Abstract
Bacille Calmette-Guérin (BCG) is an attenuated strain of Mycobacterium bovis currently used as a vaccine against tuberculosis. Global distribution and propagation of BCG has contributed to the in vitro evolution of the vaccine strain and is thought to partially account for the different outcomes of BCG vaccine trials. Previous efforts by several molecular techniques effectively identified large sequence polymorphisms among BCG daughter strains, but lacked the resolution to identify smaller changes. In this study, we have used a NimbleGen tiling array for whole genome comparison of 13 BCG strains. Using this approach, in tandem with DNA resequencing, we have identified six novel large sequence polymorphisms including four deletions and two duplications in specific BCG strains. Moreover, we have uncovered various polymorphisms in the phoP-phoR locus. Importantly, these polymorphisms affect genes encoding established virulence factors including cell wall complex lipids, ESX secretion systems, and the PhoP-PhoR two-component system. Our study demonstrates that major virulence factors are different among BCG strains, which provide molecular mechanisms for important vaccine phenotypes including adverse effect profile, tuberculin reactivity and protective efficacy. These findings have important implications for the development of a new generation of vaccines.
Similar content being viewed by others
Background
Bacille Calmette-Guérin (BCG) is an attenuated strain of Mycobacterium bovis and is the only available vaccine against tuberculosis (TB). Since 1974, BCG vaccination has been included in the World Health Organization (WHO) Expanded Program on Immunization. It is estimated that more than 3 billion individuals have been immunized with BCG and over 100 million doses of BCG are administered annually. Multiple studies have confirmed that BCG is generally safe and can protect children against disseminated disease, including tuberculosis meningitis [1, 2]. BCG also provides cross-protection against leprosy [3]. However, the success of BCG against pulmonary TB in adults is still debated, since randomized clinical trials have reported protection efficacy ranging from 0–80% [4, 5]. Several hypotheses for the variation in observed efficacy have been proposed [6–9].
One explanation concerns the heterogeneity of the BCG strains [6]. The original BCG was derived from a virulent strain of M. bovis isolated from a cow. From 1908 through 1921, this isolate was subjected to 230 passages on glycerinated potato bile medium, which generated an attenuated strain termed BCG [10]. Distribution and widespread use of BCG started around 1924 and was accompanied by changes in the manufacturing process in production facilities. For instance, while BCG in Sweden was transferred without interruption from bile potato to bile potato medium in accordance with Calmette's original practice [11], BCG production in Denmark involved alternating rounds of growth on potato bile medium and Sauton broth until 1949 when it was grown exclusively in Sauton medium [12]. Prior to the establishment of seed stocks in the 1960s, BCG was passaged continuously, and the changes in media and transfer schedules contributed to the "in vitro evolution" of BCG [6]. It is estimated that as many as 49 production substrains have been used at one time or another in various parts of the world [13], including the four major BCG vaccines in current use (BCG-Pasteur, -Danish, -Glaxo, and -Japan) [14]. The relative protective efficacy of BCG substrains is currently unknown [6, 15].
Anecdotal reports have long indicated that BCG substrains exhibit phenotypic differences in growth characteristics, biochemical activities, ability to protect against challenge with Mycobacterium tuberculosis (M. tb), and residual virulence [16]. Over the past decade, numerous groups have sought to identify the genomic changes responsible for these phenotypes. The earliest whole genome comparisons confirmed that BCG was indeed related to, but distinct from M. tb and M. bovis [17–19]. Subsequent analyses of multiple vaccine strains have uncovered extensive genome diversity including both deletions and duplications in BCG substrains [18, 20–22]. The phylogeny established by these molecular methods is consistent with the historical records of BCG dissemination [20, 23, 24]. For example, BCG strains acquired after 1927 exhibit the RD2 deletion, while nRD18 is only deleted in strains obtained after 1933. Other genomic changes are exclusive to individual daughter strains, and are associated with vaccine production at specific locations [22, 24].
A number of molecular techniques have been used to investigate genomic polymorphisms in BCG strains. Early efforts using subtractive hybridization [18] and spotted oligonucleotide arrays [20, 22, 25] effectively identified large sequence polymorphisms, but lacked the resolution to identify smaller changes. More recently, complete genome sequencing has enabled high-resolution analysis of BCG-Pasteur 1173P2 [24], but sequences for other BCG lineages have yet to be determined. To identify potential genomic polymorphisms in other BCG substrains, we have employed a tiling array platform developed by NimbleGen Systems. This DNA microarray-based comparative genome sequencing technique allows high resolution detections of sequence polymorphisms [26–28]. Using this technique, in tandem with DNA resequencing, we have identified a number of novel genomic polymorphisms in BCG strains. Importantly, these polymorphisms affect genes that are known virulence factors and are expected to have a major impact on the immunogenicity and efficacy of individual vaccine strains.
Results
We have used NimbleGen tiling arrays to analyze the genomic variability of 13 BCG strains, including BCG-Russia, -Japan, -Moreau, -Sweden, -Birkhaug, -China, -Prague, -Glaxo, -Danish, -Tice, -Phipps, -Frappier and -Pasteur. All of these strains, except BCG-China, have previously been subjected to genomic analysis by other methods [18, 20, 22, 24, 25]. The complete genome sequence of BCG-Pasteur 1173P2 is available [24]. The same BCG-Pasteur strain was included in the analysis to serve as an internal control for our experiments in addition to validating the NimbleGen technique. In each experiment, genomic DNA from M. tb H37Rv [29] acted as the common referent.
Deletions and Duplications
A total of 42 deletions were identified. Twenty-five of these have been described previously [18, 20, 22, 24, 25]. Thirteen more represent transposons (e.g., IS6110) present in the referent strain (M. tb H37Rv), but absent from the M. bovis and BCG lineages [24, 29, 30]. Six duplications were identified, four (DU1, DU2-I, -II, -III) of which have been described previously [21, 24]. These results confirm the validity of our approach, and the utility of tiling arrays for comparative genomics. A total of 4 novel deletions and 2 duplications were identified in our analysis. These novel deletions and duplications are described below.
Two deletions specific to BCG-Moreau were identified. The first is a 975 bp deletion (Table 1) that eliminates the distal end of fadD26 (Rv2930/BCG2952) and the start of ppsA (Rv2931/BCG2953). These genes are part of the genetic locus required for the biosynthesis of phthiocerol dimycocerosates (PDIMs) and phenolic glycolipids (PGLs) [31], two cell wall lipids known to be important for the virulence of M. tb and M. bovis [32–34]. In previous work, we demonstrated that BCG-Moreau does not produce PDIMs or PGLs [35], which is now explained by the fadD26-ppsA deletion identified in the current study.
The second novel polymorphism in BCG-Moreau is an 1128 bp deletion within Rv3887c/BCG3942c (Table 1). Although intact in other BCG substrains, this region overlaps with a 2.4-kb deletion (termed RDpan) found in some M. bovis strains [36], including the sequenced strain, AF2122/97 [30]. The Rv3887c/BCG3942c gene encodes a membrane transport protein and is part of the ESX-2 type VII secretion system [37]. The role of the ESX-2 system in virulence is unknown considering its variable presence among clinical M. bovis isolates from both France and England [36]. However, loss of the Rv3887c membrane transporter likely eliminates the secretion of ESAT-6- and CFP-10-like antigens [37] and influences the immunogenicity of the vaccine strain.
Two novel deletions were identified in BCG-Sweden and BCG-Birkhaug. These polymorphisms are identical between the two BCG strains, which is consistent with their genealogy [24]. The first deletion comprises 110 bp and disrupts the promoter and translational start site of whiB3 (Rv3416/BCG3486) [see Additional file 1]. The other is a 245 bp deletion within trcR (Rv1033c/BCG1091c) (Table 1). Both genes encode transcriptional regulators known to impact virulence.
WhiB3 belongs to a family of seven M. tb transcriptional regulatory proteins that contain iron-sulfur clusters and are predicted to regulate gene expression in response to environmental stimuli [38]. WhiB3 responds to oxygen and nitric oxide, and is important for regulation of carbon metabolism [39]. The deletion of whiB3 in M. bovis attenuates in vivo growth in guinea pigs [40]. TrcR is the response regulator of the TrcR-TrcS two-component system. Deletion of trcS from M. tb generates a hypervirulent phenotype such that the strain exhibits increased lethality in SCID mice [41].
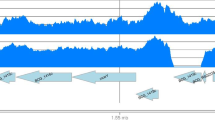
Although the genomic profiles of BCG-Birkhaug and BCG-Sweden are similar, we have also found that BCG-Birkhaug is distinguished by a strain-specific duplication, named DU-Birkhaug. This spans the origin of replication and is analogous to the DU1 duplication in BCG-Pasteur [21, 24] (Fig. 1A). However, the borders of the DU-Birkhaug are different. Whereas DU1 encompasses 29.6 kb from Rv3910 to pknB/Rv0014, DU-Birkhaug spans a slightly different region, from trxB/Rv3913 to rodA/Rv0017c. Most of the genes in these regions are involved with DNA replication and cell division. Unlike DU1, DU-Birkhaug also appears to be in a genomic location distant to its original copy. Initial PCR-based attempts to characterize the boundaries of this duplication assumed that the second copy was nearby failed to detect a product (data not shown). As such, the genome location of DU-Birkhaug remains unknown.
Novel duplications identified in BCG-Birkhaug and BCG-Tice by NimbleGen tiling array. Sections of the ratio plot are shown. The ratio of the reference (M. tb H37Rv) probe intensity (Cy5) was divided by the test (BCG strain) probe intensity (Cy3). Reference probes and test probes that do not span a mutation should represent full-length perfect match hybridization, and thus should have similar intensities, with a reference/test ratio near 1. If the test genome contains an amplification event (increased copy number when compared to the reference), then the reference/test ratio will shift below 1. (A) Novel duplication (DU-Birkhaug) identified in BCG-Birkhaug, which is analogous to the DU-Pasteur (DU1) but has different borders. The same genomic region of BCG-Sweden, which is closely related to BCG-Birkhaug, is shown for comparison. (B) Novel duplication (DU-Tice) identified in BCG-Tice. Three other BCG strains belonging to the same group (DU2-IV) are shown for comparison. (C) The precise border of DU-Tice is mapped by PCR amplification using primers specific to the junction. The two copies are immediately adjacent to each other and overlap by 1 bp.
Our analysis also revealed a novel duplication in the genome of BCG-Tice termed DU-Tice. It comprises a 22-kb duplication that encompasses Rv1782-Rv1800 (Fig. 1B). The precise boundaries and location of this duplication were determined using primers at the junction (Fig. 1C). Interestingly, DU-Tice encodes the ESX-5 secretion system [37, 42]. This includes several conserved membrane transporters (Rv1782, Rv1783, Rv1795, and Rv1797), a membrane associated ATPase (Rv1784), a set of PE/PPE genes (Rv1787-Rv1792) and the ESAT-6 and CFP-10 family proteins (esxM and esxN) [37]. ESX-5 is absent from the genome of the fast-growing, non-pathogenic M. smegmatis, but present in both the M. avium complex and M. marinum. The role of ESX-5 in virulence has been demonstrated in M. marinum [37, 42]. It has been suggested that the ESX clusters evolved via gene duplication [43] and DU-Tice offers the first snapshot of such an event.
To our knowledge, we have conducted the first genomic analysis of BCG-China, which is a descendant of BCG-Danish obtained from the Statens Serum Institut around 1947. Consistently, BCG-China exhibits the DU2-III duplication and deletion of RD2 (data not shown), which is similar to other BCG-Danish derivatives, including BCG-Prague (obtained in 1946 from passage 725) [44], BCG-Glaxo (obtained in 1954, from passage 1077) and BCG-Danish (lyophilized in 1961, from passage 1331) [45]. However, BCG-China and -Prague do not contain the previously described deletion of Rv1810, which is characteristic of BCG-Glaxo and -Danish. As such, the Rv1810 deletion must have occurred between 1947 and 1954. Coincidentally, this period corresponds to the replacement of potato bile medium by Sauton medium for BCG production in Denmark [12].
Polymorphisms of phoP-phoR
The PhoP-PhoR system is one of the 11 two-component systems found in the M. tb genome [29]. The PhoR protein is a transmembrane histidine kinase that transmits signals from the environment by autophosphorylation. The phosphoryl group is then transferred to PhoP, a response regulator that regulates the expression of multiple genes [46]. Recently, several studies have demonstrated that the PhoP-PhoR system, particularly PhoP, plays an essential role in M. tb virulence [26, 46–48]. A single point mutation (S219L) in the DNA binding region of PhoP partially accounts for the attenuation of the H37Ra strain of M. tb [26]. Furthermore, a phoP mutant of M. tb was found to be more attenuated than BCG-Pasteur in SCID mice infections [47]. Our NimbleGen analysis revealed some weak signals in the phoP-phoR region (not shown), which prompted us to resequence these genes. The DNA fragment containing the promoter region of phoP, the ORFs of phoP and phoR, and the intergenic region was PCR amplified from each BCG strain and determined by DNA sequencing.
Our sequence analysis revealed a number of polymorphisms in the phoP-phoR locus in various BCG strains compared to the genome sequence of M. bovis. The three early BCG substrains, BCG-Russia, -Japan, and -Moreau, contain an identical IS6110 (1,356 bp) insertion at nucleotide 851593 of the M. tb genome, which is 18 bp upstream of the start codon of phoP (Fig. 2). This IS6110 element is identical to many other copies of IS6110 found in various locations in the M. tb genome. It is flanked by a 3-bp direct repeat (GAA) on both sides and is in an inverse orientation of phoP-phoR (Fig. 2). The presence of an IS6110 element in the promoter region of phoP in BCG-Russia, -Japan, and -Moreau has been described previously, but its insertion site and orientation were not determined until now [49]. Although not present in M. tb H37Rv or M. bovis AF2122/97, an IS6110 insertion in the phoP promoter was found in a clinical strain of M. bovis termed B strain, which was responsible for a severe nosocomial outbreak of multidrug resistant TB in humans in Spain [50, 51]. However, unlike the three BCG strains, the IS6110 insertion in the M. bovis B strain is located at 75 bp upstream of the start codon of phoP and is in the same orientation as phoP-phoR [50]. The potential effect of IS6110 on phoP expression is described in the 'Discussion' section.
IS 6110 insertion in the phoP promoter in BCG-Russia, -Moreau, and -Japan. (A) Schematic representation of the phoP-phoR locus with IS6110 inserted in an inverse orientation 18 bp upstream from phoP start codon. (B) Nucleotide sequence surrounding IS6110. The IS6110 sequence is boxed. The GAA direct repeats flanking the IS6110 insertion site is underlined and in boldface. The ATG start codons of phoP and phoR are indicated by arrows and in boldface.
Three other novel phoP-phoR polymorphisms that likely impact their functions were also uncovered by our sequencing analysis. An identical, 11-bp deletion within the ORF of phoR was uncovered in BCG-Sweden and BCG-Birkhaug (ACCGGACTGGG, nucleotides from 853689 to 853699, M. tb genome coordinates). This deletion changes the amino acid sequence of 54 residues (residues 432 to 485) in the C-terminal of PhoR. This polymorphism is different than the previously described 10 bp deletion within phoR present in BCG-Danish and BCG-Glaxo, which affects residues 91–485 [24]. BCG-Frappier also contains a single nucleotide deletion (A at 852701, M. tb genome coordinates), causing a frame-shift mutation that affect residues 103–485 of PhoR. Together, these results indicate that besides BCG-Danish and BCG-Glaxo, BCG-Sweden, -Birkhaug, and -Frappier also contain a defective phoR gene.
A single nucleotide insertion within the ORF of phoP was uncovered in BCG-Prague (G, between nucleotides 852067 and 852068, M. tb genome coordinates) [see Additional file 2]. This frame shift mutation changes the C-terminal sequence (residues 154–247) of PhoP, which is the DNA binding domain (residues 144–247) [52–54]. As such, BCG-Prague is a natural phoP mutant.
Single point mutations in PhoP or PhoR are also found in various BCG strains and are summarized in Table 2. In contrast, sequences of the phoP-phoR locus of BCG-Phipps, -Tice, and -Pasteur are identical to the published sequence of BCG-Pasteur and M. bovis [24, 30].
Discussion
The loss of the RD1-encoded ESX-1 protein secretion system during 1908–1921 contributes to the attenuation of BCG ([55], see also Fig. 3). However, because reintroduction of ESX-1 into BCG does not restore full virulence, other genetic lesions are also involved [56]. Whole genome sequence comparison reveals 2,223 single nucleotide polymorphisms (SNPs) between BCG-Pasteur and M. tb H37Rv, and 736 SNPs between BCG-Pasteur and M. bovis AF2122/97 [24]. NimbleGen analysis revealed 1,010 SNPs between BCG-Pasteur and M. tb H37Rv. Of which, 945 SNPs were correctly identified when comparing to the complete genome sequence of BCG-Pasteur. Thus the NimbleGen technique has a limited ability to detect SNPs but the majority of identified SNPs are accurate. Our NimbleGen analysis also revealed numerous SNPs (ranging from ~400 to ~1,800) between individual BCG strains and M. tb H37Rv, and the majority of changes detected by the NimbleGen technique are also present in BCG-Pasteur (data not shown). This suggests that the loss of RD1 and the accumulation of a number of point mutations during the 230 passages in vitro likely account for the initial loss of virulence by 1921. Subsequent dissemination of BCG to various parts of the world, accompanied by changes in the manufacturing process, further affected the residual virulence and immunogenicity of individual BCG strains. As such, some strains are more virulent than others in animal models of infection [57] and also exhibit differential ability to induce adverse reactions (reactogenicity) following vaccination in neonates [58]. Our current work begins to provide some explanation for these observed differences (Fig. 3).
Refined genealogy of BCG vaccines. The genealogy is modified from a previous model [24]. Genetic markers identified in this work are highlighted.
Consistent with a previous study [58], we find that the earliest distributed BCG strains, BCG-Russia, -Japan, and -Moreau, all contain a second copy of IS6110 that is inserted in the promoter region of phoP. A similar, albeit distinct, insertion of IS6110 in the phoP promoter was also found in a virulent strain called M. bovis B strain [50]. The presence of IS6110, which is in the same orientation as phoP, increases the expression of phoP and (the resulting increase in virulence) was thought to be responsible for the outbreak of M. bovis B strain in humans [50]. Similarly, the expression level of phoP was found to be higher in BCG-Japan than in BCG-Pasteur [24]. However, in BCG-Japan, -Russia, and -Moreau, the IS6110 is in the inverse orientation of phoP (Fig. 2). As such, how IS6110 upregulates phoP expression in BCG is not immediately apparent and likely involves a different mechanism. One possibility is the elimination of phoP autoregulation. It was shown that PhoP protein, albeit from H37Ra, binds to three 9-bp direct repeats within the phoP promoter sequence and represses its own expression [52]. In BCG, IS6110 is inserted between the PhoP binding sites and the start codon of phoP, which could impair the repression by PhoP and subsequently increase phoP expression. Alternatively, an unidentified promoter sequence within IS6110 in the same orientation of phoP could drive the expression of phoP. The presence of the second copy of IS6110 in these early BCG strains also suggests that the original BCG isolated in 1921 might have been derived from a highly virulent M. bovis strain containing the same IS6110 element. This IS6110 was subsequently lost in other BCG strains (Fig. 3) and is not present in most clinical strains of M. bovis and M. tb isolated in modern times [50, 59]. Given the important role of PhoP in M. tb virulence, higher expression of phoP could explain why BCG-Russia is generally considered more virulent than other BCG strains [6]. However, in the other early strains, BCG-Moreau and BCG-Japan, the loss of lipid virulence factors PDIMs and PGLs appears to have a more pronounced effect on virulence. Consequently, these two strains, together with BCG-Glaxo, which also lacks PDIMs and PGLs, and as we have described previously, are more attenuated and less reactogenic than other BCG strains [35]. The deletion of fadD26-ppsA described here provides a genetic mechanism for the defective PDIM/PGL biosynthesis in BCG-Moreau. However, this region is intact in BCG-Japan and BCG-Glaxo, indicating that other mechanisms may also lead to the PDIM/PGL defect.
BCG-Sweden was obtained from the Institut Pasteur in 1926 while Konrad Birkhaug acquired the strain that bears his name around 1927 [6]. Previous studies indicated that these strains differ from other early BCG strains (i.e., BCG-Russia, -Japan, -Moreau) only by the loss of the IS6110 element described above. Our current work reveals three novel deletions shared by BCG-Sweden and BCG-Birkhaug (Fig. 3), which distinguish them from other early strains. Two deletions affect the regulatory proteins WhiB3 and TrcR, and have different impacts on virulence. The whiB3 gene appears to be important for virulence. The M. bovis whiB3 mutant is attenuated for growth in guinea pigs but not in mice [40]. Conversely, the trcRS two-component system has a negative impact on virulence. Deletion of trcS from M. tb generates a hypervirulent phenotype in SCID mice [41]. BCG-Sweden was used in Sweden from 1926 until 1978 and was then replaced by BCG-Danish because of the high frequency of osteitis associated with the former strain [60]. The deletion of trcR may contribute to the reactogenicity of BCG-Sweden.
The other deletion found in BCG-Sweden and BCG-Birkhaug affects the phoR gene of the phoP-phoR two-component system. Remarkably, three other late BCG strains, BCG-Danish, -Glaxo, and -Frappier also contain a defective phoR gene. Together a total of five BCG strains are natural phoR mutants. However, three distinct mutations are found among these five strains, which correspond to their genealogy (Fig. 3). The role of phoR in virulence is less understood than for phoP. Among its many functions, phoP is required for the biosynthesis of trehalose-containing cell wall lipids [48, 61, 62]. Contrastingly, phoR does not seem to be required for this function [62]. Nevertheless, the fact that the phoR mutation has been acquired by different groups of BCG strains by three independent events and genetic mechanisms suggests that there was a common selective pressure and an important role for this gene during the in vitro evolution of BCG.
Another BCG strain that contains a major mutation in the phoP-phoR system is BCG-Prague. A single nucleotide insertion in the ORF of phoP changes the C-terminal sequence, which contains the DNA binding domain of PhoP [52–54]. As such, BCG-Prague is a natural phoP mutant and likely to be more attenuated than other BCG strains. This is consistent with the study by Lagranderie et al., which showed in mice models of infection that BCG-Prague exhibited more attenuated phenotypes compared to three other BCG strains (BCG-Russia, -Pasteur, and -Glaxo) [57]. Compared to 11 other BCG strains, including BCG-Russia, -Moreau, -Japan, -Sweden, -Danish, -Glaxo, and -Pasteur that have been analyzed in the current study, BCG-Prague consistently exhibited the weakest ability to induce delayed type hypersensitivity to tuberculin in children [63] or in guinea pig models [64]. Because of the traditional presumption that tuberculin reactivity is associated with vaccine potency, BCG-Prague, which was used in Czechoslovakia between 1951–1980 and appeared to be effective, was replaced by BCG-Russia in 1981 [58]. An immediate increase of BCG-induced osteitis cases was observed in Czechoslovakia following the switch of BCG-Prague to BCG-Russia [65]. The phoP mutation detected in the current study may explain the weak tuberculin sensitivity induced by BCG-Prague. It was recently shown that a phoP mutant of M. tb was more attenuated than BCG-Pasteur and confers an equivalent protection in mice against M. tb challenge. In the guinea pig model, the M. tb phoP mutant showed superior protection to BCG-Pasteur against a high dose challenge with M. tb [47]. Consequently, the M. tb phoP mutant is now being evaluated as a vaccine candidate to replace BCG [66]. Since BCG-Pasteur contains an intact phoP gene, and in light of our finding, it would be worthy to compare the M. tb phoP mutant with BCG-Prague in terms of safety and protective efficacy.
The novel duplication uncovered in BCG-Tice (DU-Tice) may have an impact on its residual virulence and immunogenicity. DU-Tice contains the entire ESX-5 secretion system, which is one of the five type VII secretion systems found in the M. tb complex [37]. Importantly, besides the RD1-encoded ESX-1, ESX-5 is the only other ESX system that has been shown to be involved in virulence thus far [37]. ESX-5 is conserved in other pathogenic mycobacteria and reported to facilitate the cell-to-cell spread of M. marinum in infected macrophages, a function shared by ESX-1 [42]. However, ESX-5 does not complement the loss of virulence caused by ESX-1 deletion, suggesting that they play distinct roles in virulence [37]. Horwitz and co-workers have used BCG-Tice as the host strain to overexpress antigen 85B. This resulted in a recombinant strain termed rBCG30 that exhibits superior protective efficacy over BCG-Tice and is currently being evaluated as a vaccine candidate in human clinical trials [67–71]. The rBCG30 Tice strain also showed significantly stronger immune response and better protection against M. tb challenge than the rBCG30 strain based on BCG-Connaught [69]. The duplication of ESX-5 in BCG-Tice, which could increase the residual virulence and immunogenicity, may partially account for the benefit associated with rBCG30 Tice.
Conclusion
Our current work has uncovered six large sequence polymorphisms not described previously, including two deletions exclusive to BCG-Moreau, two deletions shared by BCG-Sweden and BCG-Birkhaug, as well as the DU-Birkhaug and DU-Tice duplications. Moreover, we have uncovered a number of polymorphisms in the phoP-phoR locus in various BCG strains. Remarkably, these polymorphisms affect genes that are well known to have major impact on the virulence of M. tb or M. bovis. These include genes involved in the biosynthesis of lipid virulence factors PDIMs/PGLs, genes that encode the ESX family type VII secretion system, and the phoP-phoR two-component regulatory system. As such, the current collection of BCG comprises natural mutants of established virulence factors identified thus far. BCG-Moreau, -Japan, and -Glaxo are PDIMs/PGLs deficient mutants. BCG-Prague is a phoP mutant. BCG-Sweden, -Birkhaug, -Danish, -Glaxo, and -Frappier are defective in phoR. BCG-Sweden and BCG-Birkhaug are also whiB3 mutants. These findings have important implications on the current effort and future development of TB vaccines. Currently, among major efforts, a phoP mutant of M. tb and several recombinant BCG strains including rBCG30 Tice, rBCG-Aeras 403 Danish, rBCGΔ Ure::CHly Pasteur, BCG::RD1 Pasteur, and rBCGΔ Sod Tice are being evaluated as new generation TB vaccines in preclinical or clinical trial studies [72]. In addition, a mutant of M. bovis deficient in PDIMs/PGLs is being considered as a vaccine to protect wildlife against bovine tuberculosis [73]. Our previous study [35] and current work provide direct evidence that BCG vaccine strains are different in major virulence factors, and likely have different vaccination properties including safety, immunogenicity, and efficacy. Since new vaccine candidates are evaluated for their vaccination properties relative to BCG, the appropriate choice of BCG strain for these studies is critical. Furthermore, because it is likely that BCG will continue to play a role in tuberculosis control by being included in forthcoming clinical trials, as either a primer to be boosted by new components (e.g. subunit or DNA vaccine) or as an integral component (e.g. recombinant BCG) of new vaccines, greater attention must be given to the benefits that a particular strain may – or may not – offer.
Methods
Bacterial strains
The mycobacterial strains used in this study were: Mycobacterium tuberculosis H37Rv, BCG-Russia (ATCC 35740), BCG-Moreau/Rio de Janeiro, BCG-Japan, BCG-Sweden, BCG-Birkhaug (ATCC 35731), BCG-Denmark 1331 (ATCC 35733), BCG-China, BCG-Prague, BCG-Glaxo (ATCC 35741), BCG-Tice (ATCC 35743), BCG-Frappier (ATCC 35735), BCG-Connaught, BCG-Phipps (ATCC 35744), and BCG-Pasteur 1173. All BCG strains except BCG-China have been previously described [20]. BCG-China was obtained from Shanghai Institute of Biological Product, which is the main manufacturer for BCG in China.
Genomic DNA extraction and labeling
Mycobacteria were cultured in Middlebrook 7H9 broth supplemented with 10% ADC. Chromosomal DNA was extracted using QIAGEN Genomic-tip 100/G kit (Qiagen) and then labeled with a random primer reaction. DNA (1 μg) was mixed with 1 O.D. of 5'-fluorescence dye labeled random nonamer (Cy3 for BCG strains and Cy5 for reference strain H37Rv) (TriLink Biotechnologies) in 62.5 mM Tris-HCl, 6.25 mM MgCl2 and 0.0875% ß-mercaptoethanol, denatured at 98°C for 5 min, chilled on ice, and incubated with 100 units Klenow fragment (NEB) and dNTP mix (6 mM each in TE) for 2 h at 37°C. Reactions were terminated with 0.5 M EDTA (pH 8.0), precipitated with isopropanol, and resuspended in water. A fifty-fold amplification was typically achieved.
Design of mutation mapping microarray
Mutation mapping microarrays were designed with NimbleGen algorithms that select a 29-mer oligonucleotide every 7 bases on each strand of the reference genome sequence (Genbank Accession AL123456) [29]. All probes were synthesized in parallel on a four-array set using a Digital Light Processor™ (Texas Instruments, Plano Texas) and photoprotected by phosphoramidite chemistry (Maskless Array Synthesis) (NimbleGen Systems, Madison WI) in a random probe layout [74, 75].
Microarray hybridization
Labeled genomic DNA was hybridized to arrays in the NimbleGen Hybridization Buffer at 42°C for 16 hr using a MAUI hybridization system (BioMicro Systems, Inc. Salt Lake City, Utah). Labeled genomic DNA (5 μg) from the reference strain M. tb H37Rv and from each BCG strain were co-hybridized to each array. Arrays were washed with NimbleGen wash buffer, and were then spun dry in a microarray high-speed centrifuge (TeleChem International, Inc., Sunnyvale, CA) and stored until scanned.
Analysis of mapping array data and design and hybridization of resequencing microarrays
Microarrays were scanned at 5 μm resolution using the Genepix® 4000B scanner (Axon Instruments, Union City CA), and pixel intensities were extracted using NimbleScan™ v2.4 software (NimbleGen). Probes that spanned potential mutations were identified by NimbleGen software. Probe sequences corresponding to all possible candidate mutation sites were selected for resequencing. The strategy that was used to automatically generate the sequencing array is similar to that described previously [28]. Briefly, 8 probes per base position were analyzed, 4 per genome strand. These probes contain all possible alleles at a centrally located position. The length, melting temperature and mismatch position of each probe were optimized. When target DNA is hybridized to these arrays the perfectly matched probe will hybridize more strongly than the three corresponding mismatched probes for each strand. The differential signal intensity between the perfectly matched probe and mismatched probes allows the base to be determined precisely. These resequencing arrays were synthesized, hybridized with labeled genomic DNA from each BCG strain and scanned as above. Sequence base assignments were made using a machine-learning algorithm [76]. Putative mutation-containing DNA segments were PCR amplified and verified by capillary sequencing [see Additional file 3]. The microarray data has been deposited in the Center for Information Biology Gene Expression Database (CIBEX; http://cibex.nig.ac.jp), with the accession number of CBX70.
References
Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV: The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995, 96: 29-35.
Trunz BB, Fine P, Dye C: Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006, 367: 1173-1180. 10.1016/S0140-6736(06)68507-3.
Zodpey SP: Protective effect of bacillus Calmette Guerin (BCG) vaccine in the prevention of leprosy: a meta-analysis. Indian J Dermatol Venereol Leprol. 2007, 73: 86-93.
Brewer TF: Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000, 31 (Suppl 3): S64-S67. 10.1086/314072.
Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F: Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994, 271: 698-702. 10.1001/jama.271.9.698.
Behr MA: BCG–different strains, different vaccines?. Lancet Infect Dis. 2002, 2: 86-92. 10.1016/S1473-3099(02)00182-2.
Brandt L, Feino CJ, Weinreich OA, Chilima B, Hirsch P, Appelberg R, Andersen P: Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun. 2002, 70: 672-678. 10.1128/IAI.70.2.672-678.2002.
Comstock GW: Field trials of tuberculosis vaccines: how could we have done them better?. Control Clin Trials. 1994, 15: 247-276. 10.1016/0197-2456(94)90042-6.
Demangel C, Garnier T, Rosenkrands I, Cole ST: Differential effects of prior exposure to environmental mycobacteria on vaccination with Mycobacterium bovis BCG or a recombinant BCG strain expressing RD1 antigens. Infect Immun. 2005, 73: 2190-2196. 10.1128/IAI.73.4.2190-2196.2005.
Bonah C: The 'experimental stable' of the BCG vaccine: safety, efficacy, proof, and standards, 1921–1933. Stud Hist Philos Biol Biomed Sci. 2005, 36: 696-721. 10.1016/j.shpsc.2005.09.003.
Lind A: The Swedish strain of BCG. Tubercle. 1983, 64: 223-224. 10.1016/0041-3879(83)90019-3.
Osborn TW: Changes in BCG strains. Tubercle. 1983, 64: 1-13. 10.1016/0041-3879(83)90044-2.
Corbel MJ, Fruth U, Griffiths E, Knezevic I: Report on a WHO consultation on the characterisation of BCG strains, Imperial College, London 15–16 December 2003. Vaccine. 2004, 22: 2675-2680. 10.1016/j.vaccine.2004.01.050.
Anonymous: BCG vaccine. WHO position paper. Wkly Epidemiol Rec. 2004, 79: 27-38.
Brewer TF, Colditz GA: Relationship between bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin Infect Dis. 1995, 20: 126-135.
Gupta KC: Effect of freeze-drying on the colony morphology of various daughter strains of BCG. J Biol Stand. 1975, 3: 349-352. 10.1016/0092-1157(75)90059-1.
Brosch R, Gordon SV, Billault A, Garnier T, Eiglmeier K, Soravito C, Barrell BG, Cole ST: Use of a Mycobacterium tuberculosis H37Rv bacterial artificial chromosome library for genome mapping, sequencing, and comparative genomics. Infect Immun. 1998, 66: 2221-2229.
Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK: Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996, 178: 1274-1282.
Philipp WJ, Nair S, Guglielmi G, Lagranderie M, Gicquel B, Cole ST: Physical mapping of Mycobacterium bovis BCG pasteur reveals differences from the genome map of Mycobacterium tuberculosis H37Rv and from M. bovis. Microbiology. 1996, 142 (Pt 11): 3135-3145.
Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM: Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999, 284: 1520-1523. 10.1126/science.284.5419.1520.
Brosch R, Gordon SV, Buchrieser C, Pym AS, Garnier T, Cole ST: Comparative genomics uncovers large tandem chromosomal duplications in Mycobacterium bovis BCG Pasteur. Yeast. 2000, 17: 111-123. 10.1002/1097-0061(20000630)17:2<111::AID-YEA17>3.0.CO;2-G.
Mostowy S, Tsolaki AG, Small PM, Behr MA: The in vitro evolution of BCG vaccines. Vaccine. 2003, 21: 4270-4274. 10.1016/S0264-410X(03)00484-5.
Behr MA, Small PM: A historical and molecular phylogeny of BCG strains. Vaccine. 1999, 17: 915-922. 10.1016/S0264-410X(98)00277-1.
Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, Dos SS, Duthoy S, Lacroix C, Garcia-Pelayo C, Inwald JK, Golby P, Garcia JN, Hewinson RG, Behr MA, Quail MA, Churcher C, Barrell BG, Parkhill J, Cole ST: Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci USA. 2007, 104: 5596-5601. 10.1073/pnas.0700869104.
Salamon H, Kato-Maeda M, Small PM, Drenkow J, Gingeras TR: Detection of deleted genomic DNA using a semiautomated computational analysis of GeneChip data. Genome Res. 2000, 10: 2044-2054. 10.1101/gr.GR-1529R.
Frigui W, Bottai D, Majlessi L, Monot M, Josselin E, Brodin P, Garnier T, Gicquel B, Martin C, Leclerc C, Cole ST, Brosch R: Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 2008, 4: e33-10.1371/journal.ppat.0040033.
Manjunatha UH, Boshoff H, Dowd CS, Zhang L, Albert TJ, Norton JE, Daniels L, Dick T, Pang SS, Barry CE: Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006, 103: 431-436. 10.1073/pnas.0508392103.
Wong CW, Albert TJ, Vega VB, Norton JE, Cutler DJ, Richmond TA, Stanton LW, Liu ET, Miller LD: Tracking the evolution of the SARS coronavirus using high-throughput, high-density resequencing arrays. Genome Res. 2004, 14: 398-405. 10.1101/gr.2141004.
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG: Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998, 393: 537-544. 10.1038/31159.
Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, Duthoy S, Grondin S, Lacroix C, Monsempe C, Simon S, Harris B, Atkin R, Doggett J, Mayes R, Keating L, Wheeler PR, Parkhill J, Barrell BG, Cole ST, Gordon SV, Hewinson RG: The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci USA. 2003, 100: 7877-7882. 10.1073/pnas.1130426100.
Onwueme KC, Vos CJ, Zurita J, Ferreras JA, Quadri LE: The dimycocerosate ester polyketide virulence factors of mycobacteria. Prog Lipid Res. 2005, 44: 259-302. 10.1016/j.plipres.2005.07.001.
Cox JS, Chen B, McNeil M, Jacobs WRJ: Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999, 402: 79-83. 10.1038/47042.
Hotter GS, Wards BJ, Mouat P, Besra GS, Gomes J, Singh M, Bassett S, Kawakami P, Wheeler PR, de Lisle GW, Collins DM: Transposon mutagenesis of Mb0100 at the ppe1-nrp locus in Mycobacterium bovis disrupts phthiocerol dimycocerosate (PDIM) and glycosylphenol-PDIM biosynthesis, producing an avirulent strain with vaccine properties at least equal to those of M. bovis BCG. J Bacteriol. 2005, 187: 2267-2277. 10.1128/JB.187.7.2267-2277.2005.
Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE: A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature. 2004, 431: 84-87. 10.1038/nature02837.
Chen JM, Islam ST, Ren H, Liu J: Differential productions of lipid virulence factors among BCG vaccine strains and implications on BCG safety. Vaccine. 2007, 25: 8114-8122.
Rauzier J, Gormley E, Gutierrez MC, Kassa-Kelembho E, Sandall LJ, Dupont C, Gicquel B, Murray A: A novel polymorphic genetic locus in members of the Mycobacterium tuberculosis complex. Microbiology. 1999, 145 (Pt 7): 1695-1701.
Abdallah AM, Gey vPN, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W: Type VII secretion–mycobacteria show the way. Nat Rev Microbiol. 2007, 5: 883-891. 10.1038/nrmicro1773.
Geiman DE, Raghunand TR, Agarwal N, Bishai WR: Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes. Antimicrob Agents Chemother. 2006, 50: 2836-2841. 10.1128/AAC.00295-06.
Singh A, Guidry L, Narasimhulu KV, Mai D, Trombley J, Redding KE, Giles GI, Lancaster JRJ, Steyn AJ: Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe-4S] cluster and is essential for nutrient starvation survival. Proc Natl Acad Sci USA. 2007, 104: 11562-11567. 10.1073/pnas.0700490104.
Steyn AJ, Collins DM, Hondalus MK, Jacobs WRJ, Kawakami RP, Bloom BR: Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth. Proc Natl Acad Sci USA. 2002, 99: 3147-3152. 10.1073/pnas.052705399.
Parish T, Smith DA, Kendall S, Casali N, Bancroft GJ, Stoker NG: Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect Immun. 2003, 71: 1134-1140. 10.1128/IAI.71.3.1134-1140.2003.
Abdallah AM, Verboom T, Hannes F, Safi M, Strong M, Eisenberg D, Musters RJ, Vandenbroucke-Grauls CM, Appelmelk BJ, Luirink J, Bitter W: A specific secretion system mediates PPE41 transport in pathogenic mycobacteria. Mol Microbiol. 2006, 62: 667-679. 10.1111/j.1365-2958.2006.05409.x.
Gey vPN, Sampson SL, Lee H, Kim Y, van Helden PD, Warren RM: Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol Biol. 2006, 6: 95-10.1186/1471-2148-6-95.
Pruchova J, Mara M, Mohelska H, Sir Z, Galliova J: Some biological, biochemical and morphological changes and their correlations in selected BCG vaccine strains. J Biol Stand. 1976, 4: 319-327. 10.1016/S0092-1157(76)80016-9.
Oettinger T, Jorgensen M, Ladefoged A, Haslov K, Andersen P: Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber Lung Dis. 1999, 79: 243-250. 10.1054/tuld.1999.0206.
Lee JS, Krause R, Schreiber J, Mollenkopf HJ, Kowall J, Stein R, Jeon BY, Kwak JY, Song MK, Patron JP, Jorg S, Roh K, Cho SN, Kaufmann SH: Mutation in the transcriptional regulator PhoP contributes to avirulence of Mycobacterium tuberculosis H37Ra strain. Cell Host Microbe. 2008, 3: 97-103. 10.1016/j.chom.2008.01.002.
Martin C, Williams A, Hernandez-Pando R, Cardona PJ, Gormley E, Bordat Y, Soto CY, Clark SO, Hatch GJ, Aguilar D, Ausina V, Gicquel B: The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine. 2006, 24: 3408-3419. 10.1016/j.vaccine.2006.03.017.
Walters SB, Dubnau E, Kolesnikova I, Laval F, Daffe M, Smith I: The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol. 2006, 60: 312-330. 10.1111/j.1365-2958.2006.05102.x.
Fomukong NG, Dale JW, Osborn TW, Grange JM: Use of gene probes based on the insertion sequence IS986 to differentiate between BCG vaccine strains. J Appl Bacteriol. 1992, 72: 126-133.
Soto CY, Menendez MC, Perez E, Samper S, Gomez AB, Garcia MJ, Martin C: IS6110 mediates increased transcription of the phoP virulence gene in a multidrug-resistant clinical isolate responsible for tuberculosis outbreaks. J Clin Microbiol. 2004, 42: 212-219. 10.1128/JCM.42.1.212-219.2004.
Rivero A, Marquez M, Santos J, Pinedo A, Sanchez MA, Esteve A, Samper S, Martin C: High rate of tuberculosis reinfection during a nosocomial outbreak of multidrug-resistant tuberculosis caused by Mycobacterium bovis strain B. Clin Infect Dis. 2001, 32: 159-161. 10.1086/317547.
Gupta S, Sinha A, Sarkar D: Transcriptional autoregulation by Mycobacterium tuberculosis PhoP involves recognition of novel direct repeat sequences in the regulatory region of the promoter. FEBS Lett. 2006, 580: 5328-5338. 10.1016/j.febslet.2006.09.004.
Sinha A, Gupta S, Bhutani S, Pathak A, Sarkar D: PhoP-PhoP interaction at adjacent PhoP binding sites is influenced by protein phosphorylation. J Bacteriol. 2008, 190: 1317-1328. 10.1128/JB.01074-07.
Wang S, Engohang-Ndong J, Smith I: Structure of the DNA-binding domain of the response regulator PhoP from Mycobacterium tuberculosis. Biochemistry. 2007, 46: 14751-14761. 10.1021/bi700970a.
Behr MA, Sherman DR: Mycobacterial virulence and specialized secretion: same story, different ending. Nat Med. 2007, 13: 286-287. 10.1038/nm0307-286.
Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST: Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003, 9: 533-539. 10.1038/nm859.
Lagranderie MR, Balazuc AM, Deriaud E, Leclerc CD, Gheorghiu M: Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun. 1996, 64: 1-9.
Milstien JB, Gibson JJ: Quality control of BCG vaccine by WHO: a review of factors that may influence vaccine effectiveness and safety. Bull World Health Organ. 1990, 68: 93-108.
Isabel O, Gomez AB, Kremer K, de Haas P, Garcia MJ, Martin C, van Soolingen D: Mapping of IS6110 insertion sites in Mycobacterium bovis isolates in relation to adaptation from the animal to human host. Vet Microbiol. 2008, 129: 333-341. 10.1016/j.vetmic.2007.11.038.
Lotte A, Wasz-Hockert O, Poisson N, Dumitrescu N, Verron M, Couvet E: BCG complications. Estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv Tuberc Res. 1984, 21: 107-193.
Chesne-Seck ML, Barilone N, Boudou F, Gonzalo AJ, Kolattukudy PE, Martin C, Cole ST, Gicquel B, Gopaul DN, Jackson M: A point mutation in the two-component regulator PhoP-PhoR accounts for the absence of polyketide-derived acyltrehaloses but not that of phthiocerol dimycocerosates in Mycobacterium tuberculosis H37Ra. J Bacteriol. 2008, 190: 1329-1334. 10.1128/JB.01465-07.
Gonzalo AJ, Maia C, Ferrer NL, Barilone N, Laval F, Soto CY, Winter N, Daffe M, Gicquel B, Martin C, Jackson M: The virulence-associated two-component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem. 2006, 281: 1313-1316.
Vallishayee RS, Shashidhara AN, Bunch-Christensen K, Guld J: Tuberculin sensitivity and skin lesions in children after vaccination with 11 different BCG strains. Bull World Health Organ. 1974, 51: 489-494.
Ladefoged A, Bunch-Christensen K, Guld J: Tuberculin sensitivity in guinea-pigs after vaccination with varying doses of BCG of 12 different strains. Bull World Health Organ. 1976, 53: 435-443.
Lotte A, Wasz-Hockert O, Poisson N, Engbaek H, Landmann H, Quast U, Andrasofszky B, Lugosi L, Vadasz I, Mihailescu P: Second IUATLD study on complications induced by intradermal BCG-vaccination. Bull Int Union Tuberc Lung Dis. 1988, 63: 47-59.
Asensio JA, Arbues A, Perez E, Gicquel B, Martin C: Live tuberculosis vaccines based on phoP mutants: a step towards clinical trials. Expert Opin Biol Ther. 2008, 8: 201-211. 10.1517/14712598.8.2.201.
Horwitz MA, Lee BW, Dillon BJ, Harth G: Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995, 92: 1530-1534. 10.1073/pnas.92.5.1530.
Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S: Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci USA. 2000, 97: 13853-13858. 10.1073/pnas.250480397.
Horwitz MA, Harth G: A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun. 2003, 71: 1672-1679. 10.1128/IAI.71.4.1672-1679.2003.
Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S: Enhancing the protective efficacy of Mycobacterium bovis BCG vaccination against tuberculosis by boosting with the Mycobacterium tuberculosis major secretory protein. Infect Immun. 2005, 73: 4676-4683. 10.1128/IAI.73.8.4676-4683.2005.
Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S: A novel live recombinant mycobacterial vaccine against bovine tuberculosis more potent than BCG. Vaccine. 2006, 24: 1593-1600. 10.1016/j.vaccine.2005.10.002.
Skeiky YA, Sadoff JC: Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006, 4: 469-476. 10.1038/nrmicro1419.
Collins DM, de Lisle GW, Aldwell FE, Buddle BM: A new attenuated Mycobacterium bovis vaccine protects brushtail possums (Trichosurus vulpecula) against experimental tuberculosis infection. Vaccine. 2007, 25: 4659-4664. 10.1016/j.vaccine.2007.04.014.
Nuwaysir EF, Huang W, Albert TJ, Singh J, Nuwaysir K, Pitas A, Richmond T, Gorski T, Berg JP, Ballin J, McCormick M, Norton J, Pollock T, Sumwalt T, Butcher L, Porter D, Molla M, Hall C, Blattner F, Sussman MR, Wallace RL, Cerrina F, Green RD: Gene expression analysis using oligonucleotide arrays produced by maskless photolithography. Genome Res. 2002, 12: 1749-1755. 10.1101/gr.362402.
Albert TJ, Norton J, Ott M, Richmond T, Nuwaysir K, Nuwaysir EF, Stengele KP, Green RD: Light-directed 5'-->3' synthesis of complex oligonucleotide microarrays. Nucleic Acids Res. 2003, 31: e35-10.1093/nar/gng035.
Molla M, Shavlik J, Richmond T, Smith S: A self-tuning method for one-chip SNP identification. Proc IEEE Comput Syst Bioinform Conf. 2004, 69-79.
Acknowledgements
This work was supported by an award from the National Natural Science Foundation of China (NSFC) (to JL), and research grants from Canadian Institutes of Health Research (CIHR) (MOP-15107 and MOP-82772 to JL), and a grant (Z0005190043521) from Beijing Municipal Science And Technology Commission to (BZ).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Authors' contributions
ASL, VT, ZW, and XY performed the experiments and participated in data analysis. DCA participated in data analysis and co-authored the manuscript. GFG oversaw the experiments. BZ oversaw the experiments and participated in data analysis. JL oversaw the experiments, analyzed the data, and wrote the manuscript.
Andrea S Leung, Vanessa Tran, Zuowei Wu, Xuping Yu contributed equally to this work.
Electronic supplementary material
12864_2008_1606_MOESM1_ESM.tiff
Additional file 1: Reference (M. tb H37Rv) to test (BCG-Birkhaug) ratio plot showing the 110 bp deletion within whiB3 gene. (TIFF 98 KB)
12864_2008_1606_MOESM2_ESM.tiff
Additional file 2: DNA sequencing chromatograph showing the single nucleotide (G) insertion within the phoP gene in BCG-Prague. This SNP was confirmed by repeating the PCR amplification and DNA sequencing. (TIFF 56 KB)
12864_2008_1606_MOESM3_ESM.doc
Additional file 3: DNA primers used for PCR amplification and DNA resequencing of identified novel polymorphisms. (DOC 20 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Leung, A.S., Tran, V., Wu, Z. et al. Novel genome polymorphisms in BCG vaccine strains and impact on efficacy. BMC Genomics 9, 413 (2008). https://doi.org/10.1186/1471-2164-9-413
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2164-9-413