Abstract
Background
Oxidative stress has been proposed to be involved in the pathogenesis of Parkinson's disease (PD). A plausible source of oxidative stress in nigral dopaminergic neurons is the redox reactions that specifically involve dopamine and produce various toxic molecules, i.e., free radicals and quinone species. α-Synuclein, a protein found in Lewy bodies characteristic of PD, is also thought to be involved in the pathogenesis of PD and point mutations and multiplications in the gene coding for α-synuclein have been found in familial forms of PD.
Results
We used dopaminergic human neuroblastoma BE(2)-M17 cell lines stably transfected with WT or A30P mutant α-synuclein to characterize the effect of α-synuclein on dopamine toxicity. Cellular toxicity was analyzed by lactate dehydrogenase assay and by fluorescence-activated cell sorter analysis. Increased expression of either wild-type or mutant α-synuclein enhances the cellular toxicity induced by the accumulation of intracellular dopamine or DOPA.
Conclusions
Our results suggest that an interplay between dopamine and α-synuclein can cause cell death in a neuron-like background. The data presented here are compatible with several models of cytotoxicity, including the formation of α-synuclein oligomers and impairment of the lysosomal degradation.
Similar content being viewed by others
Background
Parkinson's disease (PD) is a common neurodegenerative disorder characterized by symptoms that include resting tremor, slowness of movement, rigidity and postural imbalance. PD is a chronic and progressive disease caused by degeneration of several neuronal populations in the brain, most notably the dopaminergic neuromelanin-containing neurons of the substantia nigra (SN) pars compacta[1].
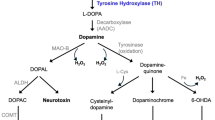
While the etiopathogenesis of idiopathic PD remains poorly understood, post mortem studies support the involvement of oxidative stress and the production of reactive oxygen species in neuronal damage [2, 3]. Redox reactions that specifically involve dopamine (DA) are a possible source of oxidative stress accounting for the more pronounced degeneration of dopaminergic neurons in PD. A critical determinant of DA toxicity is the amount of DA present in the cytoplasm, outside of the acidic stabilizing environment of the synaptic vesicles where the neurotransmitter is normally confined. In the cytoplasm, DA can undergo both spontaneous or enzymatic-mediated oxidation and, as a consequence, generate superoxide anions, hydrogen peroxide and quinones [4], which can damage cellular components such as lipids, proteins and DNA [5–8].
Neuropathologically, PD is characterized by the presence of intracellular ubiquitinated inclusions, known as Lewy bodies, composed predominantly of fibrillar α-synuclein [9, 10]. The discovery of three different missense mutations in the SNCA gene that codes for the α-synuclein protein, found in rare familial forms of PD indicates that α-synuclein is a likely causal factor in the pathogenesis of PD [11–13]. Further supporting this contention, multiplications of the SNCA gene are also causal for PD, suggesting that simply increasing the amount of protein is sufficient to trigger the disease [14]. Human α-synuclein is a small, 140-residue, natively unfolded protein abundantly expressed in neurons, where it is localized at presynaptic terminals [15–18]. The physiological role of α-synuclein is still poorly understood. Possible key functions of the protein could be the modulation of synaptic vesicle recycling, DA storage and release at nerve terminals [19–24]. In addition, α-synuclein could modulate intracellular DA handling through interactions with proteins that regulate DA synthesis and uptake, such as tyrosine hydroxylase [25], the aromatic amino acid decarboxylase [26] and plasma membrane dopamine transporter [27–29].
To explore the possibility of a synergistic effect between oxidative cellular conditions induced by the oxidation of DA and α-synuclein in PD, we investigated how wild-type (WT) α-synuclein or its A30P pathogenic mutant influences DA toxicity in a dopaminergic human neuroblastoma BE(2)-M17 cell model stably over-expressing α-synuclein.
Methods
Human Neuroblastoma Cell Lines
The production of stable cell lines overexpressing α-synuclein from parental BE(2)-M17 human dopaminergic neuroblastoma cells has been described elsewhere [30]. Cells were cultured in high-glucose DMEM (Life Technologies) supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin and 500 μg/ml G418. α-Synuclein expression was evaluated by Western blot analysis, using monoclonal anti-α-synuclein antibodies (BD Biosciences), after cell lysis and protein separation on SDS-PAGE. Films were analyzed with ImageJ (Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/) for densitometry quantification and protein levels were normalized with respect to β-actin.
Lactate dehydrogenase (LDH) activity-based cytotoxicity assay
Cells were plated in 96 well plates at 1.5 × 104 cells per well in 100 μl of OptiMEM growth medium (Life Technologies) supplemented with DA or DOPA between 25 and 200 μM. Hydrogen peroxide generated by extracellular catecholamine oxidation was removed by adding 600 U/ml catalase (Sigma-Aldrich). After 24 hr of incubation, LDH activity measurements were performed using a commercially available assay (Roche Applied Science) according to the manufacturer's instructions. The ratio of LDH activity in the supernatant to the total LDH activity was taken as the percentage of cell death. For each experiment, eight wells per concentration were averaged and each experiment was performed in triplicate.
Fluorescence-activated cell sorter (FACS) analysis
After 24 hr of incubation in the presence of 200 μM DA or DOPA, 106 cells/ml were labeled with Hoechst 33342 and propidium iodide (Vybrant Apoptosis Assay Kit #5; Molecular Probes, Invitrogen), according to manufacturer's specifications. Cells were analyzed using a dual-laser FACSVantage SE flow cytometer (Becton Dickinson, Mountain View, CA, USA). Propidium iodide was excited using a 488-nm laser light and the emission captured with a bandpass filter set at 613 ± 20 nm. Hoechst 33342 was excited using a 351-nm ultraviolet laser light and its emission captured with a bandpass filter set at 450 ± 20 nm. Cell Quest Acquisition and Analysis software (Becton Dickinson) was used to acquire and quantify the fluorescence signal intensities and to graph the data as bivariate dot density plots.
Statistical analysis
Data were analyzed using GraphPad Prism 4 software and are expressed as the mean ± SEM. One-way ANOVA followed by Newman-Keuls's post hoc test was used to determine whether groups were statistically different. P values < 0.05 were considered significant.
Results
To study the relationship between α-synuclein and catecholamine toxicity, we used dopaminergic human neuroblastoma BE(2)-M17 cell lines stably transfected with WT or A30P mutant α-synuclein, as previously described [30]. The expression levels of WT and A30P α-synuclein were, respectively, about 8 and 6 times higher than cells transfected with the empty vector (Figure 1). Each cell line (empty vector control, WT or A30P α-synuclein) was treated with increasing concentrations (25, 50, 100 and 200 μM) of DA or DOPA, the cellular precursor of DA, and the levels of cellular toxicity were determined using an LDH assay. To limit the analysis only to the intracellular effects of DA or DOPA oxidation, we added 600 U/ml of catalase in the growth medium, as described for SH-SY5Y and PC12 cell types [31, 32]. As expected, the presence of DA or DOPA in the growth medium induced cellular damage in all cell lines. The level of cell death was approximately 15% in the presence of the highest amount of catecholamines used in these experiments (200 μM) (Figure 2). Cells overexpressing WT or A30P α-synuclein showed an increased vulnerability to DA or DOPA-mediated toxicity (Figure 2), with a level of cellular death up to ~ 25% compared to controls. The toxicity induced by DA or DOPA was similar.
Expression levels of α-synuclein in stably transfected M17 cell line. Immunoblot of α-synuclein in cell lines overexpressing WT or A30P α-synuclein using a monoclonal antibody against α-synuclein (upper panel). The same immunoblot was then stripped and reprobed with an antibody for β-actin (lower panel). Quantitation of α-synuclein is expressed as a ratio between the major α-synuclein band and β-actin. Cells transfected with vector alone show moderate expression of α-synuclein compared to cells overexpressing WT or A30P α-synuclein.
α-Synuclein exacerbates DA or DOPA toxicity in M17 cells. Overexpression of WT or A30P α-synuclein produces increased sensitivity to DA or DOPA. Cells were exposed to increasing concentration of catecholamines for 24 hr and cytotoxicity was subsequently estimated using the LDH assay (see Experimental Procedures). Cell lines included cells transfected with EV, WT or A30P α-synuclein. For each experiment, eight wells per concentration were used and each experiment was performed in triplicate. Values are the mean ± SEM. * p < 0.05
To confirm that overexpression of α-synuclein increases cell vulnerability to DA or DOPA, we performed analysis by fluorescence-activated cell sorting to discriminate between necrosis and apoptosis [33]. The analysis of 2 × 104 cells, obtained after 24 hr of incubation in the presence of 200 μM DA or DOPA, is shown in additional file 1. Figure 3 summarizes the results derived from 4 independent experiments. Addition of DA or DOPA to cells transfected with empty vector increased both apoptotic and necrotic events with a decrease in viability from 89% (control) to 62% (DA) and to 70% (DOPA). Although the cellular growth rate was similar both for empty vector and α-synuclein overexpressing cells, the presence of WT or A30P α-synuclein had cytotoxic effects with a decrease in viability from 89% to 66% (WT) and to 73% (A30P). The overexpression of WT or A30P α-synuclein variants also increased cellular susceptibility to DA or DOPA, especially for apoptotic cell death. The viability of cells overexpressing WT α-synuclein decreased from 89% to 32% (DA) and to 26% (DOPA), with and a change in viability of 57% and 63%, respectively. In the case of A30P overexpression, viability decreased from 89% to 39% (DA) and to 43% (DOPA), with a change in viability of 50% and 46%, respectively. Results obtained for each condition tested are summarized in Table 1. These data suggest that the cytotoxicity of α-synuclein and DA/DOPA are greater than the sum of the individual effects.
DA and DOPA augment α-synuclein mediated apoptosis and necrosis. Decreased cell viability (A) induced by DA or DOPA is accompanied by increased apoptosis (B) and necrosis (C). After 24 hr of incubation in the presence of 200 μM DA or DOPA, cells were labeled with Hoechst 33342 and propidium iodide and analyzed by FACS. For each experiment, 1.5-3 × 104 cells were analyzed. Values are the mean ± SEM (n = 4). * p < 0.05, ** p < 0.01, *** p < 0.001 relative to EV.
Discussion
Oxidative stress and α-synuclein are considered two potential factors involved in the pathogenesis of PD. An important source of oxidative species is cytoplasmic DA which may contribute to the preferential neuronal death of catecholaminergic neurons observed in PD [34]. Previous studies have shown a direct correlation between α-synuclein and oxidized DA. In cell-free systems, oxidized DA can covalently modify α-synuclein and promote the stabilization of toxic protofibrils [35]. Moreover, degradation of α-synuclein through the chaperone-mediated autophagic pathway is impaired when the protein is modified by oxidized DA [36].
In the present work, we explored the combined effects of α-synuclein and DA in promoting cellular toxicity. The influence of α-synuclein on DA toxicity has been previously been mainly studied in transiently transfected cells or under inducible expression conditions [37–41]. In this study, we used stable transfected dopaminergic human neuroblastoma BE(2)-M17 cell lines expressing WT or A30P α-synuclein [30]. We found that the presence of either WT or mutant α-synuclein increase susceptibility to oxidative conditions induced by DA in this model.
Increased susceptibility to oxidative conditions upon WT or mutant α-synuclein overexpression is in agreement with studies using transient overexpresssion or inducible cell lines [37–41], but are in contrast with one previous study carried out using WT α-synuclein stably transfected SH-SY5Y cells [32]. The observed discrepancy is unlikely to be due to the fact that different cell lines were used as SY5Y cells have previously been shown to be susceptible to toxic effects of α-synuclein in a dopamine-dependent manner [38] but may be due to the choice of cytotoxicity assays, as catecholamines have been shown to interfere with MTT assay [32]. Because of this potential concern about interference of catecholamines with colorimetric assays, we used FACS analysis to confirm results seen in LDH release assays. This method allowed us to confirm that DA exposure and α-synuclein expression have additive toxic effects.
One possible interpretation for two observations described in this study, the toxicity measured in cells over expressing both WT and A30P α-synuclein relative to the control and the synergistic toxicity of α-synuclein and DA is that α-synuclein may have an effect on DA metabolism. It has previously been reported that overexpression of WT or mutant α-synuclein in PC12 cells [42] or in differentiated MESC2.10 cells [22], increases cytosolic catecholamine concentration. In contrast, the levels of cytosolic DA in adrenal chromaffin cells from mice over expressing human α-synuclein or from knock-out mice are unaltered compared to wild type animals [42, 43], suggesting that the observation of the DA homeostasis effect by α-synuclein may be influenced by cell line. As we have not directly measured cytoplasmic DA concentrations in this cell line, further experiments are needed to define whether α-synuclein affects DA homeostasis in this context.
Another possible mechanism that may contribute to the combined toxic effects of α-synuclein and DA is the interaction between α-synuclein and the oxidation products of DA. In support of this hypothesis, we have shown in vitro that α-synuclein is able to incorporate radiolabeled DA [44]. The mechanism(s) underlying the potentially toxic effects of DA-modified α-synuclein are unclear. One leading hypothesis is that quinone adducts of α-synuclein may stabilize protofibrils [35]. Additionally, DA-modified α-synuclein blocks degradation of long-lived by chaperone mediated autophagy [36], an effect that can be seen with A30P α-synuclein without the incubation of the protein with DA [45]. Therefore, both impairment of the autophagy degradation and the formation of α-synuclein oligomers may both contribute to the observed cytotoxicity. Detergent-stable α-synuclein oligomers can isolated from DA-treated cells [41] and non-denaturing size fractionation also identifies non-covalent oligomers of α-synuclein in catecholaminergic cells [38]. However, whether these oligomers are specific to DA treatment, and therefore the same as the putative toxic species seen in vitro, is difficult to test as DA-modified α-synuclein has not yet been shown to be stable enough to be detected in vivo. Further work is therefore needed to establish whether the effects of DA and α-synuclein are mediated through direct interaction or independent pathways.
Conclusions
In summary, we have shown that DA exposure increases the toxicity of the PD-related protein α-synuclein in dopaminergic neuroblastoma cell lines BE(2)-M17. These results support the concept that DA, likely through formation of cytosolic quinones, has synergistic toxic effects with α-synuclein. While additional studies are required to define the precise mechanism(s) by which the two stressors act in a cellular context, our results further highlight that an interaction between cytosolic DA and α-synuclein may underlie the susceptibility of SN neurons in PD.
Abbreviations
- DA:
-
dopamine
- FACS:
-
fluorescence-activated cell sorter
- LDH:
-
lactate dehydrogenase
- MTT:
-
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]
- PD:
-
Parkinson's disease
- WT:
-
wild-type.
References
Hirsch E, Graybiel AM, Agid YA: Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988, 334 (6180): 345-348. 10.1038/334345a0.
Hirsch EC: Does oxidative stress participate in nerve cell death in Parkinson's disease?. Eur Neurol. 1993, 33 (Suppl 1): 52-59. 10.1159/000118538.
Jenner P: Oxidative mechanisms in nigral cell death in Parkinson's disease. Mov Disord. 1998, 13 (Suppl 1): 24-34.
Graham DG: Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978, 14 (4): 633-643.
Lotharius J, Brundin P: Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002, 3 (12): 932-942. 10.1038/nrn983.
Ito S, Kato T, Fujita K: Covalent binding of catechols to proteins through the sulphydryl group. Biochem Pharmacol. 1988, 37 (9): 1707-1710. 10.1016/0006-2952(88)90432-7.
Hastings TG, Lewis DA, Zigmond MJ: Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA. 1996, 93 (5): 1956-1961. 10.1073/pnas.93.5.1956.
LaVoie MJ, Hastings TG: Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999, 19 (4): 1484-1491.
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M: Alpha-synuclein in Lewy bodies. Nature. 1997, 388 (6645): 839-840. 10.1038/42166.
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M: alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998, 95 (11): 6469-6473. 10.1073/pnas.95.11.6469.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al.: Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997, 276 (5321): 2045-2047. 10.1126/science.276.5321.2045.
Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O: Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998, 18 (2): 106-108. 10.1038/ng0298-106.
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, et al.: The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004, 55 (2): 164-173. 10.1002/ana.10795.
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al.: alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003, 302 (5646): 841-10.1126/science.1090278.
Nakajo S, Shioda S, Nakai Y, Nakaya K: Localization of phosphoneuroprotein 14 (PNP 14) and its mRNA expression in rat brain determined by immunocytochemistry and in situ hybridization. Brain Res Mol Brain Res. 1994, 27 (1): 81-86. 10.1016/0169-328X(94)90187-2.
Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T: The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995, 14 (2): 467-475. 10.1016/0896-6273(95)90302-X.
Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT: NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996, 35 (43): 13709-13715. 10.1021/bi961799n.
Bisaglia M, Mammi S, Bubacco L: Structural insights on physiological functions and pathological effects of {alpha}-synuclein. Faseb J. 2008, 23 (2): 329-40. 10.1096/fj.08-119784.
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al.: Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000, 25 (1): 239-252. 10.1016/S0896-6273(00)80886-7.
Murphy DD, Rueter SM, Trojanowski JQ, Lee VM: Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000, 20 (9): 3214-3220.
Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, et al.: Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002, 22 (20): 8797-8807.
Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P: Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002, 277 (41): 38884-38894. 10.1074/jbc.M205518200.
Yavich L, Tanila H, Vepsalainen S, Jakala P: Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004, 24 (49): 11165-11170. 10.1523/JNEUROSCI.2559-04.2004.
Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, et al.: Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006, 26 (46): 11915-11922. 10.1523/JNEUROSCI.3821-06.2006.
Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ: A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002, 22 (8): 3090-3099.
Tehranian R, Montoya SE, Van Laar AD, Hastings TG, Perez RG: Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J Neurochem. 2006, 99 (4): 1188-1196. 10.1111/j.1471-4159.2006.04146.x.
Lee FJ, Liu F, Pristupa ZB, Niznik HB: Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. Faseb J. 2001, 15 (6): 916-926. 10.1096/fj.00-0334com.
Wersinger C, Prou D, Vernier P, Sidhu A: Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. Faseb J. 2003, 17 (14): 2151-2153.
Fountaine TM, Wade-Martins R: RNA interference-mediated knockdown of alpha-synuclein protects human dopaminergic neuroblastoma cells from MPP(+) toxicity and reduces dopamine transport. J Neurosci Res. 2007, 85 (2): 351-363. 10.1002/jnr.21125.
Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B: The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci. 2000, 20 (16): 6048-6054.
Blum D, Torch S, Nissou MF, Benabid AL, Verna JM: Extracellular toxicity of 6-hydroxydopamine on PC12 cells. Neurosci Lett. 2000, 283 (3): 193-196. 10.1016/S0304-3940(00)00948-4.
Colapinto M, Mila S, Giraudo S, Stefanazzi P, Molteni M, Rossetti C, Bergamasco B, Lopiano L, Fasano M: alpha-Synuclein protects SH-SY5Y cells from dopamine toxicity. Biochem Biophys Res Commun. 2006, 349 (4): 1294-1300. 10.1016/j.bbrc.2006.08.163.
Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM: Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005, 25 (2): 154-162. 10.1038/sj.jcbfm.9600003.
Chen L, Ding Y, Cagniard B, Van Laar AD, Mortimer A, Chi W, Hastings TG, Kang UJ, Zhuang X: Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci. 2008, 28 (2): 425-433. 10.1523/JNEUROSCI.3602-07.2008.
Conway KA, Rochet JC, Bieganski RM, Lansbury PT: Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001, 294 (5545): 1346-1349. 10.1126/science.1063522.
Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, et al.: Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008, 118 (2): 777-788.
Tabrizi SJ, Orth M, Wilkinson JM, Taanman JW, Warner TT, Cooper JM, Schapira AH: Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum Mol Genet. 2000, 9 (18): 2683-2689. 10.1093/hmg/9.18.2683.
Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA: Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002, 8 (6): 600-606. 10.1038/nm0602-600.
Moussa CE, Wersinger C, Tomita Y, Sidhu A: Differential cytotoxicity of human wild type and mutant alpha-synuclein in human neuroblastoma SH-SY5Y cells in the presence of dopamine. Biochemistry. 2004, 43 (18): 5539-5550. 10.1021/bi036114f.
Orth M, Tabrizi SJ, Tomlinson C, Messmer K, Korlipara LV, Schapira AH, Cooper JM: G209A mutant alpha synuclein expression specifically enhances dopamine induced oxidative damage. Neurochem Int. 2004, 45 (5): 669-676. 10.1016/j.neuint.2004.03.029.
Moussa CE, Mahmoodian F, Tomita Y, Sidhu A: Dopamine differentially induces aggregation of A53T mutant and wild type alpha-synuclein: insights into the protein chemistry of Parkinson's disease. Biochem Biophys Res Commun. 2008, 365 (4): 833-839. 10.1016/j.bbrc.2007.11.075.
Mosharov EV, Staal RG, Bove J, Prou D, Hananiya A, Markov D, Poulsen N, Larsen KE, Moore CM, Troyer MD, et al.: Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J Neurosci. 2006, 26 (36): 9304-9311. 10.1523/JNEUROSCI.0519-06.2006.
Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D: Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009, 62 (2): 218-229. 10.1016/j.neuron.2009.01.033.
Bisaglia M, Mammi S, Bubacco L: Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J Biol Chem. 2007, 282 (21): 15597-15605. 10.1074/jbc.M610893200.
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D: Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004, 305 (5688): 1292-1295. 10.1126/science.1101738.
Acknowledgements
This work was funded by grants from the Italian Ministry of Education, University and Research, MIUR-PRIN and MIUR-FIRB, (L.B.) and in part by the Intramural Research Program of the NIH, National Institute on Aging, project number 1 Z01 AG000953 (M.R.C.). M.B. was supported through an EMBO short term fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MB, EG, DWM, MRC and LB designed experiments, MB, EG, DM and DWM carried out experiments, MB, EG, MRC and LB wrote the paper. All authors read and approved the manuscript.
Marco Bisaglia, Elisa Greggio contributed equally to this work.
Electronic supplementary material
12868_2009_1600_MOESM1_ESM.PDF
Additional file 1:Figure S1. DA or DOPA induced toxicity analyzed by FACS. After 24 hrs of incubation in the presence of 200 mM DA or DOPA, cells were labeled with Hoechst 33342 and propidium iodide. 2 × 104 cells were analyzed for each condition tested. The staining pattern resulting from simultaneous use of these dyes makes it possible to distinguish viable, apoptotic and necrotic cell populations. Cells transfected with the empty vector (A1-3) show moderate increase of apoptosis and necrosis after the incubation with either catecholamine. Apoptosis strongly increases in cell lines overexpressing both WT (B1-3) and A30P (C1-3) asyn variants. Necrosis is also increased after exposure to DA or DOPA, but to a lesser extent. Ap: apoptotic cells; N: necrotic cells; V: viable cells. (PDF 404 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bisaglia, M., Greggio, E., Maric, D. et al. α-Synuclein overexpression increases dopamine toxicity in BE(2)-M17 cells. BMC Neurosci 11, 41 (2010). https://doi.org/10.1186/1471-2202-11-41
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2202-11-41