Abstract
Background
Diagnosis and treatment of bloodstream infections (BSI) are often hampered by the delay in obtaining the final results of blood cultures. Rapid identification of pathogens involved in BSI is of great importance in order to improve survival of septic patients. Beacon-based fluorescent in situ hybridization (hemoFISH® Gram positive and hemoFISH® Gram negative test kits, miacom diagnostics GmbH Düsseldorf, Germany) accelerates the identification of most frequent bacterial pathogens of sepsis.
Results
In this study a total of 558 blood culture (377 blood culture positive and 181 negative) were tested with the hemoFISH® method and the results were evaluated in comparison with the traditional culture based methods. The overall sensitivity and specificity of the hemoFISH® tests were 94.16% and 100%, while, the PPV and NPV were 100 and 89.16%, respectively. As the hemoFISH® results were obtained within 45 mins, the time difference between the final results of the traditional culture method and the hemoFISH® assay was about two days.
Conclusions
Considering the good sensitivity and specificity of the hemoFISH® assays as well as the significant time saving in obtaining the final results (p-value 0.0001), the introduction of the system could be rialable in the microbiology laboratories, even alongside the traditional systems.
Similar content being viewed by others
Background
Sepsis is a serious clinical syndrome resulting from a host’s systemic inflammatory response to infection[1]. When severe, it is associated with high mortality, greater in patients with septic shock (40-70%), than in those with sepsis alone (25-30%). The syndrome is nowadays considered as a major international health care problem[2, 3]. Bloodstream infection is commonly associated with the development of sepsis and requires microbiological diagnosis usually performed by traditional culture, detection and identification of the causative pathogens of the systemic inflammatory response syndrome (SIRS)[3–5]. However, culture routinely takes several days before a positive result is available[6]. This gap between the initial clinical suspicion and the confirmation of infection by culture results could result in a poor clinical outcome of the septic patient[7, 8]. The long total turnaround time (TAT) which characterizes traditional culture methods encourages clinicians in empirical antimicrobial therapy as a safety-first strategy. The delay in appropriate antimicrobial therapy is associated with increased mortality[7, 8]. Therefore, there is an urgent need to introduce techniques, with a reduced TAT, which allow the clinicians to set therapeutic regimens in the earlier stages of sepsis. Molecular methods seem to be an appropriate choice, they are widely used in the diagnosis of BSIs, along side to the conventional methods. Molecular techniques are based on amplification of nucleic acids, species-specific hybridization, microarray technology and gene sequencing[9]. However, these techniques involve significantly increased cost and technical complexity, both of which are likely to hamper their adoption in the laboratory routine in the clinical setting. Fluorescent in-situ hybridization (FISH) technique is based on fluorescently labelled oligonucleotide probes complementarily binding to specific target sequences in the ribosomal RNA of bacteria, yeasts or other organisms. The most commonly used target for FISH in prokaryotes is 16S rRNA, as it contains both highly stable and variable regions. However, the 23S rRNA in prokaryotes and the 18S and 28S rRNA in eukaryotes, as well as mRNA have also been used as FISH targets[10]. Since the ribosomes are found in a large number in active multiplying bacteria, especially in the log phase of bacterial growth, the amplification step is not required. Target sequences will be presented naturally in the bacteria in a concentration high enough to enable visual detection of the specific fluorescent signal. FISH was first applied for detection of prokaryotes by environmental biologists for analysis of microbial communities. The method was soon introduced to medical microbiology and ever since used in various fields of diagnostics of human infectious diseases, with emphasis on situations when a speedy identification is crucial or the pathogen is difficult to culture: sepsis, meningitis, endocarditis, respiratory tract infections, especially those of cystic fibrosis patients, screening for intrapartum Streptococcus agalactiae carriage, diagnosis of zoonotic infections such as those caused by Brucella and Francisella[11–17]. Miacom® diagnostics GmbH has combined the classical FISH technology with the usage of fluorescently labelled DNA-molecular beacons as probes, making it an easy procedure known as the beacon-based FISH (bbFISH®) technology[18]. It is now possible, for the first time, to use specific probes against a wide variety of clinically relevant bacteria working directly on blood culture. The probes enter the cells, hybridize to their specific targets, making the cells visible using a fluorescence microscope. In order to assess the possible benefits of the introduction of such technology into the laboratory routine, we evaluated in the present study the performance of the bbFISH® (hemoFISH® Gram positive and hemoFISH® Gram negative) in comparison to the conventional culture of bacteria from positive blood culture vials in febrile patients. The study was conducted independently in two Italian centers: Polyclinic of Tor Vergata in Rome and Polyclinic Ospedale G.B. Rossi in Verona. We have also examined the hemoFISH® test and the conventional identification assay’s total turnaround time (TAT) performance.
Results
Blood culture results
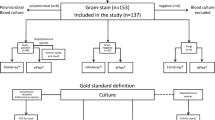
In this study 558 consecutive samples were tested: 377 positive and 181 negative. The Hospital of Verona processed 243 blood culture (88 negative and 155 positive) while the Hospital in Rome analysed a total of 315 blood cultures (93 negative and 222 positive). 393 were the isolates (239 Gram-positive and 153 Gram-negative and one yeast) identified by conventional system (Vitek 2 System), including those from 16 mixed blood cultures (those which contain two isolates).
hemoFISH® performances
The test works equally well in both centers being the overall performances substantially similar. The hemoFISH® test correctly identified 364/393 isolates, showing an overall agreement of 92.6% with the culture method. If the performances were considered referred to the specimens (not the isolates) 355/377 positive specimens were identified by hemoFISH® (94.16%). The sensitivity, the specificity the PPV and NPV were 94.16, 100, 100 and 89.16, respectively.
The individual Kit performances were as follows:
The hemoFISH®Gram negative panel correctly identified 143/153 (93.6%) of the Gram-negative for which a specific probe was present in the Kit (59 Escherichia coli, 26 Klebsiella.pneumoniae, 4 Proteus mirabilis, 2 Proteus vulgaris, 4 Salmonella spp, 5 Serratia marcescens, 14 Pseudomonas aeruginosa, 12 Acinetobacter spp, 4 Stenotrophomonas maltophilia and 1 Haemophilus influenzae) (Table 1). Two K.pneumoniae and one P.mirabilis were identified only at the genus level as Enterobacteriaceae s pp (Table 1). Other Gram-negatives, for which there were no specific probes on the panel, yet belonging to the Enterobacteriaceae group, such as Klebsiella oxytoca, Enterobacter aerogenes and Enterobacter cloacae, were correctly identified as Enterobacteriaceae spp. One Pasteurella multocida (for which no specific probe was present on the hemoFISH Gram negative panel) was misidentified as Enterobacteriaceae spp (Table 1).
Eleven Acinetobacter baumannii and one Acinetobacter lwoflii were identified as Acinetobacter spp. A misidentification was assigned to Aeromonas veronii, which was improperly identified as Enterobacteriaceae spp (Table 1).
Five specimens (2 Bacteroides fragilis, 2 Burkholderia cepacia and 1 Rhizobiom radiobacter) were identified by the traditional method, but they only gave a signal with the positive control using the miacom test (Table 1). No probes for these species are included in the test panel so these results are conform with miacom’s claims.
The hemoFISH®Gram positive panel correctly identified 221/239 Gram-positive isolates (92.5%) (Table 1). Particularly, a total of 130 coagulase negative staphylococci were identified as Staphylococcus spp (the staphylococci identification obtained using Vitek 2 system were: 70 Staphylococcus epidermidis, 23 Staphylococcus hominis, 22 Staphylococcus haemolyticus, 4 Staphylococcus warneri, 8 Staphylococcus capitis, 1 Staphylococcus auricolaris, 1 Staphylococcus saccharolyticus, 1 Staphylococcus saprophyticus) while one sample positive for Staphylococcus cohnii was not identified. 16 samples, positive per Staphylococcus aureus, were correctly identified (Table 1).
Looking at the streptococci, 30/32 samples were correctly identified as Streptococcus spp (19 Streptococcus mitis, 1 Streptococcus bovis, 2 Streptococcus oralis, 4 Streptococcus gallolyticus and 1 Streptococcus gordoni), while among 5 specimens positive for Streptococcus pneumoniae, 3 were identified as Streptococcus spp (albeit no signal was evidenced with specific probe in S.pneumonie well) and 2 were not identified (only the signal with the eubacterial probe was recorded) (Table 1).
Enterococci were detected in a total of 41/44 specimens, two Enterococcus raffinosus were not identified and one Enterococcus gallinarum was misidentified by hemoFISH as Enterococcus faecium (Vitek 2 system identified: 19 Enterococcus faecalis, 22 E.faecium, 2 E. raffinosus and one E.gallinarum) (Table 1).
Eight specimens resulted positive for Microcococcus spp, namely 4 Micrococcus luteus and 4 Micrococcus lylae, of these, two (those positive for M.luteus) gave a positive fluorescent signal on the Staphylococcus spp well (recorded as misidentifications), the remaining 6 were not identified (Table 1).
Among the Gram-positive bacilli: two Corynebaterium spp and two Bacillus spp were identified in four different specimens by Vitek 2 (one Corynebacterium amycolatum, one Corynebacterium spp, one Bacillus cereus and one Bacillus spp). Identification by hemoFISH® failed for all of them (neither the signal for the positive control was detected). While the hemoFISH® correctly identified three Clostridium perfringens (Table 1).
One sample containing Candida did not yield a specific signal with any of the hemoFISH® probes but was clearly visible via auto fluorescent signals on all fields.
A total of 29 specimens were not identified (21 strains) or misidentified (8 strains) by the hemoFISH® test (29/393; 7.4%). The global performances recorded with the hemoFISH panels, in comparison with those identified by Vitek 2 system, are summarized in the Table 1.
The overall concordance between traditional culture and hemoFISH® for the negative samples was 100%, no fluorescent specific signal was recorded on 181 negative blood cultures processed.
The average TAT from the hemoFISH® system and the one obtained using traditional culture methods as well as the Δ (means the difference in time to achieve a final result) between the two system in both hospitals are consistently different as reported in Table 2. The results were available sooner using the hemoFISH® assay (mean value 5.2) compared to the conventional assay (mean value 43.65) expressed also by a p value of 0.001 (Table 2). The Verona data was obtained calculating the work-flow on 5 days open laboratory. From all blood cultures, the growth of microorganisms was obtained after an incubation of 18-24 h and identification to the family, genus or species level was achieved after another day, except for 16 samples, which contained more than a microorganism and subcultures were required with a delay of one more day. For this reason, the average TAT obtained using traditional culture methods is 43.65 h. hemoFISH® was performed in the same blood cultures, with an average TAT of 5.2 h. The Δ TAT between the two systems is 38.45 h, with a hemoFISH® time savings of two days (compared with conventional laboratory identification). hemoFISH® provides a same-day identification of the majority of microorganisms and the turnaround time is considerably lower than microbiological culture.
Discussion
BSI, is a serious and life-threatening condition, rapid diagnosis of BSI and identification of the pathogenic microorganisms are needed to improve the patient outcome[5, 8]. Blood culture is still currently considered the “gold standard” in BSI diagnosis[8]. However, culture assays require a long time to achieve a final result[19]. On the contrary examination of positive blood cultures with specific molecular techniques based on the microscopic morphology of the detected microorganisms enables rapid and specific determination of sepsis pathogens, enhancing early adequate therapy and improving prognosis of the patients[18–20]. A timely reporting of results of a Gram stain of blood cultures to the physician already showed a decrease in mortality[20]. If the communication of a Gram stain result is combined with a presumptive diagnosis of the pathogens involved in BSI the clinician could more appropriately target the therapy. To achieve this we find plausible to put the FISH methodologies into a routine use in our laboratories. The results of our work, aimed at the evaluation of the bbFISH technology in comparison with the traditional culture techniques, confirm the diagnostic usefulness of this system. This test presents not only an excellent sensitivity and specificity, but also a greatly reduced TAT. Laboratory TAT is a reliable performance indicator, which measures the laboratory’s efficiency in producing its results[21–23]. The TAT is commonly defined as the time elapsed between ordering a laboratory test and the reporting of the results. In this study, the TAT was specified as the time lapse from when the blood culture flagged positive in the BacT/ALERT 3D® system to when the final verification of the result was reported (either by the identification of the microorganisms using the hemoFISH® assay or the conventional culture assay), this just to underline the advantage in using rapid detection assays compared to traditional systems, but avoiding any other interfering parameters not strictly imputable to the laboratory work flow. Our findings also underline how different workflows in microbiology laboratory are and how these can affect the TAT. The delay caused in TAT is primarily due to the pre- and post-analytical phases. The most common reasons for this delay were found to be the order processing time, the laboratory excessive queue and the instruments times[22, 23]. A huge impact on TAT, particularly in analytical phase, was also due to the choice of laboratory procedures. Recently, many publications have underlined the usefulness of “rapid methods” either PCR-based or those using the newly introduced technology of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry MALDI-TOF (MS) in diagnosing blood stream infections[24–26]. Moreover, delays in the reporting the tests results were generally linked to the practice of interrupting the workflow over the weekend and during the holidays. Our study, in fact, showed that the main impact in reducing the TAT is indeed in the laboratory itself, where these interruptions were longer (Verona Hospital than the Rome Hospital). No less important is the presence of skilled personal in the laboratory and their impact on reporting time, as demonstrated by the TAT recorded in the hospital of Rome. This laboratory realistically reported the timing by performing hemoFISH® tests even with those specimens processed in delay, due to the lack of personnel in the laboratory (i.e. on Saturday afternoons and Sundays). This fact has had a heavy impact on the observed average TAT (8.9 vs 1.5). Faster TAT is universally seen as desirable, as the more timely and rapidly a testing is performed, the more efficient and effective will be the treatment[22, 27, 28]. This in turn can save not only time and money for the patient and the hospital, but more importantly it can save lives, reduce patient morbidity and help reducing the further increase of antibiotic resistance as well as a long stay at the hospital[19, 20]. Thus system such as bbFISH technology which is easy to use, with a reduced TAT and which does not require sophisticated and expensive equipments (as MALDI TOF needs instead to) could be placed in a profitable manner, especially in small laboratories as well as in the hub laboratories which receive samples from other hospitals (in the latter in order to recover the time lost in the specimens transportation). The only improvement we can auspicate is the implementation of the bbFISH panels by the adding of other specific probes on the slide (such as those for identifications of Corynebacterium, Gram-negative anaerobe and Microcococcus spp) in order to reduce the number of pathogens not identified by the system. Considering our 29 strains (ten Gram-negative and 18 Gram-positive bacteria and one yeast) for which the bbFISH failed the identification, we have observed that most of them were not identified because of the absence of a specific probe (this is true for Gram-positive: Bacillus spp., Corynebacterium spp. and Micrococcus spp., but also for Bacteroides spp, Rhizobium spp, A.veronii and P.multocida among the Gram-negative). The remaining (two S.pneumoniae, one S.cohnii, two E.raffinosus two K.pneumoniae and one P.mirabilis) could be easily explained either by the sensitivity of the system or by a possible misinterpretation of the reader and finally we cannot exclude the possibility of a technical error in preparing the slide. In order to determine if the probes did really miss the corresponding bacteria or if the procedure failed for some other reason, bacteria would have to be retested from pure culture. Unfortunately at the time of data analysis, when these discrepancies were evidencied, we could have not done it anymore because the isolates had been discarded.
Conclusions
The bbFISH technology is a new successful molecular assay, supplementing traditional approaches, speeding up the diagnosis of bloodstream infections and identifying the majority of most important sepsis pathogens. This assay has the potential to provide timely and cost-effective information on infection status, thus allowing clinicians to make more informed decisions on appropriate antibiotic therapy at an earlier stage than is possible with culture-based approaches. Complications can be avoided and hospital stay may so be reduced. The bbFISH technology can be a good choice, in order to reduce the analytical phase of TAT, in those laboratories in which, due to the high cost, technologies such as PCR and MALDI TOF cannot be introduced.
Methods
Specimens
A total of 558 blood cultures from different patients were included in this study. 299 were the samples processed and recorded by the Microbiology laboratory of Foundation Polyclinic of Tor Vergata and 259 by the Microbiology laboratory of Policlinico G.B.Rossi- Azienda Ospedaliera Universitaria Integrata-Verona. Both are teaching hospitals in Rome and Verona, Italy, respectively. The blood cultures included in the study were those consecutively collected and delivered in each hospital in a three months period. The blood specimens were collected in BacT/ALERT FN Plus and BacT/ALERT FA Plus vials, which were incubated in the BacT/ALERT® 3D system, (bioMérieux; Marcy l’Etoile, France).
Hospital workflow
The Verona hospital microbiology laboratory is a 5 days open laboratory, meaning that laboratory workflow is fully covered by a microbiologist from 8.00 a.m. to 3.00 p.m., Monday to Friday, but it is off duty on Saturday afternoon and on Sunday. While, the Rome laboratory has a working time divided on 7 days, from 7.30 am to 8.00 pm, but the microbiologist, on Saturday afternoon and on Sunday, is not present.
Traditional routine methods on positive blood culture vials
The Bact/Alert 3D® (bioMerieux) system was used for blood culturing. A minimum of two culture vials per patient, one aerobic and one anaerobic, were filled directly with blood according to the manufacturer instructions. Growth of microorganisms was detected by the instrument. Cultures were continued for 5 days. When blood culture vials flagged positive, some microliters from the vial were aliquoted aseptically for light microscopy. Gram stain was performed using Previ Color (bioMérieux) according to the instructions of the manufacturer and for culturing on a variety of agar plates for different growth requirements (Agar Chocolate, Columbia supplemented with 5% of sheep blood and Schaedler agar incubated under aerobic, micro-aerobic and anaerobic condition respectively) and further identified using the VITEK 2® system (bioMerieux,). The cultivation and identification was performed by the same trained individuals.
Beacon-based fluorescent in-situ hybridization (hemoFISH®)
Miacom’s molecular probes consist of a DNA sequence folded into a hairpin-like structure that is linked to a fluorophore on the 5′ end and to a quencher on the 3′ end. Such probes are also referred to as molecular beacons. The DNA sequence is complementary to a rRNA counterpart that is unique to the family, genus or species level of a certain organism. Because each bacterial cell includes more than 10,000 copies of rRNA, no amplification step is necessary[29]. Each rRNA copy with a bound beacon contributes to a fluorescent signal and the cell can be detected as a shining object under a fluorescence microscope. In addition to the fluorescent signal the cells morphology can be examined to confirm the result. Miacom’s hemoFISH® Gram positive and hemoFISH® Gram negative panels were used to perform the assay. Tests were run as soon as possible after the blood culture vial turned positive and not later than 24 hours. On positive blood cultures, dependent on the Gram strain result, either a Gram negative (hemoFISH® Gram negative panel) or a Gram positive panel (hemoFISH® Gram positive panel) was used. Negative blood cultures were processed using both kits (the test kits used for these studies were kindly supplied by miacom diagnostics GmbH, Düsseldorf, Germany). Briefly, an aliquot from a positive/negative blood culture vial (which was either the aerobic or the anaerobic vial but the first which turned positive) was diluted in Clinical Sample Buffer and applied, dried and fixed onto an 8 field glass slides placed on a hotplate pre-warmed to 52+/-1°C. Permeabilization of bacteria including treatment with enzymatic Lysis Solution at 52+/-1°C followed by an incubation in ethanol. Hybridization with DNA-molecular beacon probes was carried out in a hotplate hybridization chamber at 52+/-1°C followed by an incubation in a Stop Solution bath for 1 minute. This step ensures that all unbound beacons are pushed back into the closed conformation. Slides were dried, covered with mounting medium and evaluated under a fluorescence microscope. Two filter sets are required. One detects the probes labeled with ATTO550 (red channel, absorption max 554 nm/emission max 576 nm), the other one those labeled with FAM (green channel, absorption max 494 nm/emission max 520 nm). Total turn-around time of the hemoFISH® assay was approximately 45 minutes (15 min of hands-on time plus the time required for microscopic observation). The list of fluorescently labeled probes used for the strain identification is the following: Enterobacteriaceae spp., E.coli, K.pneumoniae, S.marcescens, P.mirabilis, P.vulgaris, Salmonella spp., P.aeruginosa, Acinetobacter spp., S.maltophilia, H.influenzae (for the hemoFISH G(-) Panel) and Staphylococcus spp., S.aureus, Streptococcus spp., S.pneumoniae, S.pyogenes, S.agalactiae, C.perfringens, E.faecium, E.faecalis (for the hemoFISH G(+) Panel).
The first field of the slide serves as an intrinsic control of the procedure. It contains a probe that detects most Eubacteria, giving a positive signal only in the red channel. When turning to the green channel, no fluorescence should be visible. On the remaining fields, there might be pairs of probes, labeled either with FAM or ATTO 550, giving either a red or a green fluorescent signal when the specific target is encountered. If a specific target is not encountered, the unbound probes are pushed back into the initial closed conformation and no fluorescent signal is generated. Due to the use of molecular beacons, the washing step, known to be a critical and error-prone step during the FISH procedure, can be omitted.
Statistical analysis
For database processing, data from BacT/ALERT 3D® and VITEK 2® system were downloaded as text files into Microsoft Excel with subsequent transfer of it into a Microsoft Access database for analysis. Final tabulation of TAT was performed using Access with report generation, including graphs, created in Excel. The comparisons between the two techniques are expressed as proportions. Standard descriptive statistical methods (such as mean) were calculated, and a comparison of the proportions was performed using a two-tailed Chi squared test. Differences were considered to be significant for a p-value ≤ 0.05[30].
Ethical approval
Ethics committee of both hospitals gave approval for publication of this study (Verona Hospital “Protocol PTP49_Italy (Pr numB1207) signed by the secretariat (Dr Anna Fratucello) on benhalf of President Professor Roberto Leone; Roma Hospital approved on 22nd December 2011, signed by the President Professor Maria Grazia Marciani.
References
Cohen J: The immunopathogenesis of sepsis. Nat. 2002, 420: 885-891. 10.1038/nature01326.
Hotchkiss RS, Karl I: The pathophysiology and treatment of sepsis. New Engl J Med. 2003, 348: 138-150. 10.1056/NEJMra021333.
Lever A, MacKenzie I: Sepsis: definition, epidemiology and diagnosis. BMJ. 2007, 335: 879-883. 10.1136/bmj.39346.495880.AE.
Calandra T, Cohen J: The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005, 33: 1538-1548. 10.1097/01.CCM.0000168253.91200.83.
Dellinger R, Levy M, Carlet J, Bion J, Parker M, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL: Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008, 34: 17-60.
Health Protection Agency: Investigation of blood cultures (for organisms other than Mycobacterium species). National Standard Method.London, Standards Unit. 2012, B37: 6.1-Available online at http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317132857861
Kollef M, Sherman G, Ward S, Fraser V: Inadequate antimicrobial treatment of infections: a risk factor of hospital mortality among critically ill patients. Chest. 1999, 115: 462-474. 10.1378/chest.115.2.462.
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M: Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006, 34: 1589-1596. 10.1097/01.CCM.0000217961.75225.E9.
Stefani S: Diagnostic techniques in bloodstream infections: where are we going?. Int Antimicrob Agents. 2009, 34: 9-12.
Moter A, Göbel UB: Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microb Meth. 2000, 41: 85-112. 10.1016/S0167-7012(00)00152-4.
Mallmann C, Siemoneit S, Schmiedel D, Petrich A, Gescher DM, Halle E, Musci M, Hetzer R, Göbel UB, Moter A: Fluorescence in situ hybridization to improve the diagnosis of endocarditis: a pilot study. Clin Microbiol Infect. 2010, 16: 767-773. 10.1111/j.1469-0691.2009.02936.x.
Poppert S, Essig A, Stoehr B, Steingruber A, Wirths B, Juretschko S, Reischl U, Wellinghausen N: Rapid diagnosis of bacterial meningitis by real-time PCR and fluorescence in situ hybridization. J Clin Microbiol. 2005, 43: 3390-3397. 10.1128/JCM.43.7.3390-3397.2005.
Splettstoesser WD, Seibold E, Zeman E, Trebesius K, Podbielski A: Rapid differentiation of Francisella species and subspecies by fluorescent in situ hybridization targeting the 23S rRNA. BMC Microbiol. 2010, 10: 72-10.1186/1471-2180-10-72.
Wellinghausen N, Nöckler K, Sigger A, Bartel M, Essig A, Poppert S: Rapid detection of Brucella spp. in blood cultures by fluorescence in situ hybridization. J Clin Microbiol. 2006, 44: 1828-1830. 10.1128/JCM.44.5.1828-1830.2006.
Wellinghausen N, Köthe J, Wirths B, Sigge A, Poppert S: Superiority of molecular techniques for identification of Gram-negative, oxidase-positive rods, including morphologically non typical Pseudomonas aeruginosa, from patients with cystic fibrosis. J Clin Microbiol. 2005, 43: 4070-4075. 10.1128/JCM.43.8.4070-4075.2005.
Goddard KA, Townsend R, Ridgway E: Rapid diagnosis of intrapartum group B streptococcal carriage by fluorescent in situ hybridization. J Clin Pathol. 2007, 60: 842-843.
Amman RI, Krumholz L, Stahl DA: Fluorescent oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990, 172: 762-770.
Tyagi S, Kramer FR: Molecular Beacons: Probes that fluoresce upon hybridization. Nat Biotechnol. 1996, 14: 303-308. 10.1038/nbt0396-303.
Beckmann SE, Diekema DJ, Chapin KC, Doern GV: Effects of rapid detection of bloodstream infections on length of hospitalization and hospital charges. J Clin Microbiol. 2003, 41: 3119-3125. 10.1128/JCM.41.7.3119-3125.2003.
Barenfanger J, Graham DR, Kolluri L, Sangwan G, Lawhorn J, Drake CA, Verhulst SJ, Peterson R, Moja LB, Ertmoed MM, Moja AB, Shevlin DW, Vautrain R, Callahan CD: Decreased mortality associated with prompt Gram staining of blood cultures. Am J Clin Pathol. 2008, 130: 870-6. 10.1309/AJCPVMDQU2ZJDPBL.
Hawkins RC: Laboratory turnaround time. Clin Biochem Rev. 2007, 28: 179-194.
Steindel SJ, Howanitz PJ: Physician satisfaction and emergency department laboratory test turnaround time. Arch Pathol Lab Med. 2001, 125: 863-71.
Hilborne LH, Oye RK, McArdle JE, Repinski JA, Rodgerson DO: Evaluation of stat and routine turnaround times as a component of laboratory quality. Am J Clin Pathol. 1989, 91: 331-335.
Dark P, Dunn G, Chadwick P, Young D, Bentley A, Carlson G, Warhurst G: The clinical diagnostic accuracy of rapid detection of healthcare-associated bloodstream infection in intensive care using multi patho genereal-time PCR technology. BMJ Open. 2011, 1: e000181-
Kaleta EJ, Clark AE, Cherkaoui A, Wysocki VH, Ingram EL, Schrenzel J, Wolk DM: Comparative analysis of PCR-electrospray ionization/mass spectrometry (MS) and MALDI-TOF/MS for the identification of bacteria and yeast from positive blood-culture bottles. Clin Chem. 2011, 57: 1057-67. 10.1373/clinchem.2011.161968.
Christner M, Rohde H, Wolters M, Sobottka I, Wegscheider K, Aepfelbacher M: Rapid identification of bacteria from positive blood culture bottles by use of matrix-assisted laser desorption-ionization time of flightmass spectrometry fingerprinting. J Clin Microbiol. 2010, 48: 1584-91. 10.1128/JCM.01831-09.
Valenstein P: Laboratory turnaround time. Am J Clin Pathol. 1996, 105: 676-688.
Valenstein PN, Emancipator K: Sensitivity, specificity, and reproducibility of four measures of laboratory turnaround time. Am J Clin Pathol. 1989, 92: 613-618.
Travers A: The regulation of promoter selectivity in eubacteria. DNA-Protein Interactions. 1993, New York: Chapman and Hall, 109-129.http://link.springer.com/chapter/10.1007%2F978-94-011-1480-6_5,
Fontana C, Favaro M, Minelli S, Bossa MC, Altieri A, Favalli C: A novel culturing system for fluid samples. Med Sci Monit. 2009, 15: BR55-BR60.
Acknowledgements
We are grateful Dr. Claudio Puliti for his help in performing part of the experiments conduct in Rome. The test kits used for these studies were kindly supplied by miacom diagnostics GmbH, Düsseldorf, Germany. We also gratefully acknowledge ADA (the Italian distributor of bbFISH by miacom) for providing the instrumentation and/or some of the reagents used in the evaluation
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
All authors declare no financial or personal relationships with other people or organizations that could inappropriately have influenced (bias) their work.All coauthors have no specific conflict of interests.
Authors’ contributions
CF and GLC, contributed to the conception of the study, in data analysis and are also involved in drafting the manuscript. CS, MP contributed in acquisition and interpretation of data. All authors approved the final version of the manuscript.
Giuliana Lo Cascio and Carla Fontana contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sakarikou, C., Parisato, M., Cascio, G.L. et al. Beacon-based (bbFISH®) technology for rapid pathogens identification in blood cultures. BMC Microbiol 14, 99 (2014). https://doi.org/10.1186/1471-2180-14-99
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2180-14-99