Abstract
Background
Soil bacteria collectively known as Rhizobium, characterized by their ability to establish beneficial symbiosis with legumes, share several common characteristics with pathogenic bacteria when infecting the host plant. Recently, it was demonstrated that a fadD mutant of Sinorhizobium meliloti is altered in the control of swarming, a type of co-ordinated movement previously associated with pathogenicity, and is also impaired in nodulation efficiency on alfalfa roots. In the phytopathogen Xanthomonas campestris, a fadD homolog (rpfB) forms part of a cluster of genes involved in the r egulation of p athogenicity f actors. In this work, we have investigated the role in swarming and symbiosis of SMc02161, a S. meliloti fadD-linked gene.
Results
The SMc02161 locus in S. meliloti shows similarities with members of the Major Facilitator Superfamily (MFS) of transporters. A S. meliloti null-mutant shows increased sensitivity to chloramphenicol. This indication led us to rename the locus tep1 for t ransmembrane e fflux p rotein. The lack of tep1 does not affect the appearance of swarming motility. Interestingly, nodule formation efficiency on alfalfa plants is improved in the tep1 mutant during the first days of the interaction though nod gene expression is lower than in the wild type strain. Curiously, a nodC mutation or the addition of N-acetyl glucosamine to the wild type strain lead to similar reductions in nod gene expression as in the tep1 mutant. Moreover, aminosugar precursors of Nod factors inhibit nodulation.
Conclusion
tep1 putatively encodes a transmembrane protein which can confer chloramphenicol resistance in S. meliloti by expelling the antibiotic outside the bacteria. The improved nodulation of alfalfa but reduced nod gene expression observed in the tep1 mutant suggests that Tep1 transports compounds which influence nodulation. In contrast to Bradyrhizobium japonicum, we show that in S. meliloti there is no feedback regulation of nodulation genes. Moreover, the Nod factor precursor, N-acetyl glucosamine reduces nod gene expression and nodulation efficiency when present at millimolar concentrations. A role for Tep1 in the efflux of Nod factor precursors could explain the phenotypes associated with tep1 inactivation.
Similar content being viewed by others
Background
The rhizobia-legume mutualistic symbiosis is characterized by the formation of root nodules in which the bacteria fix atmospheric nitrogen to generate nitrogen sources assimilable by the plant. Although the attack of phytopathogens on plants have a different outcome (i.e. disease), similar efficient strategies have been acquired by pathogenic and mutualistic bacteria to establish compatible associations with their host plants [1]. These include signals involved in cell-cell communication in bacterial populations but also in cross-kingdom communication with host plants [1].
Recently, swarming has been described in Rhizobiaceae [2, 3]. This type of co-ordinated movement was previously associated with the virulence of pathogens. In Sinorhizobium meliloti, swarming motility was associated with the activity of a long-chain fatty acyl-CoA ligase (FadD) which upon disruption affected nodulation efficiency on alfalfa roots. The authors hypothesized that a fatty acid derivative dependent on FadD activity may act as an intracellular signal controlling motility and symbiotic factors. In fact RpfB, a close homolog of FadD in Xanthomonas campestris [4], is implicated in the synthesis of cis-11-methyl-2-dodecenoic acid, a low-molecular-mass diffusible signal factor (DSF) involved in the regulation of pathogenicity factors [5]. In X. campestris the homolog of FadD is surrounded by genes which also participate in several ways in the regulation of important virulence determinants [6]. Therefore, a closer look was taken at the genes of S. meliloti in the vicinity of the fadD locus to determine their participation in symbiosis and/or swarming. Of the putative genes in the neighbourhood, the ORF SMc02161 located upstream from fadD and transcribed divergently from this gene, shows significant identity to permeases of the Major Facilitator Superfamily (MFS) [7]. The MFS class of permeases is the second largest family of membrane transporters found, after the ABC transporters. Members of this protein superfamily are typically single-polypeptide secondary carriers, comprising of 10–14 transmembrane α-helices which are able to transport small solutes such as sugars or toxins in response to chemiosmotic ion gradients [7, 8]. In this work, the role of SMc02161 in bacterial resistance to toxics, nod gene expression and nodulation of alfalfa is described.
Results and discussion
S. meliloti ORF Smc02161 potentially codes for a transmembrane transporter with striking homology to MFS permeases
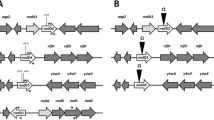
To analyze the region surrounding the fadD gene of S. meliloti, the available sequence of S. meliloti 1021 [9] was used. The analysis using BLAST [10] revealed an ORF (SMc02163) downstream of fadD with homology to phosphoglucose isomerase (pgi) while upstream a divergently coding ORF (SMc02161) showed high identity to permeases of the Major Facilitator Superfamily (MFS). In this study, we characterize specifically ORF SMc02161. Putatively, this ORF encodes for a 411 amino acid protein with 11 transmembrane motifs typical of inner membrane proteins. This protein has an ATP/GTP binding motif, an alanine rich region (PROSITE [11]) and has the multi-domain of the MFS that covers most of the protein (from amino acid 73 to 331). The product shows the highest identity (66%) with a putative MFS protein in Beijerinckia indica subsp. indica ATCC9039, and shares most identity to MFS related permeases, transmembrane proteins, sugar transporters and efflux proteins of bacteria belonging to the Rhizobiales and Burkholderiales orders. Unfortunately, the physiological functions of the closest SMc02161 homologs have not been experimentally tested. One of the few SMc02161 homologs with an experimentally assigned function is CmlR (P31141, 29% identity), a chloramphenicol resistance protein of Streptomyces lividans [12].
The S. meliloti SMc02161 mutant shows higher sensitivity to chloramphenicol
To functionally characterize SMc02161, we constructed the GR4T1 mutant in which the wild type locus was replaced with a mutated version. Considering the homology shown by SMc02161 with CmlR, we compared the sensitivity of the GR4T1 mutant with the wild type S. meliloti strain GR4 to different concentrations of antimicrobial compounds such as chloramphenicol, tetracycline, and salicylic acid. The influence of luteolin and plant root exudates on the growth of these strains was also compared. Only the presence of chloramphenicol reduced the growth of the mutant compared to that of the wild type strain (Figure 1). This suggests that the protein encoded by SMc02161 can function as an efflux pump, expelling the antibiotic chloramphenicol from the bacteria. As a result, we renamed ORF SMc02161 to tep1 for t ransmembrane e fflux p rotein. To rule out possible polar effects of the created mutation in tep1 on downstream genes, complementation of the chloramphenicol sensitivity of the mutant was attempted with a plasmid construct. However, the results were inconclusive due to severe growth problems. Nevertheless, tep1 and the downstream gene of unknown function, SMc02160, have different expression patterns [13] and close homologs of these genes in other rhizobia are not located adjacently thereby suggesting that each form independent transcriptional units.
Effect of different concentrations of chloramphenicol on the growth of S. meliloti GR4 and GR4T1. Growth of GR4 (open symbols) and GR4T1 (tep1 mutant) (closed symbols) was tested in TY broth with 0 μg/ml (triangles), 25 μg/ml (diamonds) or 50 μg/ml (squares) chloramphenicol. A representative example from 3 independent experiments is shown.
tep1 is not necessary for swarming motility in S. meliloti
To determine if the function of tep1 is related to swarming as is the fadD product encoded upstream, swarming assays were performed. The results in Figure 2 show that the fadD mutant QS77 shows conditional swarming on semi-solid minimal medium (MM) plates containing 0.7% agar, in contrast to the wild type strain GR4. Likewise, the tep1 mutant GR4T1 does not show swarming. Furthermore, the tep 1 knock out mutant in a fadD mutant background, QSTR1, shows swarming as the fadD simple mutant, QS77 (Figure 2). Therefore, it appears that any substance possibly transported by tep1 is not involved in swarming motility.
A tep1 mutation in S. meliloti improves nodule formation efficiency on alfalfa plants but shows reduced nod gene expression
To determine whether the activity of Tep1 is involved in symbiosis, the nodulation efficiency of the tep1 mutant was compared to the wild type strain. As shown in Figure 3, the mutant exhibits greater nodulation efficiency than the wild type strain during the first days of inoculation. Moreover, competition experiments in which alfalfa plants were co-inoculated with mixtures 1:1 of the wild type and mutant strains revealed that the lack of Tep1 confers a higher competitive ability to the bacterium (35% nodules occupied by the wild type strain versus 49% nodules occupied by the tep1 mutant). These results suggest that Tep1 transports some type of compound which affects the nodulation of the host plant.
To check whether the greater nodule formation efficiency shown by the tep1 mutant correlates with an altered nod gene expression, activity of the nodC: lacZ fusion [14] was studied in the presence and absence of the inducer luteolin in either the mutant or wild type strain (Table 1). In contrast to the constitutively expressed promoter of the npt gene (Pkm::lacZ) [15], the induction of the nodC::lacZ transcriptional fusion in response to luteolin was reduced 3 fold in the tep1 mutant background compared to the S. meliloti wild type strain. This suggests that the product transported by Tep1 influences the luteolin-induction of the nodC gene. It is unlikely that lower uptake and/or accumulation of the flavonoid by the tep1 mutant is responsible for the observed effect. It has been reported that in S. meliloti, luteolin mostly accumulates in the outer membrane and only a relatively small amount of the flavonoid is present in the cytoplasmic membrane, in or on which the interaction with the NodD protein takes place [16]. It has been proposed that the accumulation of the flavonoid in the outer membrane protects the bacteria against the inhibitory effect of luteolin on NADH oxidase activity. As previously mentioned, we tested the effect of different concentrations (0, 5, 50 and 100 μM) of luteolin on the growth of the wild type and tep1 mutant strains. Although in both strains growth was negatively affected with increasing concentrations of the flavonoid, no differences could be detected (data not shown), suggesting that the mutation does not lead to different cellular concentrations of the inducer. Another possible explanation for the reduction of nod gene expression in a tep1 mutant would be that the mutation results in the accumulation of a compound which inhibits or interferes with the activation of the nodC promoter.
A S. meliloti nodC mutant is affected in nod gene expression
The results described above suggest that Tep1 transports a compound that has an effect on the number of nodules developed by the plant. The same or maybe a different compound transported by Tep1 also affects the induction of the nodC gene in response to luteolin. It is known that the strong, constitutive expression of the nod genes results in reduced nodulation phenotypes on legumes [17, 18]. In Bradyrhizobium japonicum a feedback regulation of nod genes has been described [19]. The addition of chitin and lipochitin oligomers, or the expression of the β-glycosyl transferase NodC, reduces nod gene expression. These data together with the homology to sugar transporters shown by Tep1, prompted us to investigate whether the effects of the tep1 mutation could be due to alterations in the intra- and extracellular concentrations of Nod factors or Nod factor-related compounds. To our knowledge the existence or not of feedback regulation of nod genes in S. meliloti has not been investigated previously. Consequently, the expression of the nodC promoter was tested in GR4C5, a GR4-derivative nodC mutant, and compared with its activity in the tep1 mutant or in the wild type. The results (Table 2) show that in contrast to B. japonicum in which nod gene expression is elevated in a nodC mutant (1.6 fold) [19], nod gene expression is reduced 2.8 fold in the S. meliloti nodC mutant strain, reaching levels very similar to those shown by the tep1 mutant strain. This result indicates that in S. meliloti i) there is no feedback regulation of nod genes, and ii) a compound or compounds whose intracellular concentration is affected by the lack of NodC activity, interferes with nod gene induction. One of the most probable consequences of the lack of NodC activity is the accumulation of precursors of the Nod factor chitin backbone. To test whether changes in the concentration of these precursors could be responsible for the effects observed in the nodC and tep1 mutant, we decided to investigate how glucosamine and N-acetyl glucosamine influence both nod gene regulation in S. meliloti and nodulation of alfalfa plants.
Effect of glucosamine and N-acetyl glucosamine in nod gene expression in S. meliloti and on nodulation of alfalfa
To determine the possible role of core Nod factor precursors in nod gene regulation, studies were performed with glucosamine or N-acetyl glucosamine. The addition of glucosamine does not affect nod gene expression significantly in S. meliloti GR4 even when up to 50 mM glucosamine was added (data not shown). However, the addition of 5 mM N-acetly glucosamine reduces activity by more than 50% (Table 3). At higher concentrations (up to 50 mM) of N-acetly glucosamine the level of nod gene activity remains unchanged from that observed with 5 mM. Lower concentrations of the aminosugar (50 μM), only led to a slight reduction in nodC gene expression (data not shown). This indicates that in S. meliloti GR4, N-acetyl glucosamine can reduce nod gene expression.
To determine if core Nod factor precursors also affect nodulation, the nodulation efficiency of alfalfa inoculated with S. meliloti GR4 was determined in the presence of different concentrations of glucosamine or N-acetyl glucosamine. The results in Figure 4 show that at the lowest concentration (50 μM) whereas glucosamine has no effect, N-acetyl glucosamine improves nodulation. It is known that N-acetyl glucosamines function as adhesins in some bacteria and that core Nod factor plays a role in biofilm formation in S. meliloti, facts that could explain the positive effect of the aminosugar on nodulation [20]. Surprisingly, the addition of 5 mM of glucosamine or N-acetyl glucosamine to the plant mineral solution, abolished or severely affected nodulation, respectively. As far as we know this is the first time that it has been shown that glucosamine or N-acetyl glucosamine inhibits nodulation by S. meliloti. The reason why these sugars at millimolar concentrations inhibit nodulation in alfalfa is not known but worth further investigation. We speculate that at high concentrations these compounds bind to and collapse plant lectins and/or Nod factor receptors interfering with the recognition of symbiotic bacterial signals. On the other hand, it is noteworthy that the effects of high concentrations of these Nod factor precursors on nod gene expression and nodulation are consistent with the effects observed in the tep1 mutant. Therefore, as a first attempt to correlate the presence of these compounds with Tep1 activity, we decided to investigate the effect of these aminosugars on tep1 transcription.
Nodulation efficiency upon addition of different concentrations of Nod factor precursors. Just before inoculation with S. meliloti GR4, alfalfa plants were supplemented with 50 μM glucosamine (GA) (open squares), 5 mM glucosamine (filled squares), 50 μM N-acetyl glucosamine (NAGA) (open triangles), 5 mM N-acetyl glucosamine (closed triangles) or without the addition of Nod factor precursors (filled circles). A representative example from 3 independent experiments is shown.
Glucosamine and N-acetyl glucosamine activate tep1 transcription
Synthesis of transporters is often induced by the presence of their cognate substrates [21]. The expression of the tep1 gene was tested in S. meliloti GR4 harbouring pMPTR4 (tep1::lacZ transcriptional fusion) grown in different conditions. The results shown in Table 4 demonstrate that tep1 expression is higher in complex medium compared to defined minimal medium. Interestingly, the addition of glucosamine and N-acetyl glucosamine to the minimal medium increased transcription of tep1, suggesting that these aminosugars could be natural substrates of this putative transporter.
Considering all the results described here, we propose the following working hypothesis which is illustrated in Figure 5: Tep1 participates in the efflux of small compounds such as chloramphenicol and aminosugars which are core Nod factor precursors. Although these compounds have different structures, secondary multidrug (Mdr) transporters of the Major Facilitator Superfamily are known to be promiscuous in substrate recognition and transport [22]. In the tep1 mutant, chloramphenicol and Nod factor precursors accumulate inside the bacteria to concentrations which either hamper growth (chloramphenicol accumulation) or affect maximal nod gene expression (aminosugar accumulation). At the same time, the diminished efflux of aminosugars in the transport mutant leads to improved nodulation efficiency.
Conclusion
The results obtained in this work suggest that the tep1 gene encodes a transport protein belonging to the MFS family of permeases able to confer chloramphenicol resistance in S. meliloti by expelling the antibiotic outside the cell. A tep1-linked gene in S. meliloti, fadD, plays a role in swarming motility and in nodule formation efficiency on alfalfa plants. We have demonstrated that tep1 is not involved in swarming motility but like fadD affects the establishment of the S. meliloti-alfalfa symbiosis. A tep1 loss-of-function mutation leads to increased nodule formation efficiency but reduced nod gene expression suggesting that Tep1 transports compounds which influence different steps of the nodule formation process. Whether these effects are caused by the same or different compounds putatively transported by Tep1, still needs to be investigated. Curiously, nod gene expression is reduced in a S. meliloti nodC mutant with the same intensity as in the tep1 mutant. This has implications for nod gene regulation in S. meliloti as it rules out the existence of a feedback regulation as described for B. japonicum. On the other hand, it could indicate that Tep1 is involved in the transport of Nod factors or its precursors. Indeed, increased concentrations of the core Nod factor precursor N-acetyl glucosamine reduced nod gene expression. Moreover, both glucosamine and N-acetyl glucosamine inhibit nodulation at high concentrations. Therefore, this constitutes the first work which attributes a role for core Nod factor precursors as regulators for nodulation of the host plant by S. meliloti. Furthermore, the results suggest that the activity of Tep1 can modulate the nodule formation efficiency of the bacteria by controlling the transport of core Nod factor precursors.
Methods
Bacterial strains, plasmids, media and chemicals
Sinorhizobium meliloti QS77 [2] is a fadD::Tn5 insertion mutant derivative of wild-type GR4 [23]. The plasmid pRmM57 (nodC::lacZ fusion) [14] was used to test the expression of the nodC gene and pGD499 (npt::lacZ fusion) [15] to test the expression of the constitutive kanamycin resistance gene. The pMPTR4 plasmid is a pMP220 [24] derivative in which an Eco RI fragment of 0.6 kb harbouring the intergenic fadD-tep1 region was cloned to create a tep1::lacZ transcriptional fusion. The pGUS3 plasmid containing an nfeD::gusA fusion was used in competition assays [25]. Triparental bacterial matings were performed using pRK2013 as helper plasmid [26]. E. coli was grown routinely at 37°C in Luria-Bertani medium (LB) [27]. S. meliloti strains were grown at 30°C in TY complex medium [28] or in defined minimal medium (MM) [29]. Growth was determined regularly in a spectrophotometer measuring the absorbance at 600 nm. Glucosamine and N-acetyl glucosamine were obtained from Sigma-Aldrich.
Construction of a S. meliloti tep1 mutant
A null-mutant in ORF SMc02161 was obtained by allelic exchange. Firstly, a 3.6 kb Sac I fragment containing this ORF was subcloned from the fadD containing cosmid pRmersf442 [2] into pUC18 [30] to give pTrans1. To disrupt the ORF SMc02161 in pTrans1, a 2 kb Sma I fragment containing the streptomycin/spectinomycin resistance cassette from pHP45Ω [31] was inserted into a unique Eco RV site to give pTrans2. Next, the Sac I fragment containing the disrupted ORF was treated with T4 DNA polymerase (Roche Biochemicals) to make blunt ends and then cloned into the Sma I site of the suicide vector pK18mobsac [32] to give pTrans3. This vector was then used for allelic exchange by introducing it into the S. meliloti strains GR4, and the fadD mutant QS77 via triparental mating, and selecting putative mutants by streptomycin/spectinomycin resistance and sensitivity to sucrose. The resulting SMc02161 mutant GR4T1, and double fadD, SMc02161 mutant QSTR1 were confirmed by Southern hybridization with a specific probe.
Construction of a S. meliloti nodC mutant
To obtain a nodC mutant in S. meliloti, a fragment was amplified from the chromosomal DNA of S. meliloti GR4 by PCR using 5'-CAGATTC AAGGTCACGAAGTGGCTAAC-3' and 5'-ATAAGCTT GTGACAGCCAGTCGCTATTG-3' as forward and reverse primers respectively. An Eco RI-Pst I fragment of 1.5 kb derived from the PCR product and containing half of the nodB gene and most of the nodC gene was subcloned into pUC18 [30] to obtain pGRC8. To disrupt nodC, pGRC8 was digested with Sal I and treated with Klenow (Roche Biochemicals) to create blunt ends. Next, the 2 kb Sma I fragment containing the streptomycin/spectinomycin resistance cassette from pHP45Ω [31] was introduced to give pNC150. The 3.5 kb Eco RI-Pst I fragment from pNC150 containing the disrupted nodC gene was inserted into Eco RI-Pst I digested pK18mobsac [32] to give pNC200. This suicide vector was then used to obtain the S. meliloti nodC mutant GR4C5, which was confirmed by Southern hybridization.
Swarming behaviour assay
Swarming assays were performed as described previously [2]. Briefly, liquid cultures of S. meliloti, initiated from glycerol stocks, were grown at 30°C in TY broth with shaking to late logarithmic phase (optical density at 600 nm = 1–1.2). After incubation, cells were pelleted, washed twice in MM and resuspended in 0.1 volume of the latter medium. 2 μl drops of this suspension were deposited on the surface of plates containing MM with 0.7% agar and allowed to dry for 10 min. The plates were then inverted and incubated overnight (14–16 h) at 30°C and then scored for swarming motility.
Plant assays
Alfalfa (Medicago sativa L.) seeds were sterilized and germinated as described by Olivares et al. [33]. To test the infectivity of the rhizobial strains, 24 individual plants were inoculated with each rhizobial suspension (106 colony forming units (cfu)/plant). To prepare the inoculants, rhizobial strains were previously grown in liquid TY medium up to an OD600 of 0.5 and then diluted accordingly. When addition of Nod factor precursors (glucosamine and N-acetyl glucosamine) was required, these compounds were added at the same moment as the bacterial inoculum. After inoculation, the number of nodulated plants and the number of nodules per plant were recorded daily.
To determine competitive ability, 12 plants were inoculated with GR4 × GR4 (pGUS3) or GR4T1 × GR4 (pGUS3) mixtures at ratios 1:1. The plasmid pGUS3 contains the marker gene coding for β-glucuronidase (GUS). To determine nodule occupancy, roots were collected 12 days after inoculation, briefly washed with water, and incubated overnight in the dark at 37°C in 1 mM X-Gluc (5-bromo-chloro-3-indolyl-β-D-glucuronide, Apollo Scientific, UK) in 50 mM sodium-phosphate buffer (pH 7.5) with 1% SDS. Those nodules occupied by GR4 (pGUS3) stain blue whereby nodule occupancy could be determined by counting blue and white nodules.
Measurement of β-galactosidase activity
S. meliloti cells containing lacZ fusions were grown in liquid MM containing tetracycline to ensure plasmid maintenance. Bacteria were grown in liquid cultures overnight at 30°C to early logarithmic phase (OD600 of 0.2–0.4) in the presence or absence of 5 μM luteolin and different concentrations of glucosamine or N-acetyl glucosamine when required. Samples of 100 μl of the bacterial culture were taken and assayed for β-galactosidase activity by the SDS-chloroform method described by Miller [34].
References
Soto MJ, Sanjuán J, Olivares J: Rhizobia and plant-pathogenic bacteria: Common infection weapons. Microbiology. 2006, 152 (Pt 11): 3167-74. 10.1099/mic.0.29112-0.
Soto MJ, Fernández-Pascual M, Sanjuán J, Olivares J: A fadD mutant of Sinorhizobium meliloti shows multicellular swarming migration and is impaired in nodulation efficiency on alfalfa roots. Mol Microbiol. 2002, 43: 371-382. 10.1046/j.1365-2958.2002.02749.x.
Daniels R, Vanderleyden J, Michiels J: Quorum sensing and swarming migration in bacteria. FEMS Microbiol Rev. 2004, 28: 261-289. 10.1016/j.femsre.2003.09.004.
Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJG, Slater H, Dow JM, Williams P, Daniels M: A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol. 1997, 24: 555-566. 10.1046/j.1365-2958.1997.3721736.x.
Wang L-H, He Y, Gao Y, Wu JE, Dong Y-H, He C, Wang SX, Weng L-X, Xu J-L, Tay L, Fang RX, Zhang L-H: A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol. 2004, 51: 903-912. 10.1046/j.1365-2958.2003.03883.x.
Fouhy Y, Lucey JF, Ryan RP, Dow JM: Cell-cell signalling, cyclic di-GMP turnover and regulation of virulence in Xanthomonas campestris. Res Microbiol. 2006, 157: 899-904. 10.1016/j.resmic.2006.08.001.
Pao SS, Paulsen IT, Saier MH: Major facilitator superfamily. Microbiol Mol Biol Rev. 1998, 62: 1-34.
Saier MH, Beatty JT, Goffeau A, Harley KT, Heijne WHM, Huang S-C, Jack DL, Jähn PS, Lew K, Liu J, Pao SS, Paulsen IT, Tseng T-T, Virk PS: The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999, 1: 257-279.
Galibert F, Finan TM, Long SR, Pühler A, Abola P, Ampe F, Barloy-Hubler F, Barnet MJ, Becker A, Boistard P, Bothe G, Boutry M, Bowser L, Buhrmester J, Cadieu E, Capela D, Chain P, Cowie A, Davis RW, Dréano S, Federspiel NA, Fisher RF, Gloux S, Godrie T, Goffeau A, Holding B, Gouzy J, Gurjal M, Hernandez-Lucas I, Hong A, Guisar L, Hyman RW, Jones RW, Jones T, Kahn D, Kahn ML, Kalman S, Keating DH, Kiss E, Komp C, Lelaure V, Masuy D, Palm C, Peck MC, Pohl TM, Portetelle D, Purnelle B, Ramsperger U, Surzycki R, Thébault P, Vanderbol M, Vorholter F-J, Weidner S, Wells DH, Wong K, Yeh KC, Batut J: The composite genome of the legume symbiont Sinorhizobium meliloti. Science. 2001, 293: 668-672. 10.1126/science.1060966.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ: Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25: 3389-3402. 10.1093/nar/25.17.3389.
Hulo N, Bairoch A, Bulliard V, Cerutti L, Cuche B, De Castro E, Lachaize C, Langendijk-Genevaux PS, Sigrist CJA: The 20 years of PROSITE. Nucleic Acids Res. 2008, 36: D245-249. 10.1093/nar/gkm977.
Dittrich W, Betzler M, Schrempf H: An amplifiable and deletable chloramphenicol-resistance determinant of Streptomyces lividans 1326 encodes a putative transmembrane protein. Mol Microbiol. 1991, 5: 2789-2797. 10.1111/j.1365-2958.1991.tb01987.x.
Barnett MJ, Toman CJ, Fisher RF, Long SR: A dual-genome Symbiosis Chip for coordinate study of signal exchange and development in a prokaryote-host interaction. Proc Natl Acad Sci USA. 2004, 101: 16636-16641. 10.1073/pnas.0407269101.
Mulligan JT, Long SR: Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci USA. 1985, 82: 6609-6613. 10.1073/pnas.82.19.6609.
Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang XW, Finlay DR, Guiney D, Helinski DR: Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985, 13: 149-153. 10.1016/0147-619X(85)90068-X.
Hubac C, Ferran J, Trémolières A, Kondorosi A: Luteolin uptake by Rhizobium meliloti: evidence for several steps including an active extrusion process. Microbiology. 1994, 140: 2769-2774.
Knight CD, Rossen L, Robertson JG, Wells B, Downie JA: Nodulation inhibition by Rhizobium leguminosarum multicopy nodABC genes and analysis of early stages of plant infection. J Bacteriol. 1986, 166: 552-558.
Kondorosi E, Gyuris J, Schmidt J, John M, Duda E, Hoffmann B, Schell J, Kondorosi A: Positive and negative control of nod gene expression in Rhizobium meliloti is required for optimal nodulation. EMBO J. 1989, 8: 1331-1340.
Loh JT, Stacey G: Feedback regulation of the Bradyrhizobium japonicum nodulation genes. Mol Microbiol. 2001, 41: 1357-1364. 10.1046/j.1365-2958.2001.02603.x.
Fujishige NA, Lum MR, De Hoff PL, Whitelegge JP, Faull KF, Hirsch AM: Rhizobium common nod genes are required for biofilm formation. Mol Microbiol. 2008, 67: 504-515.
Neyfakh AA: Natural functions of bacterial multidrug transporters. Trends Microbiol. 1997, 5: 309-313. 10.1016/S0966-842X(97)01064-0.
Lewinson O, Adler J, Sigal N, Bibi E: Promiscuity in multidrug recognition and transport: the bacterial MFS Mdr transporters. Mol Microbiol. 2006, 61: 277-284. 10.1111/j.1365-2958.2006.05254.x.
Casadesús J, Olivares J: Rough and fine linkage mapping of the Rhizobium meliloti chromosome. Mol Gen Genet. 1979, 174: 203-209. 10.1007/BF00268356.
Spaink HP, Okker RJH, Wijffelman CA, Pees E, Lugtenberg BJJ: Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol Biol. 1987, 9: 27-39. 10.1007/BF00017984.
García-Rodríguez FM, Toro N: Sinorhizobium meliloti nfe (nodulation formation efficiency) genes exhibit temporal and spatial expression patterns similar to those of genes involved in symbiotic nitrogen fixation. Mol Plant-Microbe Interact. 2000, 13: 583-591. 10.1094/MPMI.2000.13.6.583.
Figurski DH, Helinski DR: Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979, 76: 1648-1652. 10.1073/pnas.76.4.1648.
Sambrook J, Fitsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. 1989, Cold Spring Harbor, Cold Spring Harbor Press
Beringer JE: R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974, 84: 188-198.
Robertsen BK, Aiman P, Darvill AG, McNeil M, Alberstein P: The structure of acidic extracellular polysaccharides secreted by Rhizobium leguminosarum and Rhizobium trifolii. Plant Physiol. 1981, 67: 389-400. 10.1104/pp.67.3.389.
Yanisch-Perron C, Vieira J, Messing J: Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985, 33: 103-119. 10.1016/0378-1119(85)90120-9.
Prentki P, Krisch HM: In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984, 29: 303-312. 10.1016/0378-1119(84)90059-3.
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A: Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994, 145: 69-73. 10.1016/0378-1119(94)90324-7.
Olivares J, Casadesus J, Bedmar EJ: Method for testing degree of infectivity of Rhizobium meliloti strains. Appl Environ Microbiol. 1980, 39: 967-970.
Miller J: Experiments in Molecular Genetics. 1972, Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press
Acknowledgements
This work was supported by grants BMC2001-0253 and BIO2007-62988 from the Spanish Ministerio de Ciencia y Tecnología to MJS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
PvD performed experiments and wrote the manuscript, JS and JO helped coordinate the study, participated in its design and in the writing of the manuscript. MJS performed experiments, coordinated and designed the study and participated in the writing of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
van Dillewijn, P., Sanjuán, J., Olivares, J. et al. The tep1 gene of Sinorhizobium meliloti coding for a putative transmembrane efflux protein and N-acetyl glucosamine affect nod gene expression and nodulation of alfalfa plants. BMC Microbiol 9, 17 (2009). https://doi.org/10.1186/1471-2180-9-17
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2180-9-17