Abstract
Background
Helicobacter pylori infection is one of the most common infections worldwide and is associated with gastric cancer and peptic ulcer. Bacterial virulence factors such as CagA have been shown to increase the risk of both diseases. Studies have suggested a causal role for CagA EPIYA polymorphisms in gastric carcinogenesis, and it has been shown to be geographically diverse. We studied associations between H. pylori CagA EPIYA patterns and gastric cancer and duodenal ulcer, in an ethnically admixed Western population from Brazil. CagA EPIYA was determined by PCR and confirmed by sequencing. A total of 436 patients were included, being 188 with gastric cancer, 112 with duodenal ulcer and 136 with gastritis.
Results
The number of EPIYA C segments was significantly associated with the increased risk of gastric carcinoma (OR = 3.08, 95% CI = 1.74 to 5.45, p < 10-3) even after adjustment for age and gender. Higher number of EPIYA C segments was also associated with gastric atrophy (p = 0.04) and intestinal metaplasia (p = 0.007). Furthermore, patients infected by cag A strains possessing more than one EPIYA C segment showed decreased serum levels of pepsinogen I in comparison with those infected by strains containing one or less EPIYA C repeat. Otherwise, the number of EPIYA C segments did not associate with duodenal ulcer.
Conclusions
Our results demonstrate that infection with H. pylori strains harbouring more than one CagA EPIYA C motif was clearly associated with gastric cancer, but not with duodenal ulcer.
Higher number of EPIYA C segments was also associated with gastric precancerous lesions as demonstrated by histological gastric atrophic and metaplastic changes and decreased serum levels of pepsinogen I.
Similar content being viewed by others
Background
Helicobacter pylori colonizes the stomach of more than half of the world's population and is associated with development of complications such as peptic ulcer disease, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma [1–4]. The factors that lead few individuals to develop the associated diseases, while the majority of infected people remain asymptomatic, are unknown, but they have been subject of intense research. Among the host factors, cytokine gene polymorphisms were shown to increase the risk of gastric cancer, specifically IL1B-31, IL1RN, and TNFA-307 single nucleotide polymorphisms in European populations, and IL1RN in a Brazilian population [5–9]. Pathogen strain-specific factors have been strongly investigated. Among them, the CagA protein is accepted as a risk factor for both peptic ulcer disease and gastric cancer [5, 10–12]. In a study of our group, infection by H. pylori cag A-positive strains had an odds ratio (OR) of 11.9 for gastric cancer, after adjusting for host polymorphisms and other variables, whereas the strongest host factor was IL1RN 2 allele, with an OR of 1.9 [5].
cag A belongs to a cag PAI (pathogenicity island) that codes a type IV secretion system (T4SS) associated with increased secretion of IL-8, a very strong proinflammatory chemokine that participates in the gastritis induced by H. pylori infection. The T4SS is also responsible for the entrance of CagA protein into the gastric epithelial cells where CagA is phosphorylated on the tyrosine residue within the phosphorylation motifs in the carboxi-terminal variable region of the protein. These motifs are defined as EPIYA (Glu-Pro-Ile-Tyr-Ala) A, B, C and D according to different flanking aminoacids. CagA protein nearly always possesses EPIYA A and B segments that are followed by none, one, two or three C segments, in strains circulating in the Western countries, or a D segment, in East Asian countries. The EPIYA C and D are the main sites for phosphorylation of CagA. Phosphorylated CagA forms a physical complex with SHP-2 phosphatase and triggers abnormal cellular signals leading to deregulation of cell growth, cell to cell contact and cell migration, elongation of epithelial cells and increase of epithelial cell turnover, which enhance the risk of damaged cells to acquire precancerous genetic changes. Carrying the type D EPIYA or multiple C repeats is associated with increased SHP-2 phosphatase activity induced by CagA [13, 14], which raises the possibility that infection by CagA strains possessing higher number EPIYA C segments predisposes to precancerous lesions and gastric cancer.
In fact, this hypothesis has been tested in Eastern countries, but the study results are discordant. Azuma et al. [15] found increased proportion of EPIYA D strains among patients with atrophic gastritis and gastric cancer, but other authors have been unable to reproduce these results [16, 17]. Similarly, in Western populations, significant association between gastric cancer and increased number of EPIYA C motifs could be demonstrated in two studies [18, 19], maybe either by the small number of included patients in the other studies [20–22], or by regional/ethnics differences as already demonstrated for other H. pylori virulence markers [23, 24]. Furthermore, discrepancies have been also demonstrated in studies evaluating the number of EPIYA C motifs and duodenal ulcer [19, 25], which deserves in deep investigations because duodenal ulcer and gastric cancer are mutually exclusive H. pylori-associated diseases.
Therefore we evaluated whether increased number of CagA EPIYA C phosphorylation motifs is associated with gastric cancer and/or duodenal ulcer including a large series of patients to avoid bias and to allow adjustment for age and gender in a Western population from Brazil. Since duodenal ulcer and gastric carcinoma are mutually exclusive diseases, and cag A is a risk factor for both conditions, we also evaluated whether the number of EPIYA C segments of the strains isolated from patients with duodenal ulcer differed from that of the strains isolated from gastric cancer patients. Because gastric atrophic and metaplastic changes - precancerous lesions - lead to impairment of the production of pepsinogen I (PGI) by chief and mucous neck cells in the corpus and fundic glands, we evaluated whether the higher number of EPIYA C motifs was associated with the serum pepsinogen levels.
Results
The characteristics of the patients are shown in the Table 1. The presence of H. pylori-specific ure A and 16S rRNA was successfully confirmed by PCR in all studied strains and the cag A PCRs were positive, by at least one of the method used, in all strains.
Determination of the CagA EPIYA pattern
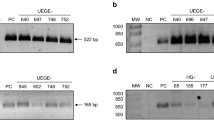
PCR amplified products from all cag A-positive strains showed distinct patterns in the 3' variable region of cag A. An electrophoresis gel representing the different CagA EPIYA types is shown in the Figure 1. The PCR results were confirmed by sequencing in seventy five randomly selected PCR products from patients of each group.
Electrophoresis of representative samples with each of the CagA EPIYA types seen in patients with H. pylori -associated diseases. Column 1: 100 bp standard; Column 2: EPIYA-AB; Columns 3, 8, 11, and 12: EPIYA-ABC; Column 4: EPIYA-ABC + -ABCCC; Columns 5 and 13: EPIYA-ABCC; Column 6: EPIYA ABCCC; Column 7: EPIYA-ABCC + -ABCCC; Column 9: EPIYA-ABC + -ABCC + -ABCCC; Column 10: EPIYA-ABC + -ABCC.
No EPIYA D was found in the H. pylori strains studied. The distribution of the EPIYA genotypes is shown in the Table 1.
Association between the numbers of EPIYA C segments and gastric cancer and duodenal ulcer
Colonization by H. pylori CagA-positive strains possessing two or three EPIYA C motifs was more frequently observed (p < 10-3) in the gastric cancer (78/188, 41.5%) than in the gastritis (25/136, 18.4%) patients. The association remained strongly significant even after adjusting for age and gender by means of logistic regression (Table 2). The Hosmer-Lemeshow test showed good fitness of the model (Chi-square = 3.98, 8 degrees of freedom, p = 0.86, with 10 steps). Otherwise, the number of EPIYA C segments did not associate with duodenal ulcer (Table 2).
Because it might be speculated that the number of EPIYA C motifs increases with increasing age, we also constructed a model with the number of EPIYA C being the dependent variable and the age, sex and H. pylori-associated diseases as independent covariables. Increased EPIYA C segments did not associate with age (p = 0.13), sex (p = 0.66) and duodenal ulcer (p = 0.29) but remained associated with gastric cancer (p < 10-3, OR = 2.81; 95% CI = 1.64 - 4.82).
Association between mixed strain colonization and diseases
Mixed strain infection was observed in 57 (13.08%) patients and it was significantly more frequent in patients with gastric cancer (38/188, 20.2%) than in those with gastritis (14/136, 10.3%) with an OR for gastric carcinoma of 2.21 (95%CI = 1.10 to 4.50). Otherwise, mixed infection was less frequently observed in duodenal ulcer patients (5/112, 4.5%) with a trend to a negative association (p = 0.09).
Association between the numbers of EPIYA C segments and serum PGI levels
The pepsinogen I serum levels were significantly higher (p = 0.01) in duodenal ulcer (mean 161.67 ± 102.36 μg/L) than in gastritis (100.37 ± 70.85 μg/L).
The patients infected by CagA strains possessing two or three EPIYA C segments showed decreased levels of PGI when compared with those with infection by CagA strains possessing ≤ 1 EPIYA C segment (duodenal ulcer: 179.67 ± 83.30 vs. 67.01 ± 34.30, respectively, p = 0.02 and gastritis: 109.26 ± 85.61 vs. 57.55 ± 34.61, respectively, p = 0.01).
Association between the numbers of EPIYA C repeats and gastric histological alterations and tumour classification
Increased number of EPIYA C segments was associated with the presence of precancerous lesions, either atrophy (p = 0.04) or intestinal metaplasia (p = 0.007), but not with the other histological parameters. Also, the infection by strains carrying increased EPIYA C motifs did not associate with intestinal or diffuse tumour type (p = 0.34).
Discussion
In this study, by evaluating a large series of patient, we demonstrated that those infected by CagA-positive H. pylori strains possessing more than one EPIYA C motif are at thrice-fold increased risk for developing gastric cancer. The strengths of our study include adequate sample size, identification of strains by means of PCR with confirmation by sequencing and adjustment for confounding factors by logistic regression analysis.
CagA is considered to be an important bacterial virulence factor associated with both gastric adenocarcinoma and duodenal ulcer disease [2, 5, 11, 12, 26]. The number and pattern of phosphorylation motifs seem to further stratify the risk associated with individual strains [18, 27].
It has been demonstrated that H. pylori CagA EPIYA patterns have a significant geographic variability and closely follow patterns of historical human migrations. EPIYA D is a characteristic Asian EPIYA pattern that virtually does not occur in the Western H. pylori strains [28]. The Brazilians form an unique Western population because, despite the multiple origins and the consequent wide diversity of phenotypic appearance, there has been a substantial degree of inter-ethnic breeding and thus most individuals cannot be ascribed to any of the founding groups on the basis of genetic background, rather they carry about 33% of genes from each of the major races that historically composed the country (Caucasians, Africans and Amerindians) [29]. With this background, it would be expected to find some CagA EPIYA D in our H. pylori strains, as it has been detected among Amerindians (in keeping with the theory that initially people from Asia populated the Americas migrating from the East Asia), but we did not detect any EPIYA D in our population.
Unfortunately, there are few studies in respect to the association between EPIYA C number and H. pylori associated diseases in Western populations with discordant results among them. Basso et al. [19] showed that higher number of EPIYA C segments was associated with gastric carcinoma in a Caucasian population from Italy, similarly to the results of Yamaoka et al. [18] evaluating American patients from Texas. Otherwise, no association was observed when Colombian patients were evaluated in the Yamaoka's study [18] in accordance with the results obtained by Acosta et al. [22], whereas Sicinschi et al. [21] observed associations between increased EPIYA C segments and precancerous lesions. Also, non-conclusive results published by Argent et al. [20] evaluating 44 strains from African patients the authors showed tendency of association between CagA with two or more EPIYA C segments and gastric cancer.
These differences may be explained by different study designs, sample size, populations and geographical diversity of H. pylori markers of pathogenicity, in respect to the CagA EPIYA pattern, highlighting the need of studying different geographical regions.
The results of this study showed that higher number of EPIYA C segments is associated with gastric cancer and with pre-malignant lesions, atrophy and intestinal metaplasia of the corpus mucosa in the group of patients with gastritis. In agreement with these findings, we also demonstrated that serum concentration of PGI was twice decreased in the patients infected by cag A-positive strains with two or three EPIYA C motifs. Because PGI is secreted by chief cells and mucous neck cells in the corpus and fundic glands, serum PGI levels reflect the functional and morphologic status of the oxyntic gastric mucosa; thus, gastric atrophic/metaplastic changes lead to decreased PGI serum levels [30, 31]. Although the corpus mucosa of patients with H. pylori associated duodenal ulcer is either mildly or not inflamed, the PGI serum levels were also decreased in duodenal ulcer patients infected by strains containing higher number of EPIYA C segments.
The results of the present study strengthen the potential role of CagA polymorphism in the development of gastric cancer in agreement with the results of the previous studies [18, 19]. However, we can not exclude the possibility that the genetic constitution of the host, more than the bacterium strain, might predispose to atrophic gastritis and the H. pylori strains carrying increasing numbers of EPIYA C repeats would have an advantage over other strains in colonizing the new gastric environment or alternatively a more complex interplay of both mechanisms.
In respect to duodenal ulcer, also the results of the studies are discordant [19, 25]. Our results are in agreement with those reported by Basso et al. [19] who also did not find association between the disease and the number of EPIYA C segments in an Italian population. Notably, none patient with duodenal ulcer of our cohort was colonized by CagA possessing three EPIYA C segments. As suggested by Yamaoka et al. [18], it is possible that strains with higher number of EPIYA C segments may be less resistant to the acid [18].
We also evaluated whether colonization by different strains (mixed infection) could be associated with disease outcomes. We found that gastric cancer patients were significantly more often colonized by mixed strains, whereas patients with duodenal ulcer had a trend toward less mixed strain colonization. One possibility is that patients with gastric cancer would have areas of gastric mucosa showing cancer transformation, alternating with areas of atrophy, intestinal metaplasia, dysplasia, and normal mucosa, each of them representing microenvironments that would be selectively advantageous to mixed infections [32, 33].
Conclusions
In conclusion, we found that infection by H. pylori CagA-positive strains harbouring multiple EPIYA C repeats is associated with gastric precancerous lesions and gastric cancer, but not with duodenal ulcer in an ethnically diverse, admixed, Western population.
Although infection by H. pylori cag A-positive strains is a risk factor for the mutually exclusive diseases, gastric cancer and duodenal ulcer, CagA strains possessing higher number of EPIYA C segments were associated with gastric cancer, but not with duodenal ulcer.
Higher number of EPIYA C segments was also associated with gastric precancerous lesions as demonstrated by histological gastric atrophic and metaplastic changes and decreased serum levels of pepsinogen I.
Methods
Patients
We included 436 patients infected with cag A-positive strains of H. pylori (188 with gastric cancer, 112 with duodenal ulcer and 136 with gastritis), among those who were submitted to endoscopy to clarify the origin of symptoms related to the upper gastrointestinal tract or who underwent gastric surgery to remove gastric carcinoma at the University Hospital/UFMG, Luxemburgo, and Mário Penna Hospitals, in Belo Horizonte, Brazil. Most of the included individuals (>80%) were of low socioeconomic level with similar cultural habits, and all were native of Minas Gerais state with the same ethnic background, approximately 33% of Portuguese, 33% of Amerindian and 33% of African ancestry, homogeneously present in each subject [29].
The study was approved by the institutional Ethics Committees and informed consent was obtained from all patients. The transport, culture, and microbiological identification of the bacterial isolates were performed as previously described [34, 35].
Histology
In the group of gastritis and duodenal ulcer patients, endoscopic biopsy samples of the antral and oxyntic gastric mucosa were obtained for histological and microbiological study. Antral and oxyntic biopsy specimens were fixed in 10% formalin and embedded in paraffin wax, and 4-μm-thick histological sections were stained with carbolfuchsin for H. pylori investigation [35] and hematoxycilin and eosin for histological evaluation according to the updated Sydney System [36]. In the group of gastric cancer patients, the fragments were obtained from the stomach removed by gastrectomy after opening it along the greater curvature within one hour of resection. The tumour was classified according to Lauren [37].
Extraction of bacterial DNA
Bacterium DNA obtained from 60 mm Petri dish growth was extracted using the QIAmp (QIAGEN, Hilden, Germany) kit according to manufacturer's recommendations with minor modifications. Distilled water was used as a reaction control. The DNA concentration was determined by spectrophotometry using NanoDrop 2000 (Thermo Scientific, Wilmington, NC) and stored at -20°C until use.
Amplification of H. pylori-specific ure A and 16S rRNA genes
The presence of specific ure A and 16S rRNA genes was evaluated according to Clayton et al.[38] and Fox et al. [39] respectively. The standard Tx30a H. pylori strain was used as a positive control, and an Escherichia coli strain and distilled water were both used as negative controls.
The thermocycler GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA) was used for all reactions. The amplified products were electrophoresed in 2% agarose gel, stained with ethidium bromide, and analyzed in an ultraviolet light transilluminator.
Amplification of the cag A gene
The cag A gene was amplified by means of two previously described primer pairs [40, 41]. A H. pylori strain from our collection (1010-95), known to be cag A-positive, was used as a positive control, and Tx30a H. pylori strain lacking cag A and distilled water were both used as negative controls. The H. pylori strains were considered to be cag A-positive when at least one of the two reactions was positive.
Amplification of the 3' variable region of cag A
For the PCR amplification of the 3' variable region of the cag A gene (that contains the EPIYA sequences), 20 to 100 ng of DNA were added to 1% Taq DNA polymerase buffer solution (KCl 50 mM and Tris-HCl 10 mM), 1.5 mM MgCl2, 100 μM of each deoxynucleotide, 1.0 U Platinum Taq DNA polymerase (Invitrogen, São Paulo, Brazil), and 10 pmol of each primer, for a total solution volume of 20 μL. The primers used were previously described by Yamaoka et al. [27]. The reaction conditions were: 95°C for 5 minutes, followed by 35 cycles of 95°C for 1 minute, 50°C for 1 minute, and 72°C for 1 minute, ending with 72°C for 7 minutes. The amplified products were electrophoresed in 1.5% agarose gel that was stained with ethidium bromide, and analyzed in an ultraviolet light transilluminator. The reaction yielded products of 500 to 850 bp according to the number of EPIYA C. This methodology also allows the detection of mixed infection.
Sequencing of the 3' variable region of cag A
A significant subset of samples (around 75 patients of each group) was randomly selected for sequencing, in order to confirm the PCR results. PCR products were purified with the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, MI) according to the manufacturer's recommendations. Purified products were sequenced using a BigDye® Terminator v3.1 Cycle Sequencing Kit in an ABI 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA). The sequences obtained were aligned using the CAP3 Sequence Assembly Program (available from: http://pbil.univ-lyon1.fr/cap3.php). After alignment, nucleotide sequences were transformed into aminoacid sequences using the Blastx program (available from: http://blast.ncbi.nlm.nih.gov/Blast.cgi) and compared to sequences deposited into the GenBank (http://www.ncbi.nlm.nih.gov/Genbank/).
Determination of the serum PGI levels
The serum concentrations of PGI were determined in the patients with gastritis and duodenal ulcer by a specific ELISA (Biohit, Helsinki, Finland) according to manufacturer's recommendations.
Statistical analysis
A sample size of at least 112 subjects in each group, in order to show a 15% difference among groups with a power of 80%, alpha of 5%, and confidence interval of 95% was calculated with the Epi Info program version 3.5.1 (Centres for Disease Control and Prevention, Atlanta, GA).
Association between the number of EPIYA C motifs and gastric cancer was initially evaluated in univariate analysis, and variables with a p-value less than 0.2 were included in the final model of logistic regression, controlling for the influences of age and sex. We also evaluated the effect of the gender and age in the number of EPIYA C segments in a model with the number of EPIYA C being the dependent variable and the age, sex and H. pylori-associated diseases as independent covariables. The logistic model fitness was evaluated with the Hosmer-Lemeshow test. Because the PGI levels were not normally distributed the data log transformed and became normal. Associations were, thus, evaluated by Student's t test (mean ± standard deviation). Association among the number of EPIYA C segments and the degree of gastric inflammation, atrophy and intestinal metaplasia was done by the two-tailed Mann-Whitney Test. The level of significance was set at a p value ≤ 0.05.
References
Pounder RE, Ng D: The Prevalence of Helicobacter-pylori Infection in Different Countries. Aliment Pharm Therap. 1995, 9: 33-39.
Megraud F, Lamouliatte H: Helicobacter-pylori and Duodenal-Ulcer - Evidence Suggesting Causation. Digest Dis Sci. 1992, 37 (5): 769-772. 10.1007/BF01296437.
Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK: Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991, 325 (16): 1127-1131. 10.1056/NEJM199110173251603.
Wotherspoon AC, Ortizhidalgo C, Falzon MR, Isaacson PG: Helicobacter-pylori-Associated Gastritis and Primary B-Cell Gastric Lymphoma. Lancet. 1991, 338 (8776): 1175-1176. 10.1016/0140-6736(91)92035-Z.
Rocha GA, Guerra JB, Rocha AMC, Saraiva IEB, da Silva DA, de Oliveira CA, Queiroz DMM: IL1RN polymorphic gene and cagA-positive status independently increase the risk of noncardia gastric carcinoma. Int J Cancer. 2005, 115 (5): 678-683. 10.1002/ijc.20935.
Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Alves CC, Campos ML, Van Doorn LJ, Caldas C, et al: A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003, 125 (2): 364-371. 10.1016/S0016-5085(03)00899-0.
El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, et al: Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003, 124 (5): 1193-1201. 10.1016/S0016-5085(03)00157-4.
Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F, et al: Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001, 121 (4): 823-829. 10.1053/gast.2001.28000.
El-Omar EM, Carrington M, Chow WH, McColl KEL, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al: Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000, 404 (6776): 398-402. 10.1038/35006081.
Nomura AMY, Perez-Perez GI, Lee J, Stemmermann G, Blaser MJ: Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease. Am J Epidemiol. 2002, 155 (11): 1054-1059. 10.1093/aje/155.11.1054.
Queiroz DMM, Mendes EN, Rocha GA, Oliveira AMR, Oliveira CA, Magalhaes PP, Moura SB, Cabral MMDA, Nogueira AMMF: cagA-positive Helicobacter pylori and risk for developing gastric carcinoma in Brazil. Int J Cancer. 1998, 78 (2): 135-139. 10.1002/(SICI)1097-0215(19981005)78:2<135::AID-IJC1>3.0.CO;2-#.
Blaser MJ, Perezperez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A: Infection with Helicobacter-pylori Strains Possessing Caga Is Associated with an Increased Risk of Developing Adenocarcinoma of the Stomach. Cancer Res. 1995, 55 (10): 2111-2115.
Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M: SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002, 295 (5555): 683-686. 10.1126/science.1067147.
Naito M, Yamazaki T, Tsutsumi R, Higashi H, Onoe K, Yamazaki S, Azuma T, Hatakeyama M: Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology. 2006, 130 (4): 1181-1190. 10.1053/j.gastro.2005.12.038.
Azuma T, Yamazaki S, Yamakawa A, Ohtani M, Muramatsu A, Suto H, Ito Y, Dojo M, Yamazaki Y, Kuriyama M, et al: Association between diversity in the Src homology 2 domain-containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J Infect Dis. 2004, 189 (5): 820-827. 10.1086/381782.
Choi KD, Kim N, Lee DH, Kim JM, Kim JS, Jung HC, Song IS: Analysis of the 3 ' variable region of the cagA gene of Helicobacter pylori isolated in Koreans. Digest Dis Sci. 2007, 52 (4): 960-966. 10.1007/s10620-005-9030-z.
Zhu YL, Zheng S, Du Q, Qian KD, Fang PC: Characterization of CagA variable region of Helicobacter pylori isolates from Chinese patients. World J Gastroenterol. 2005, 11 (6): 880-884.
Yamaoka Y, El-Zimaity HMT, Gutierrez O, Figura N, Kim JK, Kodama T, Kashima K, Graham DY: Relationship between the cagA 3 ' repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology. 1999, 117 (2): 342-349. 10.1053/gast.1999.0029900342.
Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D, et al: Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008, 135 (1): 91-99. 10.1053/j.gastro.2008.03.041.
Argent RH, Kidd M, Owen RJ, Thomas RJ, Limb MC, Atherton JC: Determinants and consequences of different levels of CagA phosphorylation for clinical isolates of Helicobacter pylori. Gastroenterology. 2004, 127 (2): 514-523. 10.1053/j.gastro.2004.06.006.
Sicinschi LA, Correa P, Peek RM, Camargo MC, Piazuelo MB, Romero-Gallo J, Hobbs SS, Krishna U, Delgado A, Mera R, et al: CagA C-terminal variations in Helicobacter pylori strains from Colombian patients with gastric precancerous lesions. Clin Microbiol Infect. 2010, 16 (4): 369-378. 10.1111/j.1469-0691.2009.02811.x.
Acosta N, Quiroga A, Delgado P, Bravo MM, Jaramillo C: Helicobacter pylori CagA protein polymorphisms and their lack of association with pathogenesis. World J Gastroentero. 2010, 16 (31): 3936-3943. 10.3748/wjg.v16.i31.3936.
Gomes LI, Rocha GA, Rocha AMC, Soares TF, Oliveira CA, Bittencourt PFS, Queiroz DMM: Lack of association between Helicobater pylori infection with dupA-positive strains and gastroduodenal diseases in Brazilian patients. Int J Med Microbiol. 2008, 298 (3-4): 223-230. 10.1016/j.ijmm.2007.05.006.
van Doorn LJ, Figueiredo C, Mégraud F, Pena S, Midolo P, Queiroz DM, Carneiro F, Vanderborght B, Pegado MD, Sanna R, De Boer W, Schneeberger PM, Correa P, Ng EK, Atherton J, Blaser MJ, Quint WG: Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology. 1999, 116 (4): 823-830. 10.1016/S0016-5085(99)70065-X.
Salih BA, Bolek BK, Arikan S: DNA sequence analysis of cagA 3 ' motifs of Helicobacter pylori strains from patients with peptic ulcer diseases. J Med Microbiol. 2010, 59 (2): 144-148. 10.1099/jmm.0.014894-0.
Hatakeyama M: Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004, 4 (9): 688-694. 10.1038/nrc1433.
Yamaoka Y, Kodama T, Kashima K, Graham DY, Sepulveda AR: Variants of the 3' region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J Clin Microbiol. 1998, 36 (8): 2258-2263.
Queiroz DM, Cunha RP, Saraiva IE, Rocha AM: Helicobacter pylori virulence factors as tools to study human migrations. Toxicon. 2010, 56 (7): 1193-1197. 10.1016/j.toxicon.2010.01.018.
Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SDJ: Color and genomic ancestry in Brazilians. P Natl Acad Sci USA. 2003, 100 (1): 177-182. 10.1073/pnas.0126614100.
Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI: Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982, 83 (1Pt2): 204-209.
Correa P, Piazuelo MB, Wilson KT: Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010, 105 (3): 493-498. 10.1038/ajg.2009.728.
Blaser MJ, Berg DE: Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001, 107 (7): 767-773. 10.1172/JCI12672.
Aras RA, Lee Y, Kim SK, Israel D, Peek RM, Blaser MJ: Natural variation in populations of persistently colonizing bacteria affect human host cell phenotype. J Infect Dis. 2003, 188 (4): 486-496. 10.1086/377098.
Queiroz DM, Mendes EN, Rocha GA: Indicator medium for isolation of Campylobacter pylori. J Clin Microbiol. 1987, 25 (12): 2378-2379.
Rocha GA, Queiroz DM, Mendes EN, Lage AP, Barbosa AJ: Simple carbolfuchsin staining for showing C pylori and other spiral bacteria in gastric mucosa. J Clin Pathol. 1989, 42 (9): 1004-1005. 10.1136/jcp.42.9.1004.
Dixon MF, Genta RM, Yardley JH, et al: Classification and grading of gastritis. The updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996, 20 (10): 1161-1181. 10.1097/00000478-199610000-00001.
Lauren P: The two histological main types of gastric cancer: diffuse and so-called intestinal type carcinoma. Acta Pathol Microbiol Scand. 1965, 64: 31-49.
Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S: Sensitive Detection of Helicobacter-pylori by Using Polymerase Chain-Reaction. Journal of Clinical Microbiology. 1992, 30 (1): 192-200.
Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, Ericson RL, Lau CN, Correa P, Araya JC, et al: Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998, 114 (4): 755-763. 10.1016/S0016-5085(98)70589-X.
Peek RM, Miller GG, Tham KT, Perez-Perez GI, Cover TL, Atherton JC, Dunn GD, Blaser MJ: Detection of Helicobacter pylori gene expression in human gastric mucosa. J Clin Microbiol. 1995, 33 (1): 28-32.
Kelly SM, Pitcher MC, Farmery SM, Gibson GR: Isolation of Helicobacter pylori from feces of patients with dyspepsia in the United Kingdom. Gastroenterology. 1994, 107 (6): 1671-1674.
Acknowledgements
This work was supported by grants of the CNPq, FAPEMIG and INCT, Brazil.
DMM, Queiroz is funded under the Sixth Framework Program of the European Union, Project CONTENT (INCO-CT-2006-032136).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
SAB performed DNA extraction, PCR and sequencing. GAR, DMMQ and SAB participate in the design of the study and wrote the manuscript. AMCR carried out pepsinogen I evaluation and reviewed the manuscript. IEBS contributed to manuscript writing. MMDAC performed histological analysis. RCO participated in the discussion of the study design. DMMQ supervised laboratory work and analyzed the data. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Batista, S.A., Rocha, G.A., Rocha, A.M. et al. Higher number of Helicobacter pylori CagA EPIYA C phosphorylation sites increases the risk of gastric cancer, but not duodenal ulcer. BMC Microbiol 11, 61 (2011). https://doi.org/10.1186/1471-2180-11-61
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2180-11-61