononuclear infiltration with predominance of macrophages. Further, expression levels of pro-fibrotic and pro-inflammatory gene, TGFβ1 increased along with COL1A1 mRNA levels. Curiously, ACTA2 (ECM deposition marker smooth muscle actin) did not show significant gene expression changes in FFD, CCl4 or the FFD-CCl4 animals and needs to be further assessed for changes in protein expression.
Interestingly, during model development, we observed a gender bias for NAFLD development with females being more susceptible than males. Further studies will be required to understand the mechanistic reasons for this susceptibility seen in females and also to precisely understand the rate of progression of NAFLD in this model. For example, assessing steatosis, inflammation and fibrosis across 8 weeks at regular intervals will allow for a more accurate interpretation of when these histological lesions develop. This could then be potentially used to identify candidate biomarkers for progression of disease from steatosis to steatohepatitis to fibrosis.
Conclusions
In summary, we present a fatty liver-induced model of hepatic fibrosis, which captures steatosis, inflammation and fibrosis seen in NAFLD. This model holds promise as a tool for screening for NAFLD therapeutics including liver anti-fibrotics.
Abbreviations
- ACTA2:
-
Alpha-actin-2
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- AOX:
-
Acyl-CoA oxidase
- CCl4:
-
Carbon Tetrachloride
- COL1A1:
-
Collagen, type I, alpha 1
- ECM:
-
Extracellular matrix
- FABP4:
-
Fatty acid binding protein 4
- FFD:
-
fast food diet
- GGT:
-
Gamma-glutamyl transpeptidase
- MAT1A:
-
Methionine adenosyltransferase 1A
- NAFLD:
-
Non-alcoholic fatty liver disease
- PIIINP:
-
Procollagen III N-Terminal Propeptide
- PTEN:
-
Phosphatase and tensin homolog
- SEM:
-
Standard error mean
- TBARS:
-
Thiobarbituric acid reactive substances
- TG:
-
Triglyceride
- TGFβ:
-
Transforming growth factor beta
- TIMP1:
-
Tissue inhibitor of metalloproteinase 1
- TNFα:
-
Tumor necrosis factor alpha.
References
Kim CH, Younossi ZM: Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008, 75 (10): 721-728. 10.3949/ccjm.75.10.721.
Sass DA, Chang P, Chopra KB: Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci. 2005, 50 (1): 171-180. 10.1007/s10620-005-1267-z.
Bellentani S, Scaglioni F, Marino M, Bedogni G: Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010, 28: 155-161. 10.1159/000282080.
Milić S, Štimac D: Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis. Clinical presentation and treatment. Dig Dis. 2012, 30: 158-162. 10.1159/000336669.
Panchal SK, Poudyal H, Arumugam TV, Brown L: Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J Nutr. 2011, 141 (6): 1062-1069. 10.3945/jn.111.137877.
Fan CY, Pan J, Chu R, Lee D, Kluckman KD, Usuda N: Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J Biol Chem. 1996, 271: 24698-24710. 10.1074/jbc.271.40.24698.
Cook WS, Jain S, Jia Y, Cao WQ, Yeldandi AV, Reddy JK: Peroxisome proliferator-activated receptor alpharesponsive genes induced in the newborn but not prenatal liver of peroxisomal fatty acyl-CoA oxidase null mice. Exp Cell Res. 2001, 268: 70-76. 10.1006/excr.2001.5266.
Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ: Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001, 98: 5560-5565. 10.1073/pnas.091016398.
Xu Z, Chen L, Leung L, Yen TS, Lee C, Chan JY: Liver specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci U S A. 2005, 102: 4120-4125. 10.1073/pnas.0500660102.
Sato W, Horie Y, Kataoka E, Ohshima S, Dohmen T, Iizuka M: Hepatic gene expression in hepatocyte-specific Pten deficient mice showing steatohepatitis without ethanol challenge. Hepatol Res. 2006, 34: 256-265. 10.1016/j.hepres.2006.01.003.
Brix AE, Elgavish A, Nagy TR, Gower BA, Rhead WJ, Wood PA: Evaluation of liver fatty acid oxidation in the leptin-deficient obese mouse. Mol Genet Metab. 2002, 75: 219-226. 10.1006/mgme.2002.3298.
Wortham M, He L, Gyamfi M, Copple BL, Wan YJ: The transition from fatty liver to NASH associates with SAMe depletion in db/db mice fed a methionine choline-deficient diet. Dig Dis Sci. 2008, 53: 2761-2774. 10.1007/s10620-007-0193-7.
Dela Pena A, Leclercq I, Field J, George J, Jones B, Farrell G: NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005, 129: 1663-1674. 10.1053/j.gastro.2005.09.004.
Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S: Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005, 42: 905-914. 10.1002/hep.20877.
Zou Y, Li J, Lu C, Wang J, Ge J, Huang Y, Zhang L, Wang Y: High fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sci. 2006, 79: 1100-1107. 10.1016/j.lfs.2006.03.021.
Fujii T, Fuchs BC, Yamada S, Lauwers GY, Kulu Y, Goodwin JM, Lanuti M, Tanabe KK: Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010, 10: 79-10.1186/1471-230X-10-79.
Hunter AL, Holscher MA, Neal RA: Thioacetamide-induced hepatic necrosis. I. Involvement of the mixed-function oxidase enzyme system. J Pharmacol Exp Ther. 1977, 200 (2): 439-448.
Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA: Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008, 295 (5): G987-95. 10.1152/ajpgi.90272.2008.
Starkel P, Leclercq IA: Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol. 2011, 25 (2): 319-333. 10.1016/j.bpg.2011.02.004.
Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G: Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011, 301 (5): G825-G834. 10.1152/ajpgi.00145.2011.
Akerboom TP, Sies H: Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77: 373-382.
Ohkawa H, Ohishi N, Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979, 95: 351-358. 10.1016/0003-2697(79)90738-3.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ: Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005, 41: 1313-1321. 10.1002/hep.20701.
Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, Yamaji R, Inui H, Fukusato T, Yamanouchi T: Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr. 2009, 139 (11): 2067-71. 10.3945/jn.109.105858.
Farrell GC, Larter CZ: Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006, 43 (2 Suppl 1): S99-S112.
Paschos P, Paletas K: Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009, 13 (1): 9-19.
Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, Van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP: Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012, 109 (46): E3186-E3195. 10.1073/pnas.1119964109.
Tsukamoto H, Matsuoka M, French SW: Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990, 10: 56-65. 10.1055/s-2008-1040457.
Constandinou C, Henderson N, Iredale JP: Modeling liver fibrosis in rodents. Methods Mol Med. 2005, 117: 237-250.
Radice S, Marabini L, Gervasoni M, Ferraris M, Chiesara E: Adaptation to oxidative stress: effects of vinclozolin and iprodione on the HepG2 cell line. Toxicology. 1998, 129: 183-191. 10.1016/S0300-483X(98)00086-9.
Shi J, Aisaki K, Ikawa Y, Wake K: Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J Pathol. 1998, 153 (2): 515-525. 10.1016/S0002-9440(10)65594-0.
Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM: Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010, 51: 1961-1971. 10.1002/hep.23535.
Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF: Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008, 48: 993-999. 10.1016/j.jhep.2008.02.011.
Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA: Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011, 140: 124-131. 10.1053/j.gastro.2010.09.038.
Han D, Hanawa N, Saberi B, Kaplowitz N: Mechanisms of liver injury. III Role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol. 2006, 291 (1): G1-G7. 10.1152/ajpgi.00001.2006.
Fraga CG, Leibovitz BE, Tappel AL: Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med. 1988, 4 (3): 155-161. 10.1016/0891-5849(88)90023-8.
Bezerra FJ, Rezende AA, Rodrigues SJ: Almeida Md: Thiobarbituric acid reactive substances as an index of lipid peroxidation in sevoflurane-treated rats. Rev Bras Anestesiol. 2004, 54 (5): 640-649. 10.1590/S0034-70942004000500004.
Hu Bscher SG: Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006, 49: 450-465. 10.1111/j.1365-2559.2006.02416.x.
Hamdy N, El-Demerdash E: New therapeutic aspect for carvedilol: antifibrotic effects of carvedilol in chronic carbon tetrachloride-induced liver damage. Toxicol Appl Pharmacol. 2012, 261 (3): 292-299. 10.1016/j.taap.2012.04.012.
Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T, Laitinen S, Kolak M, Fisher RM, Hamsten A, Auvinen P, Yki-Järvinen H: Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008, 294 (5): G1281-G1287. 10.1152/ajpgi.00074.2008.
Sahai A, Malladi P, Melin-Aldana H, Green RM, Whitington PF: Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. Am J Physiol Gastrointest Liver Physiol. 2004, 287 (1): G264-G273. 10.1152/ajpgi.00002.2004.
Plaa GL: Chlorinated methanes and liver injury: highlights of the past 50 years. Annu Rev Pharmacol Toxicol. 2000, 40: 42-65.
Weber LW, Boll M, Stampfl A: Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003, 33: 105-136. 10.1080/713611034.
Koteish A, Diehl AM: Animal models of steatosis. Semin Liver Dis. 2001, 21: 89-104. 10.1055/s-2001-12932.
Bradbury MW, Berk PD: Lipid metabolism in hepatic steatosis. Clin Liver Dis. 2004, 8: 639-671. 10.1016/j.cld.2004.04.005.
Anstee QM, Goldin RD: Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006, 87: 1-16. 10.1111/j.0959-9673.2006.00465.x.
Day CP, Saksena S: Non-alcoholic steatohepatitis: definitions and pathogenesis. J Gastroenterol Hepatol. 2002, 17 (Suppl 3): S377-S384.
Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C: Molecular basis and mechanisms of progression of nonalcoholic steatohepatitis. Trends Mol Med. 2008, 14: 72-81. 10.1016/j.molmed.2007.12.003.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-230X/14/89/prepub
Acknowledgements
We would like to extend our gratitude to Roopesh Marulasiddeshwara, Usha Narayanan, and Geeta Nirody for their contribution towards the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Tarak K Chheda, Pratibha Shivakumar, Satish Kumar Sadasivan, Vijayragav Dasarahalli Nagaraju, Harish Chanderasekharan, Yogananda Moolemath, Anup Mammen Oommen, Jagannath R Madanahalli, Venkataranganna V Marikunte declare that they have no conflict of interest.
Authors’ contributions
YM, AMO, VVM designed the research; TKC, PS, SKS, HC, performed the research; YM, VVM, JRM wrote the paper.
Electronic supplementary material
12876_2013_1121_MOESM1_ESM.doc
Additional file 1: Table S1: Scheme used for histological staging of fibrosis modified from Kleiner et al., [23]. (DOC 42 KB)
12876_2013_1121_MOESM3_ESM.doc
Additional file 3: Table S3: Body weight data of chow diet, CCL4, FFD and FFD-CCl4. All data are expressed as mean ± SEM. The data was statistically analyzed for significant using one-way ANOVA followed by Dunnett’s multiple comparison post test. (DOC 44 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Chheda, T.K., Shivakumar, P., Sadasivan, S.K. et al. Fast food diet with CCl4 micro-dose induced hepatic-fibrosis –a novel animal model. BMC Gastroenterol 14, 89 (2014). https://doi.org/10.1186/1471-230X-14-89
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-230X-14-89