Abstract
Background
The intestinal microbiota has been proposed to play a pathogenic role in coeliac disease (CD). Although antibiotics are common environmental factors with a profound impact on intestinal microbiota, data on antibiotic use as a risk factor for subsequent CD development are scarce.
Methods
In this population-based case–control study we linked nationwide histopathology data on 2,933 individuals with CD (Marsh stage 3; villous atrophy) to the Swedish Prescribed Drug Register to examine the association between use of systemic antibiotics and subsequent CD. We also examined the association between antibiotic use in 2,118 individuals with inflammation (Marsh 1–2) and in 620 individuals with normal mucosa (Marsh 0) but positive CD serology. All individuals undergoing biopsy were matched for age and sex with 28,262 controls from the population.
Results
Antibiotic use was associated with CD (Odds ratio [OR] = 1.40; 95% confidence interval [CI] = 1.27-1.53), inflammation (OR = 1.90; 95% CI = 1.72–2.10) and normal mucosa with positive CD serology (OR = 1.58; 95% CI = 1.30–1.92). ORs for prior antibiotic use in CD were similar when we excluded antibiotic use in the last year (OR = 1.30; 95% CI = 1.08-1.56) or restricted to individuals without comorbidity (OR = 1.30; 95% CI = 1.16 – 1.46).
Conclusions
The positive association between antibiotic use and subsequent CD but also with lesions that may represent early CD suggests that intestinal dysbiosis may play a role in the pathogenesis of CD. However, non-causal explanations for this positive association cannot be excluded.
Similar content being viewed by others
Background
Coeliac disease (CD) is a life-long autoimmune disease prevalent in 1 to 2% of the western population [1]. CD is a multifactorial disease where genetically predisposed individuals develop small-intestinal villous atrophy and inflammation in response to dietary gluten intake [2]. In recent decades, the prevalence of CD has more than doubled, [3] strongly indicating that environmental factors other than gluten-exposure may have a significant influence on CD development [4]. Further, data from the “Swedish celiac epidemic”, where childhood CD incidence displayed an epidemic pattern with a rapid four-fold increase in incidence in 1984 and a later abrupt decline in 1996, coinciding with changed infant feeding recommendations, have suggested that environmental factors influence CD development [5].
Today, half of all children in many Western countries receive antibiotics at least once a year [6]. Antibiotics can have both short- and long-term effects on the ecological balance between the host and the normal microbiota [7, 8]. The intestinal microbiota influences the development of the intestinal immune system, the establishment of oral tolerance and the mucosal barrier function [9]. Previous research has found a difference in the gut microbiota between individuals with CD and healthy controls, suggesting that a dysbiotic microbiota may play a pathogenic role in CD [10]. Despite the profound impact of antibiotics on the gut microbiome, there are few data on antibiotic exposure and risk of CD.
The main objective of this case–control study was to examine the association between antibiotic use and subsequent CD by comparing individuals with CD with matched controls from the general population. We also examined antibiotic use in individuals who may have early CD without villous atrophy [11] (I) small-intestinal inflammation without villous atrophy, or (II) normal small-intestinal mucosa but positive CD serology. Studying these early CD manifestations may be important because risk factors may not only influence the fully developed disease, but sometimes have an even stronger association with disease precursors. For example, cigarette smoking has been more strongly associated with colorectal adenomas compared with colorectal cancer [12, 13].
Methods
In this case–control study we linked nationwide histopathology data on individuals undergoing small intestinal biopsy to the Swedish Prescribed Drug Register in order to examine the association between use of antibiotics and CD. We hypothesized a positive association between antibiotic use and CD.
Literature search
A literature search at PubMed (http://pubmed.gov/) was performed using the following combinations of words as our major search terms: “celiac”, “coeliac”, “antibiotic” and “antimicrobial”.
Study population
Between 2006 and 2008, we searched the computerized register of Sweden’s 28 pathology departments to identify individuals with CD [14]. In this study CD was defined as small-intestinal villous atrophy (Marsh grade 3) [15]. An earlier evaluation has shown that 95% of Swedish individuals with villous atrophy have CD [14]. To examine the context of the association between antibiotic use and subsequent CD we also identified individuals with small-intestinal inflammation (Marsh grade 1–2) but without villous atrophy and individuals with normal small-intestinal mucosa (Marsh grade 0) but with positive CD serology [16]. The biopsies were performed between July 1969 and January 2008 [17]. A detailed account of the data collection process has been described elsewhere [14, 16].
In the current study we used the same dataset described in our previous study of mortality (29,096 individuals with CD, 13,306 individuals with inflammation, 3,719 individuals with normal mucosa but positive CD serology) [18]. Data on individuals with normal mucosa and positive CD serology were regional and obtained from the ascertainment areas of eight Swedish university hospitals covering approximately half of the Swedish population [16]. Positive CD serology was defined as a positive IgA or IgG AGA (antigliadin), EMA (endomysial), or TTG (tissue transglutaminase) test less than 180 days before or no later than 30 days after a normal biopsy (and with no prior or subsequent biopsy showing villous atrophy or inflammation) [16]. In a recent consensus paper individuals with normal mucosa and positive CD serology were identified as having potential CD [11].
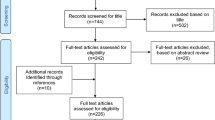
For each individual undergoing biopsy, the government agency Statistics Sweden identified up to five controls from the population matched for age, sex, calendar period of birth and county of residence. For example, a girl living in the county of Blekinge, diagnosed with CD in 2006 at the age of 13 years; was matched with five 13-year-old girls who were living in Blekinge in 2006. After exclusion of individuals with data irregularities, [18] we identified 228,632 controls (Figure 1).
Individuals undergoing biopsy and their matched controls were then linked to the Swedish Prescribed Drug Register (established on July 1st 2005) [19]. Through this linkage, we identified individuals biopsied between July 1st 2005 and January 29th 2008 (end of the study period). Thus, the final analyses included 2,933 individuals with CD, 2,118 individuals with inflammation, 620 individuals with normal mucosa but positive CD serology and 28,262 controls (Figure 1).
Antibiotic use
The Swedish Prescribed Drug Register contains prospectively recorded individual data (on e.g. date of dispensing) on more than 99% of all dispensed prescribed drugs in Sweden [19]. Antibiotics in Sweden are not sold over the counter.
We collected data on use of all systemic antibiotics (anatomical therapeutic chemical, ATC code: J01) from July 1st 2005 (launch of the Prescribed Drug Register) through January 29th 2008 (end of the study period), and up to the date of the biopsy (and the corresponding date in matched controls). Antibiotics were grouped into penicillin V, extended-spectrum penicillins, quinolones, macrolides and other systemic antibiotics (Additional file 1).
Statistical analyses
We used conditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Each stratum (one individual undergoing biopsy and up to five matched controls) was analyzed separately before a summary OR was calculated.
In our main analysis we examined the association between use of any systemic antibiotics and subsequent CD. Early-onset CD (i.e. before the age of 2 years) may have different risk factors compared with late-onset CD [5]. Additionally, antibiotic exposure early in life may have a more profound impact on the composition of the microbiota [20]. Accordingly, we performed stratified analyses by age at CD diagnosis (<2 years, 2–19 years, 20–39 years, 40–59 years and ≥60 years). We also stratified our analyses for sex. Similar sub-analyses were performed for individuals with small-intestinal inflammation and individuals with normal small-intestinal mucosa but positive CD serology. For each of these stratifications we examined for interaction via the inclusion in our models of multiplicative interaction terms, and the use of likelihood ratio tests between models with and without them.
Antibiotics differ in their influence on the intestinal microbiota. In pre-planned sub-analyses we estimated the association between CD and type of antibiotic exposure: penicillin V, extended-spectrum penicillins, quinolones, macrolides and other systemic antibiotics. This grouping of antibiotics has previously been used [21, 22] and is largely based on the ATC classification system where the subgroups indicate the different therapeutic indications of antibacterial agents. To evaluate potential causality we estimated the dose- and time-dependent association between antibiotic use and CD in two separate analyses: (1) when individuals had received 1–2 courses and at least 3 courses of antibiotics and (2) when antibiotics had been prescribed in the year (≤365 days) before biopsy.
Education level has been associated with antibiotic use [23] and may influence the risk of CD diagnosis [24]. In a sub-analysis we therefore adjusted for education using seven predefined education categories determined by Statistics Sweden.
Post-hoc analyses
Certain antiparasitic medications have similar pharmacokinetic and pharmacodynamic properties as systemic antibiotics with a strong impact on the gut microbiota. In a post-hoc analysis we therefore examined the relationship between use of any antiparasitic medications (ATC codes P01-P03, e.g. oral tinidazole) and CD, as well as specifically the use of metronidazole and CD.
Individuals with undiagnosed CD have an increased risk of comorbidity, [25] and with that, potentially increased surveillance and probability to receive antibiotic treatment. In a post-hoc analysis we therefore restricted our data to individuals who had not been admitted to a hospital during the study period. Hospital admission data were collected from the national Inpatient Register [26].
To further reduce the risk of surveillance bias we constructed a variable representing outpatient health care consumption. Hospital-based outpatient care has been recorded nationally in Sweden since January 1st 2001. We calculated the number of hospital-based outpatient visits from birth or start of the registry (whichever occurred latest) until the day before small-intestinal biopsy (or corresponding date in matched controls). We excluded visits in which CD was coded as the main reason for the visit. Individuals then were divided into four groups according to number of visits per year (those with no record of prior hospital-based outpatient care (0); >0 but <1 visit/year; 1- < 2 visits/year; and ≥2 visits/year). Those individuals with no record of hospital-based outpatient care may have undergone initial CD investigation in primary care before undergoing biopsy. For example: A patient A, undergoing biopsy in December 2006, with eight hospital-based outpatient visits in the six years between 2001 (start of registration of outpatient data) and December 2006 (time of biopsy) has an average of 1.3 visits per year (= 8 visits/6 years). In a post-hoc analysis, we added this variable to our statistical model to evaluate whether the association between antibiotic exposure and CD remained.
CD is elicited by dietary gluten and thus virtually nonexistent before the age of six months. To establish whether antibiotic use truly preceded CD, i.e. to evaluate the risk of reverse causation, we performed a sub-analysis of individuals who were exposed to antibiotics before the age of six months. In an additional post-hoc analysis we limited our exposure to antibiotic more than one year (>365 days) before CD diagnosis.
SPSS version 20.0 was used for all statistical analyses.
Ethics
This study was conducted in accordance with the national and institutional standards and was approved by the Regional Ethical Vetting Board in Stockholm.
Results
The median age at CD diagnosis in this study was 28 years. About 40% of those with CD were diagnosed in childhood and the majority of study participants were female (Table 1).
Of the 2,933 individuals with CD, 27.0% had received at least one course of antibiotics during the study period before biopsy as compared with 21.1% in the controls, corresponding to an odds ratio for subsequent CD of 1.40 (95% CI = 1.27-1.53) (Table 2). In the individuals with inflammation but no villous atrophy 39.5% had used antibiotics as compared with 25.7% in the controls (OR = 1.90; 95% CI = 1.72–2.10). Antibiotic use also was associated with having a normal small-intestinal mucosa but positive CD serology (Table 2). Restricting our analysis to individuals with normal mucosa and positive IgA EMA or TTG did not influence the OR (Additional file 2). Adjustment for education level revealed unchanged ORs in all three groups (Additional file 2).
We found increasing ORs for repeated use of antibiotics and subsequent CD diagnosis (1–2 courses of antibiotics: OR = 1.36, 95% CI = 1.23-1.50; ≥3 courses of antibiotics: OR = 1.58, 95% CI = 1.31 - 1.92). Also in individuals with a biopsy showing inflammation or normal mucosa, but with positive CD serology, we found increasing ORs for repeated use of antibiotics, indicating a dose–response effect (Table 2).
The association between antibiotic treatment and subsequent CD was similar in males and females (Males: OR = 1.48, 95% CI = 1.27-1.72; Females: OR = 1.36; 95% CI = 1.21-1.52; p-value for interaction: 0.38). The stratified analyses by sex for individuals with small-intestinal inflammation and individuals with normal mucosa but positive CD serology are also presented in Table 2 (p-value for interaction, inflammation: 0.16; normal mucosa: 0.15). The stratified analyses by age at biopsy revealed only small differences between age groups and ORs for antibiotic treatment and development of CD, small-intestinal inflammation or normal mucosa but positive CD serology (Additional file 3). ORs for previous antibiotic treatment did not differ appreciably according to year of CD diagnosis (Additional file 3).
Overall, penicillin V was the most frequently prescribed antimicrobials, being used by 9.0% of the controls and nearly 10% of those with CD. Use of penicillin V was not associated with CD (OR = 1.12; 95% = 0.98 – 1.27). However, we found an association between use of each of the remaining types of antibiotic and subsequent CD, with essentially similar ORs, irrespective of antibiotic type (Table 3).
Twenty-five percent of the individuals with CD had received at least one course of antibiotics in the year before CD diagnosis compared with 18.7% of the matched controls (OR = 1.42; 95% CI = 1.29 - 1.56). ORs for type of antibiotic, according to ATC code, used in the year (≤365 days) before CD diagnosis are presented in Table 3.
Post-hoc analyses
In a post-hoc analysis 115 individuals with CD (3.9%) and 259 controls (1.8%) had an earlier record of antiparasitic medication, equivalent to an OR of 2.12 for subsequent CD (95% CI = 1.72 - 2.62). Looking specifically at the earlier use of metronidazole revealed a slightly stronger association with CD (OR = 2.25; 95% CI = 1.71-2.96) (metronidazole use in the year before CD diagnosis: OR = 2.38; 95% CI = 1.78-3.19; and ≥ three courses of metronidazole: OR = 1.90; 95% CI = 0.62-5.78). Use of metronidazole was similarly associated with small-intestinal inflammation and normal mucosa but positive CD serology (Additional file 4).
To reduce the confounding effect of comorbidity we restricted our data to individuals with no hospital admissions (CD: n = 2,047; controls: n = 12,069). However, this post-hoc analysis revealed only a marginally changed OR for subsequent CD in relation to antibiotic use (OR = 1.30; 95% CI = 1.16 – 1.46). Post-hoc adjustment for number of outpatient visits before biopsy slightly changed the OR for CD (OR = 1.19; 95% CI = 1.08-1.31). Further, antibiotic use more than one year before biopsy examination was also associated with subsequent CD (OR = 1.30; 95% CI = 1.08-1.56).
We also estimated the OR for subsequent CD based on use of any antibiotics during the first six months of life. Only 3 of 16 (18.8%) children born after July 2005 and subsequently diagnosed with CD had been exposed to any antibiotics during their first six months of life, as compared with 7/80 (8.8%) children in the controls (OR = 2.26; 95% CI = 0.55-9.25).
Discussion
This is the first study to find a positive association between antibiotic use and subsequent CD. Antibiotic exposure was also linked to small-intestinal inflammation and to normal mucosa with positive CD serology, both of which may represent early CD. The consistent association between the multiple groups, the slightly stronger association between repeated use of antibiotics compared with no use as well as the association with use of certain antibiotics (e.g., metronidazole) and CD may suggest that antibiotic exposure, possibly through a changed gut microbiota, plays a pathogenic role in early CD development. However, given the lack of time-response effect, within the limited time window studied, we cannot rule out non-causal explanations for our findings.
Observational studies on drugs are particularly susceptible to the concerns of reverse causation and confounding-by-indication. Reverse causation defines the causality bias if the exposure is a response to manifestations of the undiagnosed disease. In CD it is difficult to date the true onset of disease and thereby to establish whether antibiotic use truly preceded CD or whether the antibiotic was given for the symptoms of as yet undiagnosed CD. Several studies have shown a mean diagnostic delay of 5–11 years from onset of CD symptoms until diagnosis, [27] a time associated with an increased number of consultation visits [28] and possibly an increased likelihood of receiving antibiotic prescriptions. To reduce the risk of reverse causation and the effect of comorbidity, which may act as a confounder by increasing the possibility of receiving antibiotic prescriptions, we performed two post-hoc analyses restricted to individuals exposed to antibiotics in the first six months of life or individuals without hospital admission. Although these post-hoc analyses revealed largely unchanged ORs, they do not rule out residual comorbidity or reverse causation.
Observational studies on drugs may also be subject to confounding-by-indication in which the indication for treatment and not the treatment per se is associated with the outcome. Individuals with undiagnosed CD have an increased risk of several diseases that may, in concert, increase their likelihood to receive antibiotics [25]. For example, because antibiotics are frequently misused in viral infections, [29] confounding may be introduced when antibiotics are erroneously used to combat adenovirus or rotavirus infections, both proposed as risk factors for CD development [2]. However, the Swedish Medical Products Agency do not recommend antibiotic treatment in diarrhoeal illnesses, except for cases of severe bacterial gastroenteritis [30]. Further, just as for diagnosed CD, undiagnosed CD may be associated with bacterial infections, [31] which may have also influenced our results. Finally, the fact that all three cohorts were similarly associated with antibiotic use raises the possibility that an external factor, i.e. gastrointestinal symptoms such as diarrhoea, increases the “risk” of both antibiotic use and the performance of a small bowel biopsy.
It is well-established that the intestinal microbiota influences the maturation of the intestinal immune system [32]. Meanwhile several studies have found an imbalanced composition of the intestinal microbiota in those with CD [33]. In vitro studies suggest that intestinal dysbiosis may, in the presence of gliadin, increase intestinal epithelial permeability [10] and enable epithelial translocation of gliadin peptides potentially triggering CD [2]. Other data suggest that the distinct intestinal microbiota in CD may have pro-inflammatory properties that affect the immune response elicited by gluten [34]. Although this study lacks conclusive evidence for a causal association between antibiotic use and subsequent CD, our results do not refute the hypothesis that the intestinal microbiota affects CD development. A causal association may also be supported by the slightly stronger association to subsequent CD and certain antibiotics (e.g., metronidazole) that have a major impact on the anaerobic bacteria of the colon. Consequently, today’s prevalent use of antibiotics and their potential public heath impact on CD development warrant attention in future research.
Antibiotic use has been associated with the development of several immunological diseases, including inflammatory bowel disease [35] and asthma [36]. More importantly with regard to CD, most [22, 37] but not all studies, [38] have failed to find an association between antibiotic use and subsequent type 1 diabetes, a disease that otherwise shares many aetiological traits with CD [39].
A major strength of this study is our use of multiple groups on the CD spectrum (CD, small-intestinal inflammation and normal mucosa with positive CD serology) [18]. With this study design, we were able to examine the association of antibiotic treatment by the degree of mucosal abnormality. Multiple groups also improved our evaluation of potential causality. Another strength is the use of prospectively recorded exposure and outcome data, which eliminate the risk of recall bias. Furthermore, this study provided detailed information on antibiotic use, including time and age of exposure, type of antibiotics and number of courses.
The use of biopsy data enabled us to identify a representative population with CD. In Sweden, more than 95% of gastroenterologists obtain a small-intestinal biopsy before CD diagnosis [14], implying that biopsy records have a high sensitivity for diagnosed CD. We regard the risk of misclassification in CD as low. In an earlier validation study 108 (95%) of 114 individuals with villous atrophy had CD [14]. Misclassification could be more of a concern in inflammation because villous atrophy may be patchy and not all inflammation is related to CD or to a pre-coeliac state. Furthermore, any potential misclassification of histopathology should be non-differential regarding antibiotic use and therefore should not lead to spurious associations, but to an underestimation of the true effect.
Our third cohort included individuals with normal small-intestinal mucosa, but positive CD serology. Most of these individuals had a single positive AGA serology with a lower specificity for CD than TTG or EMA. Thus, it may be argued that this condition does not represent a pre-coeliac state. However, when Hill et al. reviewed 26 studies of CD serology, they observed a median AGA specificity of 93% [40].
Antibiotic exposure was determined by the Swedish Prescribed Drug Register, which includes nationwide high-quality data on all dispensed prescribed medications [19]. Self-medication, i.e. obtaining an antibiotic without prescription, is very rare in Sweden, estimated to be 0.3% of all antibiotics used [41]. A limitation of our study is the recent start of the Swedish Prescribed Drug Register (established in July 2005) and the left truncation of exposure data in which individuals diagnosed with CD early in the study period (and their matched controls) will have little chance of being classified as antibiotic users because of lack of antibiotic data before July 2005. However, this loss of prior antibiotic data should not be differentially related to future CD status, and therefore only bias our results toward the null.
Conclusions
In conclusion, we found a positive association between antibiotic use and subsequent CD, as well as with inflammation, and with having a normal mucosa but positive CD serology. One explanation could be that antibiotic exposure, possibly through changes in the gut microbiota, plays a role in early CD development, but non-causal explanations cannot be ruled out. Within the limited time window studied, the lack of a time-response effect raises the possibility of reverse causation, in particular, prescription of antibiotics to individuals with manifestations of undiagnosed CD.
Abbreviations
- ATC:
-
Anatomical therapeutic chemical (pharmaceutical classification)
- AGA:
-
Antigliadin antibody
- CD:
-
Coeliac disease
- CI:
-
Confidence interval
- EMA:
-
Endomysial antibody
- OR:
-
Odds ratio
- TTG:
-
Tissue transglutaminase antibody.
References
Walker MM, Murray JA, Ronkainen J, Aro P, Storskrubb T, D'Amato M, Lahr B, Talley NJ, Agreus L: Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology. 2010, 139: 112-119. 10.1053/j.gastro.2010.04.007.
Di Sabatino A, Corazza GR: Coeliac disease. Lancet. 2009, 373: 1480-1493. 10.1016/S0140-6736(09)60254-3.
Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, Brantner TL, Kim WR, Phelps TK, Lahr BD, Zinsmeister AR, Melton LJ, Murray JA: Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009, 137: 88-93. 10.1053/j.gastro.2009.03.059.
Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, Lohi O, Bravi E, Gasparin M, Reunanen A, Maki M: Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007, 26: 1217-1225. 10.1111/j.1365-2036.2007.03502.x.
Ivarsson A, Persson LA, Nystrom L, Ascher H, Cavell B, Danielsson L, Dannaeus A, Lindberg T, Lindquist B, Stenhammar L, Hernell O: Epidemic of coeliac disease in Swedish children. Acta Paediatr. 2000, 89: 165-171. 10.1111/j.1651-2227.2000.tb01210.x.
Marra F, Patrick DM, Chong M, Bowie WR: Antibiotic use among children in British Columbia, Canada. J Antimicrob Chemother. 2006, 58: 830-839. 10.1093/jac/dkl275.
Sullivan A, Edlund C, Nord CE: Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. 2001, 1: 101-114. 10.1016/S1473-3099(01)00066-4.
Jernberg C, Lofmark S, Edlund C, Jansson JK: Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010, 156: 3216-3223. 10.1099/mic.0.040618-0.
Round JL, Mazmanian SK: The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009, 9: 313-323. 10.1038/nri2515.
Sanz Y, De Pama G, Laparra M: Unraveling the ties between celiac disease and intestinal microbiota. Int Rev Immunol. 2011, 30: 207-218. 10.3109/08830185.2011.599084.
Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, Lundin KE, Murray JA, Sanders DS, Walker MM, Zingone F, Ciacci C: The Oslo definitions for coeliac disease and related terms. Gut. 2013, 62: 43-52. 10.1136/gutjnl-2011-301346.
Johnson ME, Feinn R, Anderson JC: Clinical factors associated with non-polypoid colonic adenomas >/=6 mm: a prospective study in an asymptomatic population using a high-definition colonoscope. Am J Gastroenterol. 2011, 106: 2018-2022. 10.1038/ajg.2011.254.
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J, Willett WC: A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst. 1994, 86: 183-191. 10.1093/jnci/86.3.183.
Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A: Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009, 9: 19-10.1186/1471-230X-9-19.
Marsh MN: Grains of truth: evolutionary changes in small intestinal mucosa in response to environmental antigen challenge. Gut. 1990, 31: 111-114. 10.1136/gut.31.1.111.
Ludvigsson JF, Brandt L, Montgomery SM: Symptoms and signs in individuals with serology positive for celiac disease but normal mucosa. BMC Gastroenterol. 2009, 9: 57-10.1186/1471-230X-9-57.
Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A: The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009, 24: 659-667. 10.1007/s10654-009-9350-y.
Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F: Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009, 302: 1171-1178. 10.1001/jama.2009.1320.
Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundstrom A, Westerholm B, Rosen M: The new Swedish prescribed drug register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007, 16: 726-735. 10.1002/pds.1294.
Bedford Russell AR, Murch SH: Could peripartum antibiotics have delayed health consequences for the infant?. BJOG. 2006, 113: 758-765. 10.1111/j.1471-0528.2006.00952.x.
Hviid A, Svanstrom H, Frisch M: Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011, 60: 49-54. 10.1136/gut.2010.219683.
Hviid A, Svanstrom H: Antibiotic use and type 1 diabetes in childhood. Am J Epidemiol. 2009, 169: 1079-1084. 10.1093/aje/kwp038.
Huether CA, Ivanovich J, Goodwin BS, Krivchenia EL, Hertzberg VS, Edmonds LD, May DS, Priest JH: Maternal age specific risk rate estimates for Down syndrome among live births in whites and other races from Ohio and metropolitan Atlanta, 1970–1989. J Med Genet. 1998, 35: 482-490. 10.1136/jmg.35.6.482.
Sandberg-Bennich S, Dahlquist G, Kallen B: Coeliac disease is associated with intrauterine growth and neonatal infections. Acta Paediatr. 2002, 91: 30-33. 10.1111/j.1651-2227.2002.tb01635.x.
Sanders DS, Patel D, Stephenson TJ, Ward AM, McCloskey EV, Hadjivassiliou M, Lobo AJ: A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol. 2003, 15: 407-413. 10.1097/00042737-200304000-00012.
Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO: External review and validation of the Swedish national inpatient register. BMC Publ Health. 2011, 11: 450-10.1186/1471-2458-11-450.
Green PHR, Stavropoulos SN, Panagi SG, Goldstein SL, McMahon DJ, Absan H, Neugut AI: Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001, 96: 126-131. 10.1111/j.1572-0241.2001.03462.x.
Dickey W, McConnell JB: How many hospital visits does it take before celiac sprue is diagnosed?. J Clin Gastroenterol. 1996, 23: 21-23. 10.1097/00004836-199607000-00007.
Misurski DA, Lipson DA, Changolkar AK: Inappropriate antibiotic prescribing in managed care subjects with influenza. Am J Manag Care. 2011, 17: 601-608.
The Medical Products Agency: Recommendations for treatment of infectious diarrheal illnesses. 1997, Stockholm: Läkemedelsverket
Thomas HJ, Wotton CJ, Yeates D, Ahmad T, Jewell DP, Goldacre MJ: Pneumococcal infection in patients with coeliac disease. Eur J Gastroenterol Hepatol. 2008, 20: 624-628. 10.1097/MEG.0b013e3282f45764.
Guarner F, Malagelada JR: Gut flora in health and disease. Lancet. 2003, 361: 512-519. 10.1016/S0140-6736(03)12489-0.
Schippa S, Iebba V, Barbato M, Di Nardo G, Totino V, Checchi MP, Longhi C, Maiella G, Cucchiara S, Conte MP: A distinctive 'microbial signature' in celiac pediatric patients. BMC Microbiol. 2010, 10: 175-10.1186/1471-2180-10-175.
De Palma G, Cinova J, Stepankova R, Tuckova L, Sanz Y: Pivotal advance: Bifidobacteria and gram-negative bacteria differentially influence immune responses in the proinflammatory milieu of celiac disease. J Leukoc Biol. 2010, 87: 765-778. 10.1189/jlb.0709471.
Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE: Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012, 130 (4): e794-e803. 10.1542/peds.2011-3886.
Marra F, Lynd L, Coombes M, Richardson K, Legal M, Fitzgerald JM, Marra CA: Does antibiotic exposure during infancy lead to development of asthma?: a systematic review and metaanalysis. Chest. 2006, 129: 610-618. 10.1378/chest.129.3.610.
Cardwell CR, Carson DJ, Patterson CC: No association between routinely recorded infections in early life and subsequent risk of childhood-onset Type 1 diabetes: a matched case–control study using the UK General Practice Research Database. Diabet Med. 2008, 25: 261-267. 10.1111/j.1464-5491.2007.02351.x.
Kilkkinen A, Virtanen SM, Klaukka T, Kenward MG, Salkinoja-Salonen M, Gissler M, Kaila M, Reunanen A: Use of antimicrobials and risk of type 1 diabetes in a population-based mother-child cohort. Diabetologia. 2006, 49: 66-70. 10.1007/s00125-005-0078-2.
Cronin CC, Shanahan F: Insulin-dependent diabetes mellitus and coeliac disease. Lancet. 1997, 349: 1096-1097. 10.1016/S0140-6736(96)09153-2.
Hill ID: What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations?. Gastroenterology. 2005, 128: S25-S32. 10.1053/j.gastro.2005.02.012.
Svensson E, Haaijer-Ruskamp FM, Lundborg CS: Self-medication with antibiotics in a Swedish general population. Scand J Infect Dis. 2004, 36: 450-452. 10.1080/00365540410020721.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-230X/13/109/prepub
Acknowledgements
This work was supported by: The American Scandinavian Foundation [BL], the Celiac Sprue Association [BL, JFL], and the National Center for Research Resources, a component of the National Institutes of Health (grant number: KL2 RR024157) [BL]; National Institutes of Health (grant number: R01DK090989) [MB], and the Diane Belfer Program for Human Microbial Ecology [MB]; The Swedish Society of Medicine [JFL], the Swedish Research Council [JFL], the Sven Jerring Foundation [JFL], the Örebro Society of Medicine [JFL], the Karolinska Institutet [JFL], the Clas Groschinsky Foundation [JFL], the Juhlin Foundation [JFL], the Majblomman Foundation [JFL] and the Uppsala-Örebro Regional Research Council [JFL]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ICMJE criteria for authorship read and met: KM, WY; BL; PG; MB; TC; JFL. Agree with the manuscript’s results and conclusions: KM, WY; BL; PG; MB; TC; JFL. Designed the experiments/the study: KM, JFL. Collected data: JFL. Analyzed the data: KM. Wrote the first draft of the paper: KM. Contributed to the writing of the paper: WY; BL; PG; MB; TC; JFL. Contributed to the design of the study and interpretation of the data analyses: WY; BL; PG; MB; TC. Interpretation of data; approved the final version of the manuscript: KM, WY; BL; PG; MB; TC; JFL. Responsible for data integrity: KM, JFL. Supervised the project including data analyses: JFL. Obtained funding: JFL. All auhtors read and approved the final manuscript.
Electronic supplementary material
12876_2013_978_MOESM2_ESM.pdf
Additional file 2: Odds ratios for prior antibiotic use in individuals with normal mucosa and positive coeliac disease serology. Odds ratios (ORs) for prior antibiotic use with adjustment for education level. (PDF 68 KB)
12876_2013_978_MOESM3_ESM.pdf
Additional file 3: Odds ratios for prior antibiotic use in individuals with coeliac disease, small-intestinal inflammation, and normal small-intestinal mucosa a . Stratified analyses by age at biopsy. Odds ratios for prior antibiotic use in individuals with coeliac disease. Stratified analyses by year of diagnosis. (PDF 73 KB)
12876_2013_978_MOESM4_ESM.pdf
Additional file 4: Odds ratio for prior use of metronidazole in individuals with small-intestinal inflammation and normal mucosa a .(PDF 64 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mårild, K., Ye, W., Lebwohl, B. et al. Antibiotic exposure and the development of coeliac disease: a nationwide case–control study. BMC Gastroenterol 13, 109 (2013). https://doi.org/10.1186/1471-230X-13-109
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-230X-13-109