Abstract
Background
A primary goal of acute treatment for depression is clinical remission of symptoms. Most meta-analyses of remission rates involve randomized controlled trials (RCTs) using patients from psychiatric settings, but most depressed patients are treated in primary care. The goal of this study was to determine remission rates obtained in RCTs of treatment interventions for Major Depressive Disorder (MDD) conducted in primary care settings.
Methods
Potentially relevant studies were identified using computerized and manual search strategies up to May 2003. Criteria for inclusion included published RCTs with a clear definition of remission using established outcome measures.
Results
A total of 13 studies (N = 3202 patients) meeting inclusion criteria were identified. Overall remission rates for active interventions ranged between 50% and 67%, compared to 32% for pill placebo conditions and 35% for usual care conditions.
Conclusions
Remission rates in primary care studies of depression are at least as high as for those in psychiatric settings. It is a realistic goal for family physicians to target remission of symptoms as an optimal outcome for treatment of depression.
Similar content being viewed by others
Background
Major depressive disorder (MDD) is one of the most common and disabling of medical conditions [1]. The Canadian Community Health Survey recently reported a one-year prevalence rate of 4.5% for MDD, indicating that over 1.1 million Canadians suffer significant distress and impairment in function due to depression [2]. The economic costs of depression are estimated at over $5 billion annually [3]. Depression is currently the fourth-ranked medical condition contributing to global burden of disease, and is estimated to rise to second overall by the year 2010 [4].
There are many effective treatments for MDD, including psychotherapy and antidepressants. Traditionally, efficacy in randomized controlled trials (RCTs) for depression has been determined on the basis of score changes in rating scales such as the Hamilton Depression Rating Scale (HDRS) [5] or the Montgomery-Asberg Depression Rating Scale (MADRS) [6]. Clinical outcome has been usually assessed by clinical response rates, typically defined as a 50% or greater reduction from baseline scores on these rating scales [7]. Although obtaining clinical response represents an important therapeutic milestone, it does not necessarily indicate a complete recovery from MDD, since many patients with clinical response will still be left with substantial residual symptoms of depression. Studies have shown that the presence of residual symptoms after an episode of MDD is associated with higher risk of relapse, recurrence, chronicity, suicide, development of cardiovascular disease, and poor quality of life [8–10].
Such findings suggest that the goals of acute treatment (approximately the first 8–12 weeks or so of treatment) for MDD should be clinical remission, a clinical state distinguished by minimal residual symptoms, rather than just response [11–13]. Clinical remission is typically defined as a score within the normal range on a given outcome measure (e.g., 17-item HDRS score of 7 or less; MADRS score of 12 or less; Clinical Global Impression [CGI] [14] severity score of "Normal, not at all ill"), although there is still some uncertainty as to the validity of these cutoff scores for symptom remission [15]. The achievement of remission is of considerable clinical importance as it predicts decreased risk of relapse and greater psychosocial functioning than typically observed in patients who have achieved clinical response alone [16–18]. Clinical remission is now identified and promoted as a clinical target for successful management of MDD in many clinical practice guidelines [13, 19–21].
Increasing numbers of treatment studies are now explicitly reporting both clinical response and remission rates in assessment of outcome. A meta-analysis of 8 antidepressant studies of venlafaxine versus selective serotonin reuptake inhibitors [SSRIs] and placebo reported mean remission rates of 45%, 35%, and 25%, respectively [22]. A subsequent meta-analysis of 32 RCTs comparing venlafaxine, SSRIs and other antidepressants reported a mean overall remission rate of 42% [23]. Finally, a meta-analysis of 6 RCTs comparing antidepressants and psychotherapy in patients with MDD reported mean remission rates of 46% for each treatment [24].
All the studies in these systematic reviews involved patients in psychiatric or mixed settings. However, most people suffering from MDD will be managed in the primary care setting [25]. Approximately 5% to 10% of all patients consulting a general practitioner have MDD, with prevalence estimates being two to three times higher when other depressive disorders (i.e., minor depression or dysthymia) are included [26]. It remains unclear whether the remission rates reported in psychiatric settings can be extrapolated to primary care environments, although it is of clinical importance for primary care physicians to know whether obtaining remission is a realistic goal for their patients. There has been a recent surge in studies assessing a variety of treatment interventions for depression in primary care settings, making this an opportune time to perform a meta-analysis to address this question. Hence, the primary objective of this study was to determine remission rates obtained in RCTs of treatment interventions for MDD conducted in primary care settings.
Methods
Potentially relevant studies were identified using computerized and manual search strategies. The computerized search conducted in June, 2003 included the databases: Medline, Psych Info, Embase, Biosis, Cochrane Database of Systematic Reviews, and Cochrane Controlled Trials Register and Current Controlled Trials (1981–May 2003). The search terms used were 'depressive disorder' or 'depression' combined with 'primary care' and 'remission' and/or variants. The bibliographies of relevant articles were also manually searched. Two reviewers (MYD and RWL) collected and independently assessed abstracts for inclusion criteria. Disagreements were resolved with consensus.
Inclusion criteria
Studies were included if they were RCTs with original data comparing one or more interventions (e.g., antidepressant vs. cognitive behavioral therapy) and published in English. Only studies of predominantly adult populations, as opposed to exclusively child or elderly patient populations, were included. Although the focus was principally upon patients with MDD (studies primarily dealing with minor depression and dysthymia were excluded), the criteria for a diagnosis of MDD was intentionally broad in order to capture the heterogeneity of the sample and allow the results to be as generalizable as possible. Included studies also had to use a standardized outcome measure (e.g., HDRS, MADRS, Beck Depression Inventory [BDI] [27]) and provide explicit criteria for remission. While the definition of remission varied among the studies (Table 1), for the purpose of this meta-analysis we accepted each study's definition of remission, which usually was a score within the normal range on the outcome measure.
Data extraction
Two independent reviewers (MYD and EEM) extracted data from studies using a checklist developed for this study, with disagreements resolved by a third reviewer (RWL). A conservative measure of remission rate was calculated from each study using an intent-to-treat analysis [28], even if this method was not used in the study. For example, some studies calculated remission rates using only patients who returned for one follow-up visit post-randomization, or who had completed a course of treatment. The denominator used for remission rate was the total number of patients randomized to treatment, whether or not they were counted in the ensuing analysis. The numerator was the number of patients in remission reported in the study, regardless of the denominator used in the study analysis.
The type of intervention was classified as placebo, "usual care" by clinician (standard treatment by a patient's own physician), psychotherapy treatment only, antidepressant treatment only, psychotherapy plus antidepressant treatment, or program intervention (e.g., collaborative care using other health professionals; educational programs targeted at quality improvement for prescribing practices).
Statistics
Each set of rates was pooled based on a Bayesian approach to meta-analysis using the Fastpro software program (version 1.7) by Eddy and Hasselblad. Readers interested in a more detailed discussion of this approach should refer to Eddy et al [29]. The pooled means and confidence intervals were calculated using Jeffrey's prior and a random effects model.
Results
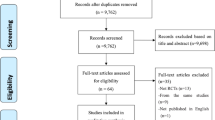
The initial electronic and bibliographic search found 63 articles of which 47 warranted more detailed review based on the published abstract. Of these, 34 articles were excluded due to methodology (not RCTs, 4 studies), lack of remission criteria (18 studies), diagnostic criteria (not MDD, 11 studies) and age criteria (geriatric, 4 studies) (some studies were excluded for multiple reasons, see Additional File 1). A final count of 13 studies met the full inclusion criteria (Table 1). In total, 3202 primary care outpatients (75% female, 25% male) were included in the analysis. The mean age of the participants was 32.1 years (range 18–73 years). The average length of follow-up was 32 weeks (range 6–104 weeks).
The study interventions and methodologies were too heterogeneous to allow for a meaningful statistical comparison of results between treatments. Figure 1 shows mean remission rates for specific interventions. Overall remission rates for active interventions, regardless of type, ranged between 50% and 67%, compared to 32% for pill placebo conditions and 35% for usual care conditions. There were a sufficient number of antidepressant arms in the studies to permit the summary of remission rates by duration of follow-up period. For antidepressant studies with follow-up of 6 months or less, mean remission rate was 51.4% (95% C.I., 43.1%–59.6%); for antidepressant studies with greater than 6 months of follow-up, mean remission rate was 62.3% (95% C.I., 48.9%–74.8%).
Remission rates for specific treatment conditions from randomized controlled trials (RCTs) of interventions for depression in primary care settings. The white lines represent the mean remission rates and the boxes represent the 95% confidence interval. N is the number of treatment arms in the RCTs (Note: Psychotx = Psychotherapy, Antidepr = Antidepressants, pts = patients).
Discussion
This review of research assessing remission of depressive symptoms in primary care populations identified 13 studies meeting the inclusion criteria. Overall remission rates (regardless of type of intervention but excluding placebo or usual care arms) ranged between 50% and 67%. These rates are equivalent to, or indeed greater than, those reported in meta-analyses of studies examining pharmacological or psychological interventions for depression in psychiatric populations, in which the overall remission rates ranged between 35% and 46% [22–24]. On the one hand, we might have predicted this finding as studies conducted in primary care settings tend to include more patients with mild to moderate depression (although we excluded studies that focused exclusively upon minor depression or dysthymia), whereas patients referred to psychiatric settings are more likely to have moderate to severe depression. Primary care treatment trials also tend to be longer, favouring a higher remission rate; whereas the mean follow-up period of studies included in the current analysis was 9 months, it was only 7 weeks and 10 weeks in the two previous meta-analyses of pharmacological interventions for MDD [22, 23], and 16 weeks in the meta-analysis of antidepressant versus psychotherapeutic interventions [24]. Conversely, we might have predicted that we would observe lower remission rates in the current meta-analysis as it included a number of studies with more lenient exclusion criteria than typically used in psychiatric clinical trials. In particular, the program intervention studies tend to include more heterogeneous patient populations as they do not routinely exclude patients with psychiatric or medical comorbidities, factors that may lessen the likelihood of obtaining remission of depressive symptoms [30].
While it was not within the scope of the current study to compare the effectiveness of different treatment interventions in improving remission rates, we can report on the trends we observed in the data. Antidepressant and psychotherapy interventions delivered in isolation showed similar remission rates (54% for both). Combination antidepressant plus psychotherapy interventions showed somewhat higher rates (67%), although this category included only 1 arm with only 35 patients. Program interventions had a mean remission rate of 50%, and all treatment interventions fared better than either placebo (32%) or usual care (35%).
The studies identified in our review were quite heterogeneous in nature, ranging from those that looked solely at the effects of a particular pharmacological agent, through to complex program initiatives that incorporated a variety of interventions at different levels of care. This heterogeneity limits our ability to make broad comments about remission rates in primary care, but was not unexpected, as we wanted to capture the diversity of treatment interventions for depression currently being tested in this setting. Other potential limitations of the study include that fact that we only assessed published studies written in English and that we used a conservative measure of remission rate. Finally, we also used the definition of remission as specified by each individual study. While these definitions were similar to those widely used in RCTs conducted in psychiatric settings, and thus are useful for comparison, there is current controversy about depression scales and which cutoff scores indicate true remission of symptoms [15].
Conclusions
This meta-analysis serves to answer an important clinical question about the feasibility of obtaining remission of symptoms of MDD in primary care patients. Our results indicate that this is a realistic goal in this population, although further research is still required to determine whether certain treatment modalities (or combinations of treatment interventions) are superior to others in achieving higher remission rates. Future research should also focus upon developing pragmatic strategies for general practitioners to implement evidence-based guidelines concerning the treatment of depression to clinical remission.
References
Parikh SV, Lam RW: Clinical guidelines for the treatment of depressive disorders, I. Definitions, prevalence, and health burden. Can J Psychiatry. 2001, 46 Suppl 1: 13S-20S.
Statistics Canada : Canadian Community Health Survey: Mental health and well-being. www statcan ca/Daily/English/030903/d030903a htm, accessed November 9,. 2003
Desjardins B, Laurier C: The burden of depression in Canada. Value in Health. 2002, 5: 229-
Murray CJ, Lopez AD: Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997, 349: 1498-1504. 10.1016/S0140-6736(96)07492-2.
Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psych. 1967, 6: 278-296.
Montgomery SA, Asberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979, 134: 382-389.
Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM: Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991, 48: 851-855.
Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB: Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998, 50: 97-108. 10.1016/S0165-0327(98)00138-4.
Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, Leon AC, Maser JD, Mueller T, Solomon DA, Keller MB: Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness?. Am J Psychiatry. 2000, 157: 1501-1504. 10.1176/appi.ajp.157.9.1501.
Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB: A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998, 55: 694-700. 10.1001/archpsyc.55.8.694.
Rush AJ, Trivedi MH: Treating depression to remission. Psychiatric Annals. 1995, 25: 704-705.
Thase ME: The clinical, psychosocial, and pharmacoeconomic ramifications of remission. Am J Manag Care. 2001, 7: S377-S385.
Reesal RT, Lam RW: Clinical guidelines for the treatment of depressive disorders. II. Principles of management. Can J Psychiatry. 2001, 46 Suppl 1: 21S-28S.
Guy W: ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Edited by: GuyW. 1976, Rockville, MD, US Department of Health, Education and Welfare, 217-222.
Zimmerman M, Posternak MA, Chelminski I: Implications of using different cut-offs on symptom severity scales to define remission from depression. Int Clin Psychopharmacol. 2004, 19: 215-220. 10.1097/01.yic.0000130232.57629.46.
Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T, Friedman E: Relapse after cognitive behavior therapy of depression: potential implications for longer courses of treatment. Am J Psychiatry. 1992, 149: 1046-1052.
Fava GA, Fabbri S, Sonino N: Residual symptoms in depression: an emerging therapeutic target. Prog Neuropsychopharmacol Biol Psychiatry. 2002, 26: 1019-1027. 10.1016/S0278-5846(02)00226-9.
Miller IW, Keitner GI, Schatzberg AF, Klein DN, Thase ME, Rush AJ, Markowitz JC, Schlager DS, Kornstein SG, Davis SM, Harrison WM, Keller MB: The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry. 1998, 59: 608-619.
American Psychiatric Association : Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000, 157: 1-45.
Bauer M, Whybrow PC, Angst J, Versiani M, Moller H-J, Disorders WFSBP Task Force on Treatment Guidelines for Unipolar Depressive: World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: Acute and continuation treatment of major depressive disorder. World Journal of Biological Psychiatry. 2002, 3: 5-43.
Depression Guideline Panel: 1993, Washington, Agency for Health Care Policy and Research, Treatment of Major Depression. Clinical Practice Guideline. Depression in Primary Care
Thase ME, Entsuah AR, Rudolph RL: Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001, 178: 234-241. 10.1192/bjp.178.3.234.
Smith D, Dempster C, Glanville J, Freemantle N, Anderson I: Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: a meta-analysis. Br J Psychiatry. 2002, 180: 396-404. 10.1192/bjp.180.5.396.
Casacalenda N, Perry JC, Looper K: Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry. 2002, 159: 1354-1360. 10.1176/appi.ajp.159.8.1354.
Barrett JE, Barrett JA, Oxman TE, Gerber PD: The prevalence of psychiatric disorders in a primary care practice. Archives of General Psychiatry. 1988, 45: 1100-1106.
Katon W, Schulberg H: Epidemiology of depression in primary care. Gen Hosp Psychiatry. 1992, 14: 237-247. 10.1016/0163-8343(92)90094-Q.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry. 1961, 4: 561-571.
Collaboration Cochrane: Cochrane Manual. 2004, Oxford, Cochrane Collaboration
Eddy DM, Hasselbald V, Shachter R: Meta-analysis by the confidence interval method. The statistical synthesis of evidence. 1992, San Diego, Academic Press Inc.
Cuffel BJ, Azocar F, Tomlin M, Greenfield SF, Busch AB, Croghan TW: Remission, residual symptoms, and nonresponse in the usual treatment of major depression in managed clinical practice. J Clin Psychiatry. 2003, 64: 397-402.
Dowrick C, Dunn G, Ayuso-Mateos JL, Dalgard OS, Page H, Lehtinen V, Casey P, Wilkinson C, Vazquez-Barquero JL, Wilkinson G: Problem solving treatment and group psychoeducation for depression: multicentre randomised controlled trial. Outcomes of Depression International Network (ODIN) Group. British Medical Journal - Clinical Research. 2000, 321: 1450-1454. 10.1136/bmj.321.7274.1450.
Benkert O, Szegedi A, Kohnen R: Mirtazapine compared with paroxetine in major depression. J Clin Psychiatry. 2000, 61: 656-663.
Patris M, Bouchard JM, Bougerol T, Charbonnier JF, Chevalier JF, Clerc G, Cyran C, Van Amerongen P, Lemming O, Hopfner Petersen HE: Citalopram versus fluoxetine: a double-blind, controlled, multicentre, phase III trial in patients with unipolar major depression treated in general practice. Int Clin Psychopharmacol. 1996, 11: 129-136.
Wade A, Lemming O.Michael, Hedegaard K.Bang: Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. International Clinical Psychopharmacology. 2002, 17: 95-102. 10.1097/00004850-200205000-00001.
Chilvers C, Dewey M, Fielding K, Gretton V, Miller P, Palmer B, Weller D, Churchill R, Williams I, Bedi N, Duggan C, Lee A, Harrison G: Antidepressant drugs and generic counselling for treatment of major depression in primary care: randomised trial with patient preference arms. BMJ. 2001, 322: 772-775. 10.1136/bmj.322.7289.772.
Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, Tomlinson D: Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care. BMJ. 1995, 310: 441-445.
Mynors-Wallis LM, Gath DH, Day A, Baker F: Randomised controlled trial of problem solving treatment, antidepressant medication, and combined treatment for major depression in primary care. BMJ. 2000, 320: 26-30. 10.1136/bmj.320.7226.26.
Schulberg HC, Pilkonis PA, Houck P: The severity of major depression and choice of treatment in primary care practice. J Consult Clin Psychol. 1998, 66: 932-938. 10.1037//0022-006X.66.6.932.
Scott AI, Freeman CP: Edinburgh primary care depression study: treatment outcome, patient satisfaction, and cost after 16 weeks. BMJ. 1992, 304: 883-887.
Katon W, Von Korff M., Lin E, Simon G, Walker E, Unutzer J, Bush T, Russo J, Ludman E: Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999, 56: 1109-1115. 10.1001/archpsyc.56.12.1109.
Katzelnick DJ, Simon GE, Pearson SD, Manning WG, Helstad CP, Henk HJ, Cole SM, Lin EH, Taylor LH, Kobak KA: Randomized trial of a depression management program in high utilizers of medical care. Arch Fam Med. 2000, 9: 345-351. 10.1001/archfami.9.4.345.
Kutcher S, Leblanc J, Maclaren C, Hadrava V: A randomized trial of a specific adherence enhancement program in sertraline-treated adults with major depressive disorder in a primary care setting. Prog Neuropsychopharmacol Biol Psychiatry. 2002, 26: 591-596. 10.1016/S0278-5846(01)00313-X.
Rost K, Nutting P, Smith JL, Elliott CE, Dickinson M: Managing depression as a chronic disease: a randomised trial of ongoing treatment in primary care. BMJ. 2002, 325: 934-10.1136/bmj.325.7370.934.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2296/5/19/prepub
Acknowledgments
Dr. Michalak was supported by a Canadian Institutes of Health Research/Wyeth Canada Postdoctoral Fellowship Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
MYD and EEM conducted the data extraction, wrote the initial draft of the manuscript, interpreted results, and revised the manuscript. PW provided statistical consultation and analysis, and revised the manuscript. JEA interpreted the results and revised the manuscript. RWL conceived the initial idea, developed the method, interpreted results, revised the manuscript, and provided financial resources for the study. All authors read and approved the final manuscript.
Competing interests
RWL is on advisory/speaker boards or has received research funds from: AstraZeneca, Biovail, Canadian Network for Mood and Anxiety Treatments, Eli Lilly, GlaxoSmithKline, Janssen-Ortho, Litebook, Inc., Lundbeck, Merck, Organon, Roche, Shire, Servier, and Wyeth.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Dawson, M.Y., Michalak, E.E., Waraich, P. et al. Is remission of depressive symptoms in primary care a realistic goal? A meta-analysis. BMC Fam Pract 5, 19 (2004). https://doi.org/10.1186/1471-2296-5-19
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2296-5-19