Abstract
Background
Anticancer bisdioxopiperazines, including ICRF-154, razoxane (Raz, ICRF-159) and ICRF-193, are a family of anticancer agents developed in the UK, especially targeting metastases of neoplasms. Two other bisdioxopiperazine derivatives, probimane (Pro) and MST-16, were synthesized at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China. Cytotoxic activities and mechanisms of Raz (+)-steroisomer (ICRF-187, dexrazoxane), Pro and MST-16 against tumor cells were evaluated by MTT colorimetry, flow cytometry and karyotyping.
Results
Pro was cytotoxic to human tumor cell lines in vitro (IC50<50 μM for 48 h). Four human tumor cell lines (SCG-7901, K562, A549 and HL60) were susceptible to Pro at low inhibitory concentrations (IC50 values < 10 μM for 48 h). Although the IC50 against HeLa cell line of vincristine (VCR, 4.56 μM), doxorubicin (Dox, 1.12 μM) and 5-fluoruouracil (5-Fu, 0.232 μM) are lower than Pro (5.12 μM), ICRF-187 (129 μM) and MST-16 (26.4 μM), VCR, Dox and 5-Fu shows a low dose-related – high cytotoxic activity. Time-response studies showed that the cytotoxic effects of Pro are increased for 3 days in human tumor cells, whereas VCR, Dox and 5-Fu showed decreased cytotoxic action after 24 h. Cell cycle G2/M phase arrest and chromosome segregation blocking by Pro and MST-16 were noted. Although there was similar effects of Pro and MST-16 on chromosome segregation blocking action and cell cycle G2/M phase arrest at 1- 4 μM, cytotoxicity of Pro against tumor cells was higher than that of MST-16 in vitro by a factor of 3- 10 folds. Our data show that Pro may be more effective against lung cancer and leukemia while ICRF-187 and MST-16 shows similar IC50 values only against leukemia.
Conclusion
It suggests that Pro has a wider spectrum of cytotoxic effects against human tumor cells than other bisdioxopiperazines, especially against solid tumors, and with a single cytotoxic pathway of Pro and MST-16 affecting chromosome segregation and leading also to cell G2/ M phase arrests, which finally reduces cell division rates. Pro may be more potent than MST-16 in cytotoxicity. High dose- and time- responses of Pro, when compared with VCR, 5-Fu and Dox, were seen that suggest a selectivity of Pro against tumor growth. Compounds of bisdioxopiperazines family may keep up their cytotoxic effects longer than many other anticancer drugs.
Similar content being viewed by others
Background
Bisdioxopiperazines, including ICRF-154, razoxane (ICRF-159, Raz); ICRF-186 and ICRF-187), two stereo-isomers of Raz, and ICRF-193, developed in the UK, were some of the earliest agents found against a murine spontaneous metastatic model (Lewis lung carcinoma) in 1969 [1]. Many papers and projects have dealt with their potential use and mechanisms since that time. Three main mechanisms of bisdioxopiperazine action have been investigated, including assisting in radiotherapy, [2, 3] overcoming multi-drug resistance (MDR) of daunorubicin and doxorubicin to leukemias [4, 5] and inhibiting topoisomerase II [6, 7]. More importantly, Raz has been licensed for cardioprotectant of anticancer anthrocyclines in more countries. Since bisdioxopiperazines represents a unique family of antimetastatic agents that are structurally conservative in their pharmacological actions, two new derivatives, probimane [1,2-bis (N4-morpholine-3, 5-dioxopeprazine-1-yl) propane; AT-2153, Pro] and MST-16, 1, 2- bis (4- isobutoxycarbonyloxymethyl- 3, 5- dioxopiperazin-1- yl) ethane were synthesized at this institute in Shanghai, China. [8, 9]. Apart from data of anti-tumor activity [10–12], the pharmacological mechanisms of Pro as Raz, like the detoxication of Adriamycin (ADR), induced cardiotoxicities and synergism with ADR against leukemias were reported at Henan Academy of Medicine, Henan, China [13]. As the main researchers of Pro, we reported some novel biological actions of Pro, including the inhibition of the activity of calmodulin (CaM), a cell-signal regulator, which can explain anticancer actions and the combined cytotoxic effect of Pro with ADR [13, 14] inhibiting lipoperoxidation (LPO) of erythrocytes [15], down-regulating sialic acid synthesis in tumors [16] and blocking the binding of fibrinogen to leukemia cells [17]. MST-16, as a licensed drug in Japan since 1994, was permitted for direct use in leukemia chemotherapy, mainly against adult T-cell leukemia treatment [18]. Structural formulae of the three bisdioxopiperazines are represented in Figure 1.
As a new bisdioxopiperazine, the pharmacological characters and features of Pro are intriguing. Increased understanding of the advantages and disadvantages of the two compounds is a first step for promoting applications of Pro and MST-16. Therefore, in depth pharmacological evaluation was carried out. Tumors studied are from 7 different organs of origin – two gastric tumor cell line (SCG-7901, MKN-28), a lung tumor cell line (A549), a colon cancer cell line (HCT-116), two mammary tumor cell lines (MDA-MB-435, MDA-MB-468), one hepatic tumor cell line (BEL7402), two leukemia cell line (HL-60 and K562) and an uteric cervical tumor cell line (HeLa). In addition, time- and concentration-dependent relations to classify the effectiveness of different therapeutic schedules and schemes of Pro and MST-16 therapy have been addressed.
Results
Cytotoxic effects of Pro and MST-16 against human tumor cell lines
Data on the anticancer effects of Pro using 10 human tumor cell lines in vitro are showed in Figure 2 and Table 1. Pro had anticancer effects in vitro at clinical acceptable concentrations (IC50 values < 50 μM) by MTT methods. The IC50 values of Pro are 1.3672 ± 0.6230 μM, 24.314 ± 5.465 μM, 14.476 ± 3.085 μM, 45.325 ± 5.335, 22.169 ± 1.250, 0.02947 ± 0.02456 μM, 5.3417 ± 1.245 μM, 4.786 ± 1.556, 42.457 ± 2.325 μM and 18.238 ± 1.112 μM representing tumor cells of SCG-7901 and MKN- 28 (two human gastric tumor cell lines), HCT-116 (a human colon tumor cell line), MDA-MB-435 and MDA-MB-468 (two human mammary tumor cell lines), A549 (a human lung tumor cell line) and HL60 and K562 (two human leukemia cell lines), BEL-7402 (a human hepatic tumor cell line) and HeLa cell (a human uteric cervical tumor cell line) respectively (Figure 2). Among these tumor cell lines, Pro is more effective to SCG-7901 (a gastric cancer cell line), A549 (a lung cancer cell line) and HL60 and K562 (two leukemia cell lines), the IC50 values being ≤10 μM.
Comparison of the cytotoxic effects of bisdioxopiperazines with other drugs
The cytotoxic effects against tumor cell lines (p388, HL-60 and HeLa cells) are included in Table 1. Although IC50s of Dox, VCR and 5-Fu are lower than that of Pro, the greatest inhibitory rates of Pro at high concentrations are seen (Table 2). No inhibitory difference between low and high concentrations of Dox, VCR and 5-Fu was observed (Table 3). Generally, the LD50 of VCR and Dox in experimental animals and humans are dramatically lower than Pro. These results suggest more difficult management and wider toxicities of these drugs in their application in the clinics, suggesting Pro may avoid these drawbacks.
Comparison of anti-tumor effects of probimane and MST-16 and their time- response relationships
Cytotoxic effects (IC50) of probimane and MST-16 against tumor cells were compared (Figure 3).
In addition, the time- response curves indicate that the anti-tumor effects of Pro increase to a climax over 3 days of drug exposure (Figures 4, 5 and 6). The cytotoxic effects of Pro persist or rise with time, whereas those of VCR, Dox and 5-Fu decrease after 24 h (Table 4). IC50 of both Pro and MST-16 reduces dramatically by 72 h from 48 h. (Figures 7 and 8). The reductions of IC50 for both agents Pro or MST-16 depend on the cell line. IC50 ratios of Pro and MST-16 for 3 days relative to 2 days against the most metastatic phenotype tumor cell line MDA-MB-435, are 8.9 and 7.5 times higher, and 2.6 times for the medium metastatic phenotype of MDA-MB-468 cells.
G2 and M phase arrests induced by Pro or MST-16
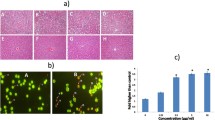
Our data shows that both probimane (Pro) and MST-16 can arrest tumor cells in G2 and M phases of the cell cycle. Dose- and/ or concentration- dependency are observed in G2 and M arrests (Figures 9 to 12), and the arresting effect of Pro on MDA-MB-435 and HCT-116 is only 2 times higher for MST-16 at equivalent concentrations. Pro at 4 μM can increase G2/M accumulation from 16.8 % (vehicle control) to 86.4 % after 24 h (p < 0.001, n = 3).
Chromosome segregation inhibition by Pro and MST-16
Chromosome linkages, aggregations and segregation in tumor cells were blocked by both Pro and MST-16. Figure 13 and 14 show linkages and segregation blockade of chromosomes in cells treated with Pro and MST-16 at 4 μM. Despite this, chromosomes began to separate with each other, and their morphology became slimmer at lower concentration 1μM in both human mammary tumors of MDA-MB-436 cells and MDA-MB-468 cell lines in vitro. This chromosome poisoning action of Pro, MST-16 and ICRF-187 was seen at 1- 4 μM. In vehicle control group, chromosomes of tumor cells separated from each other very well. Although we only show typically one or two cells, the chromosomal characteristics in each group have an overall consistency (> 80 %) in each piece of preparation from cell treated with Pro, MST-16 and ICRF-187. They are common characteristics and phenotypes induced by the three compounds. In addition, there seems no difference in overall chromosome effects of Pro and MST-16 at equivalent concentrations (Figures 13 and 14), suggests that Pro and MST-16 act equally in this pathway.
Discussion
Increased understanding over the mechanisms of bisdioxopopiperazines can greatly improve their indications and narrow down contraindicates in clinical practice. The explanations for the anticancer actions of bisdioxopiperazine are currently focusing on anti-angiogeneses [19, 20] and tumor cell DNA alterations caused by topoisomerase II. Generally speaking, most angiogenesis inhibitors often have low cytotoxicity and are ineffective against larger tumor masses, and are better combined with cytotoxic drugs clinically [21, 22]. This work on the anticancer activity of Pro and MST-16 shows that they act through the blocking of chromosomal segregation and G2/M phase arrests, causing complete inhibition of tumor cell division. Pro, MST-16 and ICRF-187 play similar roles at equi-molar concentrations. This pathway may be related to topoismerase II inhibition [23] as a possible mode of tumor growth inhibition, but is not suggested as a systematic approach through a cascade series. Two findings in this study need further discussion; (i) the effective ranges of Pro and MST-16 in the blocking of chromosome segregation, and causing G2/M phase arrests are 1- 4 μM, similar for Pro and MST-16. This suggests the two processes operate in the same course or cascade, and most possibly are directly linked; (ii) cyto-toxicity test (MTT) showed that Pro was more effective than MST-16. Lacking parallels in the effective dose ranges of Pro and MST-16 between cyto-toxicities and chromosome segregation – induced tumor inhibition can be explained by the fact that these effects of Pro and MST-16 do not strictly follow the same pathway given in Figure 15. Stronger cytotoxic effects of Pro against many other human tumor cell lines than original bisdioxopiperazines derivatives, especially on solid tumors, suggest some as yet undiscovered mechanism that Pro may have, and Pro may have better applications and require fewer drug combinations in the future.
This work shows that anticancer activities of Pro against lung cancer and leukemia are relatively greater than against other tumor typies. Cytotoxic and antimetastatic activities of Pro against lung tumor models in vivo have also been found [24]. Lung cancer is the most prevalent among all cancer categories, and is one of the deadliest cancers in the clinics. Targeted at lung cancers, Pro may offer better medical and economic benefits in the future.
For clinical chemotherapy, the paramount task is the balancing between treatment outcome and risks [25]. To optimize chemotherapeutic protocols containing bisdioxopiperazines, knowledge of its pharmacological parameters in terms of concentration- and time- responses are prerequisites. We found that Pro and MST-16 might act and accumulate longer in tumor cells than most of anticancer drugs. The peak of cytotoxicity of both Pro and MST-16 is on day 3, and not usually on day 2. This result and our early work of auto-radiography that Pro [26] persists longer in tumor tissues suggest that longer intervals may be used between treatments and less nursing responsibilities may arise, while maintaining high levels of tumor growth inhibitions. The long- term cytotoxic effects of Pro and MST-16 are more obvious in high metastatic tumor cell lines, which can explain the selective effects of compounds to tumor metastases. Early reports suggest that MST-16 needs to transform into ICRF-154 to exhibit its anticancer effects [27]. This work proves that MST-16 does not degraded to ICRF-154, and has a lower cytotoxic effects against tumor cells than Pro. Yet MST-16 can maintain a high activity in the cascade of the proposed mechanism – chromosome segregation blockage and cellular G2/M phase arrest, leading to inhibition of cell division (Figure 15). It further suggests this mechanism is not a pivotal pathway for cytotoxic activity against tumors.
Conclusion
We suggest that Pro has a wider spectrum of cytotoxic effects against human tumor cells than other bisdioxopiperazines, especially on solid tumors. The cytotoxic pathway of Pro and MST-16 appears to be through chromosome segregation blocking and G2/ M phase arrests. Pro may be more potent than MST-16. High dose- and time- related responses of Pro than VCR, 5-Fu and Dox are seen that suggest a selectivity by Pro against tumor growth. It suggests that the family of bisdioxopiperazines may sustain their cytotoxic effects longer than other anticancer drugs.
Methods
Pro and MST-16 were synthesized in this institute. Other chemical agents were purchased from sources stated below. The tumor cell lines were obtained from various sources and serially passaged in this lab.
MTT method
The cells were maintained in RPMI 1640 (Gibco, Invitrogen Corporation, NY, USA) medium supplemented with 10 % FCS, streptomycin (100 μg/ml) and penicillin (100 units/ml). A density of 105 tumor cells /ml (90 μl) were seeded in 96-well plates for 24 h. Pro or MST-16 (10 μl), final concentrations indicated below, were added to each well for incubating for the next 48 h. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma Company, USA) (5 mg/ ml, 20 μl) was added to each well. Four h later, 50 μl compound solution (10 % SDS- 5 % isobutyl alcohol-1 N HCl) were added and incubated under 5 % CO2 atmospheric condition for 24 h. Optical density at 570 nm was measured with a tunable microplate reader, VERSAmax, USA, each group was in triplicate samples and Pro or MST-16 were divided into 5 concentrations.
Cell cycle analysis by cytometry
Tumor cells in exponential phase were exposed to Pro or MST-16. After 6 -24 h, cells were collected (300 × g, 10 min) and incubated with ice-cold PBS. Then fixed with ethanol and collected and washed with PBS by centrifugation (300 × g, 10 min). Cell deposition was added with PBS 1 ml and RNAse (5 μl) at 37°C bath for 15 min. Cells were dyed with 5 μl PI (2 mg/ml) in dark. Cells were measured for their DNA content by cytometry (Becton/Dickinson – FACS Calibur) after passing through a cell filter.
Chromosome preparation protocols
Cell chromosome preparation was by a routine procedure. Human mammary tumor cells (MDA-MB-435 and MDA-MB-468) were seeded into a 6-well plate and maintained under an atmosphere of 5 % CO2 condition. When tumor cells covered about 60- 70 % of the surface, bisdioxopiperazines were added. Drug – treated cells were treated with hypotonic KCl, 0.075 M at 37°C for 30 min. Cell nuclei were fixed with fresh-prepared fixative solution [methanol/acetic acid, 3:1] for 5 min. Cell nuclei were collected by centrifugation (900 × g 15 min) and washed with fixative solution by centrifugation (1500 × g 20 min). Cell nuclei were dropped onto a cooled glass plate and placing overnight under a dehydrogenated atmosphere. The scattered chromosomes were dyed with a Giemsa solution for 15- 20 min and washed with tap water. Chromosomal behaviors were viewed and photographed by microscopy with an oil-lense (LEICA, Qwin image processing analysis system, Germany).
Statistics
IC50 of agents were calculated by software in this lab and X ± SD was calculated from data of two groups.
Abbreviations
- ADR (Dox):
-
adriamycin
- VCR:
-
vincristine
- 5-Fu:
-
5-fluorouracil
- CaM:
-
calmodulin
- LPO:
-
lipoperoxidation
- Raz (ICRF-159 or ICRF-187):
-
razoxane
- Pro:
-
probimane
- PI:
-
propidium iodide
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)- 2,5- diphenyl tetrazolium bromide
References
Herman EH, Witiak DT, Hellmann K, Waradek VS: Properties of ICRF-159 and related Bis(dioxopiperazine) compounds. Advances in Pharmacology and Chemotherapy. 1982, 19: 249-290.
Hellmann K, Rhomberg W: Radiotherapeutic enhancement by razoxane. Cancer Treatment Review. 1991, 18: 225-240. 10.1016/0305-7372(91)90014-Q.
Schuchter LM, Hensley ML, Meropol NT, Winer EP: America Society of clinical oncology chemotherapy and radiotherapy protectants: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2002, 20: 2895-2903. 10.1200/JCO.2002.04.178.
Sargent JM, Williamson CJ, Yardley C, Taylor CG, Hellmann K: Dexrazoxane significantly impares the induction of doxorubicin resistance the human leukemia line, K562. Brit J Cancer. 2001, 84: 959-964. 10.1054/bjoc.2001.1697.
Pearlman M, Jendiroba D, Pagliaro L, Keyhani A, Liu B, Freireich EJ: Dexrazoxane in combination with anthracyclines lead to a synergistic cytotoxic response in acute myelogenous leukemia cell lines. Leuk Res. 2003, 27: 617-626. 10.1016/S0145-2126(02)00273-4.
Van Hille B, Etievant C, Barret JM, Kruczynski A, Hill BT: Characterization of the biological and biochemical activities of F 11782 and bisdioxopiperazines, ICRF-187 and ICRF-193, two types of topoisomerase II catalytic inhibitors with distinctive mechanisms of action. Anti-cancer Drugs. 2000, 11: 829-841. 10.1097/00001813-200011000-00007.
Renodon-Corniere A, Sorensen TK, Jensen PB, Nitiss JL, Sokilde B, Sehested M, Jensen LH: Probing the role of linker substituents in bisdioxopiperazine analogs for activity against wild- type and mutant human topoisomerase II alpha. Mol Pharmacol. 2003, 63: 1159-1168. 10.1124/mol.63.5.1159.
Ji RY: Probimane. Drugs Fut. 1988, 13: 418-419.
He H, Wang MY, Zhang TM: Cytokinetic effects of 1, 2- bis (4-isobutoxycarbonyloxymethyl- 3, 5- dioxopiperazin-1- yl) ethane (MST-16) on leukemia L1210 cells in mice. Acta Pharmacol Sin. 1988, 9: 369-373.
Wang MY, Liu TX, Li GT, Zhang TM: Effects of bimolane and probimane on the incorporation of [3H]TdR, [3H]UR and [3H]Leu into Ehrlich ascites carcinoma cells in vitro.,. Acta Pharmacol Sin. 1988, 9: 367-369.
Zhang TM, Wang MY, Wang QD: Antineoplastic action and toxicity of probimane and its effect on immunologic functions in mice. Acta Pharmacol Sin. 1987, 8: 369-374.
Yang KZ, Huang BY, Huang TH, Wu YD: Short-term results of malignant lymphoma treated with probimane. Chin J Cancer. 1990, 9: 192-193.
Zhang Y, Liu J, Wang J, Ye QX, Zhang TM: Effects of probimane (Pro) and doxorubicin (Dox) in combination on DNA synthesis and cell cycle of tumor cells. Chin Pharmacol Bull. 1997, 13: 535-537.
Lu DY, Chen EH, Cao JY, Zhou JJ, Shen ZM, Xu B, Horie K: Comparison between probimane and razoxane on inhibiting calmodulin activity of rabbit erythrocyte membrane. Chin J Pharmacol Toxicol. 2001, 15: 76-78.
Lu DY, Chen EH, Cao JY, Jin W, Tian F, Ding J: The inhibition of probimane on lipid peroxidation of rabbit and human erythrocytes. J Shanghai Univ (Eng). 2003, 7: 301-304.
Lu DY, Liang G, Zhang MJ, Xu B: Serum contents of sialic acids in mice bearing different tumors. Chin Sci Bull (Eng). 1994, 39: 1220-1223.
Lu DY, Chi J, Lin LP, Huang M, Xu B, Ding J: Effect of anticancer drugs on the binding of 125I-fibrinogen to two leukemia cell lines in vitro. J Int Med Res. 2004, 32: 488-491.
Okamoto T, Nishimura Y, Yamada S, Yamada S, Itoh T, Mori A, Saheki K, Okada M, Takatsuka H, Wada H, Tamura A, Fujimori Y, Kakishita E: Long-term administration of oral low-dose topoisomerase II inhibitors, MST-16 and VP- 16, for refractory or relapsed non-Hodgkin's lymphoma. Acta Haematol. 2000, 104: 128-130. 10.1159/000039746.
Braybrooke JP, O'Byrne KJ, Propper DJ, Blann A, Saunders M, Dobbs N, Han C, Woodhull J, Mitchell K, Crew J, Smith K, Stephens R, Ganesan T, Talbot DC, Harris AL: A phase II study of razoxane, an antiangiogenic topoisomerase II inhibitor, in renal cell cancer with assessment of potential surrogate markers of angiogenesis. Clin Cancer Res. 2000, 6: 4697-4704.
Hellmann K: Dynamics of tumor angiogenesis: effect of razoxane- induced growth rate slowdown. Clin Exp Metastasis. 2003, 20: 95-102. 10.1023/A:1022632413888.
Taraboletti G, Margosio B: Antiangiogenic and antivascular therapy for cancer. Current Opinion in Pharmacology. 2001, 1: 378-384. 10.1016/S1471-4892(01)00065-0.
Mark J: A boost for tumor starvation. Science (Washington DC). 2003, 301: 452-454. 10.1126/science.301.5632.452.
Ishida R, Sato M, Narita T, Utsumi KR, Nishimoto T, Morita T, Nagata H, Andoh T: Inhibition of DNA topoisomerase II by ICRF-193 induces polyploidization by uncoupling chromosome dynamics from other cell cycle events. J Cell Biol. 1994, 126 (6): 1341-1351. 10.1083/jcb.126.6.1341.
Lu DY, Xu B, Ding J: Anti-tumor effects of two bisdioxopiperazines against two experimental lung cancer models in vivo. BMC Pharmacology. 2004, 4: 32-10.1186/1471-2210-4-32.
Cavazzana-Calvo M, Thrasher A, Mavilio F: The future of gene therapy, balancing the risks and benefits of clinical trials. Nature. 2004, 427: 779-781. 10.1038/427779a.
Lu DY, Xu B, Zhang X, Chen RT: Distribution of 14C labeled at dioxopiperazine or methyl morphorline group of probimane by whole body autoradiography. Acta Pharmacol Sin. 1993, 14: 171-173.
Narita T, Koide Y, Yaguchi S, Kimura S, Izumisawa Y, Takasa M, Inaba M, Tsukagoshi S: Antitumor activities and schedule dependence of orally administered MST-16, a novel derivative of bis (2, 6-dioxopiperazine). Cancer Chemother Pharmacol. 1991, 28: 235-240.
Acknowledgements
All the experiment was supported by National Foundation of Science and Technology in China and completed in Professor Jian Ding's lab, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, PR China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
The work was designed by Da-Yong Lu and Jian Ding.
The manuscript was written by Da-Yong Lu.
Cytotoxic effects of compounds against human tumors was evaluated by Da-Yong Lu, Min Huang, Chen- Hui Xu, Wei- Yi Yang, Mei- Hong Li.
Cell cycle phase determination and plotting were completed by Da-Yong Lu and Lin- Jiang Tong.
Chromosome morphology was prepared and observed by Da-Yong Lu and Chao- Xin Hu.
The project was partly administered by Li Ping Lin and Xiong Wen Zhang.
Anticancer bisdioxopiperazines were provided by Wei Lu.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lu, D.Y., Huang, M., Xu, C.H. et al. Anti-proliferative effects, cell cycle G2/M phase arrest and blocking of chromosome segregation by probimane and MST-16 in human tumor cell lines. BMC Pharmacol 5, 11 (2005). https://doi.org/10.1186/1471-2210-5-11
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2210-5-11