Abstract
Background
Hydrogen peroxide (H2O2) is a reactive oxygen species (ROS), which is part of the oxidative burst encountered upon internalization of Salmonella enterica serovar Typhimurium (S. Typhimurium) by phagocytic cells. It has previously been established that, the ArcAB two-component system plays a critical role in ROS resistance, but the genes regulated by the system remained undetermined to date. We therefore investigated the ArcA regulon in aerobically growing S. Typhimurium before and after exposure to H2O2 by querying gene expression and other physiological changes in wild type and ΔarcA strains.
Results
In the ΔarcA strain, expression of 292 genes showed direct or indirect regulation by ArcA in response to H2O2, of which 141were also regulated in aerobiosis, but in the opposite direction. Gene set enrichment analysis (GSEA) of the expression data from WT and ΔarcA strains, revealed that, in response to H2O2 challenge in aerobically grown cells, ArcA down regulated multiple PEP-PTS and ABC transporters, while up regulating genes involved in glutathione and glycerolipid metabolism and nucleotide transport. Further biochemical analysis guided by GSEA results showed that deletion of arcA during aerobic growth lead to increased reactive oxygen species (ROS) production which was concomitant with an increased NADH/NAD+ ratio. In absence of ArcA under aerobic conditions, H2O2 exposure resulted in lower levels of glutathione reductase activity, leading to a decreased GSH (reduced glutathione)/GSSG (oxidized glutathione) ratio.
Conclusion
The ArcA regulon was defined in 2 conditions, aerobic growth and the combination of peroxide treatment and aerobic growth in S. Typhimurium. ArcA coordinates a response that involves multiple aspects of the carbon flux through central metabolism, which ultimately modulates the reducing potential of the cell.
Similar content being viewed by others
Background
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a Gram-negative, facultative anaerobe and intracellular bacterium that causes gastroenteritis, bacteremia and enteric fever in the murine model [1]. During its infective cycle, S. Typhimurium is internalized by phagocytes where it is exposed to a series of antimicrobial compounds including reactive oxygen species (ROS) which trigger the production of superoxide (O2-) by phagocytic NADPH oxidase. O2- is unstable with a half life in the order of milliseconds, and under acidic conditions, as those found within the Salmonella containing vacuole (SCV), two molecules of O2- react to generate H2O2[2]. Additionally, the S. Typhimurium genome codes for both cytoplasmic (sodA and sodB) and periplasmic (sodCI and sodCII) superoxide dismutases, that catalyze the generation of H2O2 and molecular oxygen from O2-[3–5].
The response of the bacterium to H2O2 has been mostly related to the transcription factor OxyR [6], however, several studies in Escherichia coli (E. coli), S. Typhimurium, S. Enteritidis and Haemophilus influenzae indicate that the response regulator ArcA is required for H2O2 resistance [7–10]. Furthermore, in E. coli H2O2 resistance depends on the cognate sensor ArcB [7]. The ArcAB two-component system is composed of the response regulator ArcA and the hybrid sensor kinase ArcB [11, 12]. ArcAB responds to shifts in oxygen concentration [13, 14], however, the signal that activates the system remains elusive since some studies show no correlation between ArcAB activity and the ubiquinone pool [13], while others indicate that the system’s activity depends on the ubiquinone and/or menaquinone pools [15–17]. Upon reduction of the redox-active cysteine residues between two monomers, ArcB undergoes autophosphorylation in an ATP-dependent intramolecular reaction at a conserved histidine residue located at position 292 [18, 19]. The signal is transferred to residues D576 and H717 of ArcB and finally to residue D54 of ArcA [12, 19, 20]. Phosphorylated ArcA (ArcA-P) forms a tretamer of dimers in a 1:1 ratio of ArcA and ArcA-P, which binds to promoter regions, thereby regulating gene expression [21, 22].
Several studies in E. coli, S. Typhimurium, H. influenzae and Shewanella oneidensis have used global gene expression profiling to determine the ArcA regulon under anaerobic conditions, showing that the effect of ArcA is pleiotropic and coordinates a response that includes changes in cellular metabolism, motility and chromosomal replication, among others [8, 10, 23–26]. In S. Typhimurium 14028s grown under anaerobic conditions, ArcA regulates either directly or indirectly more than 392 genes. Additionally, an arcA mutant has a longer doubling time than the wild type strain, lacks flagella, is non-motile and remains fully virulent [8].
In contrast to the vast amount of information about the role of ArcA in anaerobiosis, little is known about the genes or biochemical processes that ArcA regulates in response to H2O2. Previous studies have mainly shown that arcA or arcB mutant strains are more sensitive to the toxic compound [8–10]. One study conducted in E. coli used a proteomic approach and determined that ArcA regulates the expression of fliC, oppA and gltI in response to H2O2[7], while in S. Typhimurium ArcA negatively regulates ompD and ompW[27, 28]. To gain further insights into the role of ArcA in ROS resistance, we compared transcriptional changes in S. Typhimurium 14028s wild-type and ΔarcA strains with and without peroxide exposure under aerobic conditions. As expected, the ΔarcA mutation affected multiple pathways confirming that ArcA has a pleiotropic effect and plays a role as a global regulator. Interestingly, the genes regulated by ArcA in response to H2O2 differ from those regulated under anaerobic conditions [8]. A Gene Set Enrichment Analyses using the KEGG database predicted that 10 pathways were up-regulated and 2 down-regulated by ArcA in response to H2O2 treatment in aerobiosis. Finally, biochemical analyses showed that under aerobic conditions ArcA modulates the redox potential of the cell by regulating the levels of NADH and of intracellular ROS. After H2O2 exposure under aerobic conditions, ArcA was found to regulate turnover of reduced glutathione (GSH).
Methods
Bacterial strains and growth conditions
Pre-cultures of strains 14028s wild type and ΔarcA were streaked from cryo-vials stored at −80°C onto LB agar plates and allowed to grow at 37°C for 12 h. One colony was picked and grown in a 250 ml Erlenmeyer flask containing 25 ml of LB broth for 16 h at 37°C on a rotary shaker at 200 rpm. Exactly 500 μl of the cultures were then transferred into 500 ml Erlenmeyer flasks containing 50 ml of LB broth and grown in a temperature controlled rotary shaker at 200 rpm (LSI-3016R, Labtech Shaking Incubator, Indonesia). Optical density (OD600) was measured until reaching the desired OD600 for treatment with H2O2 (~ 0.4, corresponding to an incubation time of about 2.5 h). These conditions closely resemble those used in a previous study with E. coli, where 50 ml of culture grown at 200 rpm in a shaking incubator at 37°C to an OD546 of ~ 0.4 exhibited a pO2 of ≥ 90% [29]. Solid media contained agar (20 g l-1), and plates were incubated at 37°C. When necessary, growth media was supplemented with the appropriate antibiotics.
Microarray analysis
Overnight cultures of strains 14028s and ΔarcA were diluted (1:100) and cells were grown to OD600 ~ 0.4 as described. At this point, H2O2 (1 mM) was added and cells were grown for 20 min. Control cells received no treatment. Experiments were performed in triplicate on different days. After exposure to the toxic compound, 5 ml of ice cold 5% (v/v) phenol pH 4.3 / 95% (v/v) ethanol was added to 25 ml of culture and left on ice for 20 min. Subsequently, 8 ml of this solution were centrifuged for 10 min at 8000 rpm, the supernatant was removed and the bacterial pellet was resuspended with 200 μl of 10 mM Tris–HCl (pH 8.0) that included 4 μl of lysozyme (50 mg/ml). The reaction was incubated for 10 min at 37°C, and total RNA was extracted using the High Pure RNA Isolation kit (Roche) following the manufacturer’s instructions. RNA was eluted in 105 μl of water and treated with DNaseI (Roche) at 37°C for 30 min. Total RNA was recovered using the Qiagen RNeasy kit (Qiagen), following the manufacturer’s instructions. RNA was eluted in 80 μl and subjected to a second round of DNaseI treatment (Ambion Turbo DNA-free kit) at 37°C for 30 min, purified, recovered using the Qiagen RNeasy kit (Qiagen) following the manufacturer’s instructions and eluted in 55 μl of water.
Exactly 20 μg of total RNA were used for labeling with Cy3 or Cy5. Briefly, the RNA volume was adjusted to 30 μl, 2 μl of random hexamers N6 (Sigma, 2 μg/μl) were added and the mixture was incubated for 10 min at 70°C. Subsequently, cDNA was generated using Superscript II (Invitrogen) following the manufacturer’s instructions. Final nucleotide concentrations of the reaction were 0.5 mM dATP, dTTP, dGTP and 0.2 mM dCTP. After addition of the master mix, 4 μl of 5 mM dye labeled dCTP (Cy3 or Cy5) were added to the reaction and the mixture was incubated at 42°C for 60 min. After this time, 2 μl of Superscript II were added and the reaction was incubated at 42°C for an additional 60 min. The reaction was stopped by adding 3 μl of 1 M NaOH and incubating at 70°C for 10 min. The pH was neutralized by adding 3 μl of 1 M HCl. The labeled cDNA was purified using the Qiagen PCR purification kit following the manufacturer’s instructions. The purified labeled cDNA (4 μg) was hybridized to a ~ 387.000 50-mer NimbleGen microarray (Roche NimbleGen), tiling the S. Typhimurium 14028s genome at overlapping intervals of about 12 bases on both strands, as previously described [30].
Data acquisition and analysis
Arrays were scanned using a GenePix 4000B laser scanner (Molecular Devices, Sunnyvale, California) at 5 μm resolution. Signal intensities were quantified using NimbleScan software v2.4 (Roche NimbleGen). Intensity values were background subtracted, normalized within (median) and between (quantile) the arrays using WebarrayDB [31], and converted to log2 values. For each array, the background was calculated as follows: log2 median intensity value for negative control probes + (3 * log2 intensity value standard deviation negative control probes). Negative control probes correspond to the probes located on the non-coding strand of each gene in the array. Genes with intensity values over the background were included in the analysis. After array data acquisition and normalization, two-way ANOVA was performed using MeV TM4 software [32], to determine uncorrected p-values. For the analysis, two categories were considered (genotype and treatment), each with two sub-categories. False Discovery Rate (FDR) adjusted q values were calculated using QVALUE in Bioconductor [33]. Genes with a q value ≤ 0.05 for interaction and a ratio of ≥ 2 between the fold change of strains 14028s wild type and ΔarcA ((wild type treated/wild type control)/(ΔarcA treated/ ΔarcA control)) were considered to be differentially regulated in response to H2O2. Genes with a q-value of ≤ 0.05 for genotype and a fold change of ≥ 2 between strains ΔarcA and wild type (ΔarcA control/wild type control) without treatment were considered to be differentially regulated in aerobiosis. The microarray data has been deposited in GEO (http://www.ncbi.nlm.nih.gov/geo/) and is accessible via GEO Accession Number GSE34134.
Prediction of metabolic pathways altered in the different strains by treatment with H2O2 or due to the mutation of arcA was performed using the software Gene Set Enrichment Analysis (GSEA) [34], with the KEGG database for S. Typhimurium LT2 as a reference. Briefly, GSEA is a computational method that determines whether an a priori defined set of genes shows statistically significant, concordant differences between two biological states [34]. To determine the pathways regulated by ArcA in response to H2O2, the log2 values of all replicas were averaged and treated as follows: (log2 14028s wild type H2O2 aerobic - log2 14028s wild type aerobic) - (log2 ΔarcA H2O2 aerobic - log2 ΔarcA aerobic). Positive Normalized Enrichment Score (NES) values represent pathways up-regulated by ArcA, while negative NES values represent pathways negatively regulated upon H2O2 treatment under aerobic conditions. To determine the pathways regulated under aerobic conditions, the log2 values of all replicas of untreated cells were averaged and treated as follows: (log2 ΔarcA aerobic - log2 14028s wild type aerobic). Pathways with an FDR of ≤ 0.25 as determined by GSEA were considered to present significant changes.
Real time quantitative RT-PCR
qRT-PCR was performed using the primers listed in Additional file 1: Table S1 as previously described [28], with a minor modification of the PCR program. Briefly, relative quantification was performed using Brilliant II SYBR Green QPCR Master Reagent Kit and the M×3000P detection system (Stratagene). 16S rRNA was used for normalization. The reaction mixture was carried out in a final volume of 20 μl containing 1 μl of diluted cDNA (1:1000), 0.24 μl of each primer (120 nM), 10 μl of 2 × Master Mix, 0.14 μl of diluted ROX (1:200) and 8.38 μl of H2O. The reaction was performed under the following conditions: 10 min at 95°C followed by 40 cycles of 30 s at 95°C, 30 s at 58°C and 30 s at 72°C. Finally, a melting cycle from 65°C to 95°C was performed to check for amplification specificity. Amplification efficiency was calculated from a standard curve constructed by amplifying serial dilutions of RT-PCR products for each gene. These values were used to obtain the fold-change in expression for the gene of interest normalized with 16S levels according to Pfaffl [35].
Promoter analysis
A positional weight matrix was generated using the ArcA-binding sites predicted in E. coli for which footprinting experiments are available reviewed in [36]. Additionally, the binding sites predicted for members of the ArcA regulon in S. Typhimurium 14028s in anaerobiosis were also included [8], as was that of the ompW promoter region, which was shown to be functional [28]. The upstream sequences of the genes regulated by ArcA in response to aerobiosis or H2O2 exposure under aerobic conditions (Additional file 2: Table S2) were retrieved (positions −400 to −1 with respect to the translation start site) from the sequenced and annotated genome of S. Typhimurium 14028s [37]. Promoter regions with less than 20 nt between the translation start site of the ORF under analysis and the end or start of the upstream ORF were not included in the analysis. Binding sites at the promoter regions of genes regulated by ArcA in response to aerobiosis or H2O2 exposure under aerobic conditions (Additional file 2: Table S2) were predicted using the Matrix-scan software [38] available at http://rsat.ulb.ac.be/. The parameters used for the analysis were those given by default by the software. Binding sites with a p-value of ≤ 0.0001 were considered significant and reported as predicted ArcA binding sites.
Biochemical determinations
Overnight cultures of strains 14028s wild type and ΔarcA were diluted (1:100) and cells were grown to OD600 ~ 0.4. At this point, H2O2 (1.0 mM) was added and cells were grown for 20 min. Control cells received no treatment. Experiments were performed in triplicate on different days. After treatment, 6 ml of cultures were withdrawn for each analysis and used for measurement of NADH, glutathione (GSH) and glutathione reductase (GR) activity. NADH was measured using commercially available kits by Abcam. The ratio between reduced glutathione and oxidized glutathione (GSH/GSSG) and GR activity were measured using commercially available kits by Cayman Chemicals. In all cases, measurements were performed following the instructions provided by the manufacturers without modifications.
Measurement of intracellular ROS was performed using the oxidant-sensitive probe H2DCFDA, as previously described, with minor modifications [39]. Briefly, aerobically grown cells in LB at OD600 ~ 0.4 were incubated with 10 μM H2DCFDA. At 10 min intervals aliquots were taken, washed with 10 mM potassium phosphate buffer, pH 7.0, resuspended in the same buffer, and disrupted by sonication. Cell extracts (100 μl) were mixed with 1 ml phosphate buffer and fluorescence was measured using a TECAN Infinite 200 PRO Nanoquant microplate reader (excitation, 480 nm; emission, 520 nm). Emission values were normalized based on the bacterial concentration as determined by the Optical Density (OD) of the culture at 600 nm.
Results
To analyze the role of ArcA in the transcriptional response to aerobiosis and H2O2, the ArcA regulon of S. Typhimurium was determined by microarray analysis. Expression profiles were measured from three independent samples of aerobically grown wild-type (14028s) and ΔarcA strains with or without 1 mM H2O2. After normalization, 3949 genes showed intensity values over the background in at least one array and were included in the analysis. The results were validated by randomly selecting eight genes and measuring the transcript levels by qRT-PCR (Additional file 1: Table S1). A statistically significant correlation was observed between microarray and qRT-PCR data (r2 = 0.7, p-value ≤ 0.0001), despite quantitative differences in the level of change, suggesting that the results obtained by microarray analysis reflect the actual changes in gene expression.
In the wild type strain, expression of 381 and 667 genes was up- or downregulated, respectively, in response to H2O2 under aerobic conditions (fold change ≥ 2, FDR q-value treatment ≤ 0.05, Additional file 2: Table S2). Several genes known to be upregulated by H2O2 and required for its resistance were among the upregulated genes in the wild type and ΔarcA strains, including katE, katG and mntH[40]. A previous study investigated the effect of H2O2 on gene expression in a different S. Typhimurium strain, 4/74 [41]. That study found 309 genes to be upregulated after H2O2 exposure, and 428 genes to be downregulated. The concurrence with our results in strain 14028s is about 30%: 119/381 upregulated 14028s genes and 191/667 downregulated 14028s genes had been found to be similarly regulated in strain 4/74. The observed differences in the number of genes differentially expressed might be explained by several factors including OD600 of treatment (0.4 vs 0.1), time of H2O2 challenge (20 min vs 12 min), threshold for considering a gene differentially expressed (fold change of ≥ 2 vs ≥ 3), and different S. Typhimurium strains used in the studies (14028s vs 4/74).
Role of ArcA during aerobic conditions
The expression of 220 and 122 genes was up- or downregulated, respectively, by ArcA under aerobic conditions (Additional file 2: Table S2). Comparison with the ArcA regulon of strain 14028s in anaerobiosis [8] showed that 63 genes were regulated under both conditions, but the expression of 38 genes was regulated in the opposite direction. Of the 220 genes upregulated by ArcA in aerobiosis, only 15 are positively regulated by ArcA under anaerobic conditions (Figure 1A), while of the 122 genes downregulated by ArcA under aerobic conditions, only 10 are also downregulated by ArcA in anaerobiosis (Figure 1B).
Overlap of the ArcA regulon in response to H 2 O 2 , aerobiosis and anaerobiosis. Numbers indicate the amount of genes that show statistically significant differential expression in a ΔarcA mutant vs a wild type strain 14028s in the respective condition. The number of genes (A) positively or (B) negatively regulated by ArcA in each condition is shown. Genes regulated by ArcA in anaerobiosis were obtained from [8]. Genes regulated by ArcA in aerobiosis with or without H2O2 are detailed in Additional file 2: Table S2. Genes with a fold change of ≥ 2 and an FDR q value of ≤ 0.05 for each category were considered to be differentially expressed.
To deduce the biological pathways altered in the arcA mutant as compared to the aerobically grown wild type strain under aerobic conditions, a Gene Set Enrichment Analysis (GSEA) was performed using the KEGG database for S. Typhimurium LT2 as a reference. It should be noted that to determine the pathways regulated by ArcA under aerobic conditions, we compared the transcript levels of the arcA mutant to those in the wild type strain (log2 ΔarcA aerobic – log2 wild type aerobic). Thus, pathways repressed by ArcA possess positive NES values (i.e., > 0, glycolysis), while pathways activated by ArcA have negative NES values (i.e., < 0, ABC transporters). GSEA showed that 12 and 8 pathways were up- or downregulated, respectively, by ArcA under aerobic conditions (Table 1). Among the pathways upregulated by ArcA were those implicated in the transport of amino acids and sugars, oligopeptides and metals, including PTS and ABC transporters, among others (Table 1, negative NES values). Under aerobic conditions, ArcA mainly repressed pathways implicated in central metabolism and nucleotide biosynthesis (Table 1, positive NES values). In particular, the transcript levels of genes encoding proteins of the payoff phase of glycolysis [42] and glycerolipid metabolism were higher in the arcA mutant grown under aerobic conditions than in strain 14028s (Figure 2, Addition file 2 Table S2), including pykF (pyruvate kinase), aceEF-lpdA (pyruvate dehydrogenase complex), eno (enolase), glpD and glpABC (glycerol 3-phosphate dehydrogenase). The data suggests that the aerobically grown ΔarcA mutant presents a higher flux through glycolysis and increased levels of NADH than the wild type strain. Interestingly, genes of the Krebs cycle, which are negatively regulated by ArcA under anaerobic conditions [8], were not repressed in aerobiosis (Table S2). Together, these results indicate that ArcA has a major role in regulating gene expression under aerobic conditions and that the genes regulated in aerobiosis are different from those regulated in anaerobiosis.
Effect of ArcA on the expression of genes of central metabolism, sugar uptake and reductive pathways in aerobiosis with or without H 2 O 2 . Selected genes involved in glycolysis, glycerolipid-, GSH- and thioredoxin- (Trx-[SH]2) metabolism, ABC transport and PTS are depicted. Statistically significant differences between strains 14028s and ΔarcA in aerobiosis with or without H2O2 are shown (Table S2). Fold changes are depicted for the aerobically grown wild type strain after H2O2 treatment (upper value), ΔarcA strain after H2O2 treatment (middle value), and aerobically grown untreated ΔarcA/14028s (lower value). Boxes indicate genes regulated by ArcA (Fold change ≥ 2, FDR q-value ≤ 0.05) in aerobiosis (blue), aerobiosis with H2O2 (red) and under both conditions (black).
Role of ArcA in the response to H2O2 under aerobic conditions
It has been well established that an aerobically grown ΔarcA strain is sensitive to H2O2 treatment [7–10], however, the genes regulated by ArcA under this condition have not been determined. Our analysis shows that ArcA directly or indirectly regulates the expression of 292 genes in response to H2O2 in aerobically grown cells, 117 and 175 genes were up- or downregulated, respectively (Figure 1, Additional file 2: Table S2). Surprisingly, almost no correlation was observed between the genes regulated by ArcA in aerobiosis with or without H2O2: only 6 genes were upregulated under both conditions (Figure 1A), while 7 genes were downregulated (Figure 1B). Furthermore, comparison of the ArcA regulon in aerobiosis, anaerobiosis [8] and aerobiosis with H2O2 showed that no genes were up or downregulated in all three conditions (Figure 1A and B). This suggests that the genes regulated by ArcA in response to various stimuli are different and do hardly overlap (Figure 1), which underscores the importance and versatility of ArcA-mediated regulation.
To determine the pathways regulated by ArcA in response to H2O2 under aerobic conditions, the intensity values were treated as follows: (log2 14028s wild type H2O2 aerobic - log2 14028s wild type aerobic) - (log2 ΔarcA H2O2 aerobic - log2 ΔarcA aerobic). Therefore, in contrast to the pathways regulated by ArcA under aerobic conditions, the pathways positively regulated by ArcA in response to H2O2 under aerobic conditions have positive NES values, while the pathways negatively regulated by ArcA in response to H2O2 under aerobic conditions have negative NES values. Based on the transcriptomic data (Additional file 2: Table S2), GSEA deduced that 10 and 2 pathways were up- or downregulated, respectively, by ArcA in aerobically grown cells after H2O2 exposure (Table 1). The pathways deduced to be upregulated by ArcA in response to H2O2 are implicated in nucleotide and siderophore biosynthesis, central and glutathione metabolism, among others, while the pathways downregulated by ArcA were PTS and ABC transporters (Figure 2, Table 1). Interestingly, only one gene (ahpF) required for H2O2 degradation was upregulated by ArcA in aerobiosis after H2O2 treatment (Additional file 2: Table S2), suggesting that ArcA is not required for ROS scavenging. Of particular interest are the genes most upregulated by ArcA in aerobiosis with H2O2 in the pathways of nucleotide and glutathione metabolism, coding for the alternative ribonucleotide reductase (nrdEF) and glutathione reductase (gor). In addition, the gene coding for thioredoxin reductase (trxB), required for reduction of oxidized thioredoxin (Trx-[S]2), was also upregulated by ArcA after peroxide exposure. In E. coli, nrdEF is upregulated in response to H2O2[43, 44] and allows replication in iron-limiting conditions when manganese is present [44]. Gor and TrxB are required for the turnover of oxidized glutathione (GSSG) and Trx-[S]2, respectively (reduction from GSSG to reduced glutathione (GSH), and Trx-[S]2 to reduced thioredoxin (Trx-[SH]2), respectively), which participate in the reduction of disulfide bonds [45]. This suggests that in aerobically grown cells exposed to H2O2, ArcA regulates the GSH/GSSG and Trx-[S]2/Trx-[SH]2 ratio, modulating the redox status of the cell, and the expression of nrdEF.
Promoter analysis
To determine the genes that may be directly regulated by ArcA, we identified the subset of ArcA-dependently differentially expressed genes in aerobiosis with or without H2O2 with predicted ArcA-binding sites in the upstream regions (Additional file 2: Table S2), using the sequenced genome of S. Typhimurium 14028s [37] and Matrix-scan [38], as detailed in Methods. The analysis predicted that ArcA directly regulates the expression of 6 genes in aerobiosis with H2O2 and 19 genes in aerobically grown cells (Table 2).
Biochemical analysis of the ΔarcA strain
The microarray analysis predicted that in response to H2O2 under aerobic conditions, ArcA regulates the expression of genes implicated in GSH metabolism. It also predicted that in aerobiosis, ArcA regulates expression of genes coding proteins involved in glycolysis (Table 1 and Additional file 2: Table S2). To evaluate if the changes in gene expression correlated with changes in the products of these pathways, we determined the levels of GSH, glutathione reductase (GR) activity, NADH and total intracellular ROS in the wild type and ΔarcA strains in aerobiosis with and without H2O2.
The gene gor was upregulated by ArcA under aerobic conditions with H2O2 (Figure 2, Additional file 2: Table S2), therefore decreased levels of both GSH and GR activity in the arcA mutant exposed to H2O2 under aerobic conditions were expected. The levels of GR activity were indeed lower in the aerobically grown ΔarcA strain after H2O2 treatment, although the levels of GR activity were also decreased in the ΔarcA mutant under aerobic conditions (Figure 3A). GSH remained almost unaltered in the wild type strain after treatment with the toxic compound, conversely, the aerobically grown ΔarcA mutant treated with H2O2 showed significantly reduced levels of GSH and increased GSSG, consistent with lower GR activity (Figure 3A and B). In agreement, the GSH/GSSG ratio was lower in the aerobically grown ΔarcA strain after H2O2 treatment (Figure 3C), while the levels of total glutathione were similar between the wild type and ΔarcA mutant strains (4.68 and 4.45 μmol/mg. protein, respectively). This indicates that GSH turnover is altered in an aerobically grown ΔarcA strain with H2O2 due to lower GR activity.
Role of ArcA in modulating the redox status in aerobiosis and in response to H 2 O 2 . Strains 14028s and ΔarcA were grown to OD600 ~ 0.4 and treated with 1.0 mM H2O2 for 20 min. Control cells received no treatment. The levels of (A) glutathione reductase (GR) activity, (B) reduced glutathione (GSH), oxidized glutathione (GSSG), (C) the GSH/GSSG ratio and (D) the NADH/NAD+ ratio were measured. (E) Total ROS amount was measured under aerobic conditions. Values are the mean ± SD of three independent experiments. Values were normalized by protein concentration (A, B, C, D) or OD600(E). AU: arbitrary units. GR activity: nmol NADPH min-1 ml-1 mg protein-1. *p ≤ 0.05, **p ≤ 0.01, as compared to aerobically grown wild type cells.
The transcript levels of genes coding the pyruvate dehydrogenase complex (PDH), proteins of the payoff phase of glycolysis [42] and sugar uptake were higher in the aerobically grown ΔarcA mutant than in the wild type strain (Figure 2, Additional file 2: Table S2). This suggests that under aerobic conditions a ΔarcA strain has a higher flux through glycolysis, which in turn could result in higher levels of acetyl-CoA and an elevated NADH generation in the Krebs cycle. As predicted, the NADH/NAD+ ratio was significantly lowered in the aerobically grown wild type strain after peroxide treatment (Figure 3D), compared to untreated aerobically grown wild type cells. In the ΔarcA mutant, the NADH/NAD+ ratio was higher than in the wild type strain in aerobically grown cells before and after H2O2 treatment (Figure 3D). Although there was an overall decrease in the NADH/NAD+ ratio in the ΔarcA strain after H2O2 treatment, the ratio remained 2-fold higher than in wild type cells under aerobic conditions without H2O2 treatment.
In E. coli, one of the sources of O2- is oxidation of the respiratory electron transport chain and the conversion of NADH to NAD+[46]. Since under aerobic growth conditions a ΔarcA strain has higher levels of NADH (Figure 3D) and ndh transcript than the wild type strain (Additional file 2: Table S2), we hypothesized that a ΔarcA mutant might present increased levels of total ROS. In agreement, in an aerobically grown ΔarcA strain, total ROS was increased as compared to the isogenic wild type strain under the same condition (Figure 3E), indicating that the absence of ArcA generates a metabolic imbalance which leads to increased levels of ROS.
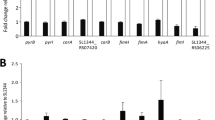
In order to complement the ΔarcA mutation, we first evaluated the mechanism by which ArcA regulates gene expression in response to ROS. Our results show that in S. Typhimurium 14028s, arcA expression is not increased either with H2O2 or hypochlorous acid (Additional file 1: Figure S1A). In addition, the levels of ArcA also remained constant after exposure to both ROS (Additional file 1: Figure S1B). This suggests that rather than changes in expression, ArcA is activated in response to ROS, most likely by phosphorylation of residue D54. To test this hypothesis, the ΔarcA mutant strain was complemented in trans with the wild type gene and a version coding a substitution of residue D54 of ArcA (D54A), and the number of colony forming units (CFU/ml) was determined after H2O2 exposure. As predicted, only complementation with the wild type gene resulted in similar CFU/ml as in strain 14028s (Additional file 1: Figure S1C), however, there were also differences in the number of CFU/ml at the initial time points. This is most likely caused by increased levels of ArcA due to complementation with a high copy number vector. Since the effect of ArcA is pleiotropic and its levels remain constant throughout all stresses evaluated (Additional file 1: Figure S1 A and B), achieving wild type levels of ArcA is required to properly address its role in the response to ROS.
Discussion
Several reports have demonstrated that the global regulator ArcA is required for H2O2 resistance [7–10], however, only a few have evaluated its role on regulating gene expression under this condition [27, 28]. One study conducted in E. coli used a proteomic approach to evaluate the mechanism underlying the role of ArcA in response to ROS [7]. Herein, we report the first genome-wide study addressing the role of ArcA in response to H2O2 under aerobic conditions. ArcA regulates different genes after ROS exposure in aerobiosis, under aerobic growth in rich media and under anaerobiosis (Figure 1, 2 and 4; Additional file 2: Table S2). In this discussion, we will focus on the genes and pathways regulated by ArcA that contribute to ROS resistance of S. Typhimurium. A full list of the genes regulated by ArcA in aerobiosis with and without H2O2 is provided in Additional file 2: Table S2.
Model showing the major differences in the processes regulated by ArcA after H 2 O 2 exposure under aerobic conditions, aerobiosis and anaerobiosis in S. Typhimurium 14028s. Each symbol represents groups of genes that are functionally related and are colored based on the degree of regulation by ArcA under each condition. Data from anaerobic conditions was obtained from [8]. Blue shows that the genes are positively regulated by ArcA, yellow shows that the genes are not affected by ArcA, and red shows that the genes are negatively regulated by ArcA. G-3-P: glyceraldehyde 3 phosphate, PDH: pyruvate dehydrogenase complex, TCA cycle: tricarboxylic acid cycle, GSSG: oxidized glutathione, Trx-[S]2: oxidized thioredoxin.
Role of ArcA in ROS scavenging
The S. Typhimurium genome codes for several genes that degrade H2O2 or organic hydroperoxide, including catalases (katG, katE and katN), alkyl hydroperoxide reductases (ahpCF and tsaA) [47] and a glutathione-dependent peroxidase (btuE) [48]. Only ahpF and katE are predicted to be regulated by ArcA in aerobiosis with or without H2O2, respectively (Additional file 2: Table S2). Expression of ahpF and katE is also known to be regulated by RpoS [49, 50]. Neither katN nor sodA, previously described as members of the ArcA regulon under anaerobic conditions [8, 51], were found to be regulated by ArcA under aerobic conditions with or without H2O2 treatment. These results are in agreement with studies in E. coli that demonstrate that an arcA mutant does not show defects in H2O2 scavenging [7].
Role of ArcA in maintaining GSH and thioredoxin levels
ArcA positively regulates the expression of the genes gor (GR) and trxB (thioredoxin reductase) in aerobiosis with H2O2, but not without the toxic compound (Figure 2 and 4 Additional file 2: Table S2). However, the levels of GR activity were lower in the arcA mutant strain grown under aerobic conditions (Figure 3A), but not the levels of GSH turnover (Figure 3B). This suggests that there are other unidentified factors that alter GR activity in the arcA mutant grown under aerobic conditions, since there are no differences in the transcript levels of the gene gor between strains 14028s and ΔarcA (Additional file 2: Table S2). In addition, this indicates that the lower levels of GR activity in the arcA mutant grown under aerobic conditions are sufficient to cope with GSH turnover, and the effect is only evident when GSH oxidation is increased, as when cells are exposed to peroxide.
In E. coli, OxyR regulates gor expression [6], while the expression of trxB has not been determined under this condition. Two putative ArcA binding sites were predicted at the promoter region of trxB (Table 2). Glutathione and thioredoxin reductases are required to reduce GSSG and thioredoxin in a NADPH-dependant manner, which in their reduced form participate in the reduction of cellular disulfide bonds [45] and of oxidized glutaredoxin. An E. coli Δgor mutant has a slight sensitivity towards paraquat and cumene hydroperoxide [52], while in stationary phase a ΔtrxB strain shows H2O2 sensitivity [53]. However, a double Δgor ΔtrxB mutant grows extremely poorly under aerobic conditions and presents increased alkaline phosphatase activity, indicative of increased disulfide bond formation, most likely due to increased ROS [54]. Since an aerobically grown ΔarcA mutant treated with H2O2 has lower transcript levels of gor and trxB, lower GR activity and lower GSH levels (Figure 2 and 3A, B and C), this might result in increased disulfide bond formation, protein inactivation and contribute to the increased sensitivity towards ROS. Since GSH is abundant in the cell and is readily oxidized by H2O2, this leads to a decrease in the levels of reduced glutathione, shifting the target of oxidation from GSH to essential macromolecules, leading to cell death [55]. This may occur earlier in a ΔarcA mutant, as its level of “protective” GSH is low, caused by decreased GR activity.
ArcA and nucleotide metabolism
The pathways that showed the highest changes in the ΔarcA mutant treated with H2O2 under aerobic conditions were purine and pyrimidine metabolism (Figure 4, Table 1). The major differences are found in the expression of the nrdAB and nrdEFHI operons, coding for aerobic and alternative aerobic ribonucleotide reductase, respectively. In E. coli and S. Typhimurium, NrdAB is indispensable for growth under aerobic conditions while NrdEF is not functional [56]. In the aerobically grown wild type strain, nrdAB was repressed while nrdEF was up-regulated after H2O2 exposure, in agreement with studies in E. coli and S. Typhimurium 4/74 [41, 43], while the regulation was lost in the ΔarcA mutant under the same conditions (Additional file 2: Table S2). NrdEF is usually repressed by Fur. However, in response to H2O2, this Fur repression is abolished and the apoprotein form of IscR upregulates expression of the operon [44]. This suggests that in response to H2O2 under aerobic conditions, ArcA may act together with Apo-IscR, up-regulating the nrdEFHI operon.
ArcA and carbon metabolism
Under aerobic conditions, the transcript levels of genes coding proteins of glycerolipid metabolism, glycolysis and the PDH complex were higher in the ΔarcA mutant than in the wild type strain (Figure 2 and 4, Additional file 2: Table S2). This suggests that the flux through glycolysis and the levels of acetyl-CoA could be increased in the ΔarcA strain. Two studies conducted in E. coli measured the flux through the PDH complex in a ΔarcA mutant under aerobic conditions with different results. One showed that there was an increase in the flux through the PDH complex [14] while in the other no differences were observed [57], although both studies determined that there was an increase in the flux through the TCA cycle. Our analysis showed that the NADH/NAD+ ratio was 2-fold higher in the aerobically grown ΔarcA mutant than in the wild type strain (Figure 3D). After H2O2 exposure, the NADH/NAD+ ratio decreased in the wild type and ΔarcA strain, but in the latter the levels remained higher than in the wild type strain under aerobic conditions (Figure 3D). Since NADH can reduce Fe3+ to Fe2+in vitro[56], and elevated NADH levels result in increased sensitivity towards H2O2[58], the higher basal levels of NADH in the ΔarcA mutant in aerobiosis and after H2O2 treatment may increase Fe+2 turnover, fueling the Fenton reaction (the formation of OH·, and Fe3+ from the nonenzymatic reaction of Fe2+ with H2O2) and leading to higher levels of ROS-derived damage.
In the respiratory chain, NADH dehydrogenase II (encoded by ndh) generates O2- and H2O2 by oxidation of its reduced FADH2 cofactor [58]. In an aerobically grown ΔarcA strain, the levels of NADH and the ndh transcript (Additional file 2: Table S2) are higher than in the wild type strain under the same condition (Figure 3D). We therefore speculated that production of intracellular ROS might be increased. In agreement, a ΔarcA mutant presents statistically significant increased levels of total ROS as compared to the wild type strain 14028s (Figure 3E). These higher levels of ROS might present further disadvantages for the bacterium when exposed to H2O2. However, several other sources of intracellular ROS besides NADH dehydrogenase II may also contribute to the higher levels of ROS observed in the ΔarcA mutant, such as fumarate-reducing flavoenzymes [59].
Conclusion
We identified the ArcA regulon in S. Typhimurium under aerobic growth with and without H2O2, and show that ArcA coordinates a response that includes changes in cellular-, glutathione-, thioredoxin-, NADH- and glycerolipid metabolism. These changes contribute to H2O2 resistance by modulating the reducing potential of the cell.
Author’s contributions
EHM and CPS conceived the project. EHM and PD conducted the analysis of microarray data and prediction of regulated pathways. EHM, BC and ILC performed the experiments. FG, RL and SP conducted partial data analysis. EHM, SP, MM and CPS wrote the paper. All authors read and approved the final manuscript. The authors declare no conflict of interest.
References
Mackaness GB, Blanden RV, Collins FM: Host-parasite relations in mouse typhoid. J Exp Med. 1966, 124: 573-583. 10.1084/jem.124.4.573.
Fridovich I: The biology of oxygen radicals. Science. 1978, 201: 875-880. 10.1126/science.210504.
Hassett D, Cohen M: Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J. 1989, 3: 2574-2582.
Imlay J: Pathways of Oxidative Damage. Annu Rev Microbiol. 2003, 57: 395-418. 10.1146/annurev.micro.57.030502.090938.
Canvin J, Langford PR, Wilks KE, Kroll JS: Identification of sodC encoding periplasmic [CuZn]-superoxide dismutase in Salmonella. FEMS Microbiol Lett. 1996, 136: 215-220. 10.1111/j.1574-6968.1996.tb08052.x.
Christman MF, Morgan RW, Jacobson FS, Ames BN: Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985, 41: 753-762. 10.1016/S0092-8674(85)80056-8.
Loui C, Chang AC, Lu S: Role of the ArcAB two-component system in the resistance of Escherichia coli to reactive oxygen stress. BMC Microbiol. 2009, 9: 183-10.1186/1471-2180-9-183.
Evans MR, Fink RC, Vazquez-Torres A, Porwollik S, Jones-Carson J, McClelland M, Hassan HM: Analysis of the ArcA regulon in anaerobically grown Salmonella enterica sv. Typhimurium. BMC Microbiol. 2011, 21: 11-58.
Lu S, Killoran PB, Fang FC, Riley LW: The global regulator ArcA controls resistance to reactive nitrogen and oxygen intermediates in Salmonella enterica serovar Enteritidis. Infect Immun. 2002, 70: 451-461. 10.1128/IAI.70.2.451-461.2002.
Wong SM, Alugupalli KR, Ram S, Akerley BJ: The ArcA regulon and oxidative stress resistance in Haemophilus influenzae. Mol Microbiol. 2007, 64: 1375-1390. 10.1111/j.1365-2958.2007.05747.x.
Iuchi S, Matsuda Z, Fujiwara T, Lin EC: The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol Microbiol. 1990, 4: 715-727. 10.1111/j.1365-2958.1990.tb00642.x.
Iuchi S, Lin EC: Purification and phosphorylation of the Arc regulatory components of Escherichia coli. J Bacteriol. 1992, 174: 5617-5623.
Rolfe MD, Ter Beek A, Graham AI, Trotter EW, Shahzad Asif HM, Sanguinetti G, de Mattos JT, Poole RK, Green J, SysMO-SUMO: Transcript profiling and inference of Escherichia coli K-12 ArcA activity across the range of physiologically relevant oxygen concentrations. J Biol Chem. 2011, 286: 10147-10154. 10.1074/jbc.M110.211144.
Alexeeva S, Hellingwerf K, Mattos JT: Requirement of ArcA for Redox Regulation in Escherichia coli under Microaerobic but Not Anaerobic or Aerobic Conditions. J Bacteriol. 2003, 185: 204-209. 10.1128/JB.185.1.204-209.2003.
Georgellis D, Kwon O, Lin EC: Quinones as the Redox Signal for the Arc Two-Component System of Bacteria. Science. 2001, 292: 2314-2316. 10.1126/science.1059361.
Malpica R, Franco B, Rodriguez C, Kwon O, Georgellis D: Identification of a quinone-sensitive redox switch in the ArcB sensor kinase. Proc Natl Acad Sci. 2004, 101: 13318-13323. 10.1073/pnas.0403064101.
Bekker M, Alexeeva S, Laan W, Sawers G, Mattos JT, Hellingwerf K: The ArcBA Two-Component System of Escherichia coli Is Regulated by the Redox State of both the Ubiquinone and the Menaquinone Pool. J Bacteriol. 2010, 191: 746-754.
Peña-Sandoval G, Georgellis D: The ArcB Sensor Kinase of Escherichia coli Autophosphorylates by an Intramolecular Reaction. J Bacteriol. 2010, 192: 1735-1739. 10.1128/JB.01401-09.
Georgellis D, Lynch AS, Lin EC: In vitro phosphorylation study of the arc two-component signal transduction system of Escherichia coli. J Bacteriol. 1997, 179: 5429-5435.
Kwon O, Georgellis D, Lin EC: Phosphorelay as the Sole Physiological Route of Signal Transmission by the Arc Two-Component system of Escherichia coli. J Bacteriol. 2000, 182: 3858-3862. 10.1128/JB.182.13.3858-3862.2000.
Lynch AS, Lin EC: Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J Bacteriol. 1996, 178: 6238-6249.
Jeon Y, Lee Y, Han J, Kim J, Hwang D: Multimerization of Phosphorylated and Non-phosphorylated ArcA is Necessary for the Response Regulator Function of the Arc Two-Component Signal Transduction System. J Biol Chem. 2001, 276: 40873-40879. 10.1074/jbc.M104855200.
Oshima T, Aiba H, Masuda Y, Kanaya S, Sugiura M, Wanner BL, Mori H, Mizuno T: Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol Microbiol. 2002, 46: 281-291. 10.1046/j.1365-2958.2002.03170.x.
Liu X, De Wulf P: Probing the ArcA-P modulon of Escherichia coli by whole genome transcriptional analysis and sequence recognition profiling. J Biol Chem. 2004, 279: 12588-12597.
Salmon KA, Hung S, Steffen NR, Krupp R, Baldi P, Hatfield GW, Gunsallus R: Global gene expression profiling in Escherichia coli K12. J Biol Chem. 2005, 280: 15084-15096. 10.1074/jbc.M414030200.
Gao H, Wang X, Yang ZK, Palzkill T, Zhou J: Probing regulon of ArcA in Shewanella oneidensis MR-I by integrated genomic analyses. BMC Genomics. 2008, 9: 42-10.1186/1471-2164-9-42.
Calderón IL, Morales E, Caro NJ, Chahuán CA, Collao B, Gil F, Villareal JM, Ipinza F, Mora GC, Saavedra CP: Response regulator ArcA of Salmonella enterica serovar Typhimurium downregulates the expression of OmpD, a porin facilitating uptake of hydrogen peroxide. Res Microbiol. 2011, 162: 214-222. 10.1016/j.resmic.2010.11.001.
Morales EH, Calderón IL, Collao B, Gil F, Porwollik S, McClelland M, Saavedra CP: Hypochlorous acid and hydrogen peroxide-induced negative regulation of Salmonella enterica serovar Typhimurium ompW by the response regulator ArcA. BMC Microbiol. 2012, 12: 63-10.1186/1471-2180-12-63.
Berney M, Weilenmann HU, Ihssen J, Bassin C, Egli T: Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl Environ Microbiol. 2006, 72: 2586-2593. 10.1128/AEM.72.4.2586-2593.2006.
Santiviago CA, Reynolds MM, Porwollik S, Choi S, Long F, Andrews-Polymenis HL, McClelland M: Analysis of pools of targeted Salmonella deletion mutants identifies novel genes affecting fitness during competitive infection in mice. PLoS Pathog. 2009, 5: e1000477-10.1371/journal.ppat.1000477.
Xia XQ, McClelland M, Porwollik S, Song W, Cong X, Wang Y: WebArrayDB: cross-platform microarray data analysis and public data repository. Bioinformatics. 2009, 25: 2425-2429. 10.1093/bioinformatics/btp430.
Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J: TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003, 34: 374-378.
Dabney A, Storey JD, Warnes GR: qvalue: Q-value estimation for false discovery rate control. R package version 1.26.0. http://www.bioconductor.org/packages/release/bioc/html/qvalue.html
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP: Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005, 102: 15545-15550. 10.1073/pnas.0506580102.
Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29: e45-10.1093/nar/29.9.e45.
Wang X, Gao H, Shen Y, Weinstock GM, Zhou J, Palzkill T: A high-throuput percentage-of-binding strategy to measure energies in DNA-protein interactions: application to genome-scale discovery. Nucleic Acids Res. 2001, 36: 4863-4871.
Jarvik T, Smillie C, Groisman EA, Ochman H: Short-term signatures of evolutionary change in the Salmonella enterica serovar Typhimurium 14028 genome. J Bacteriol. 2010, 192: 560-567. 10.1128/JB.01233-09.
Turatsinze JV, Thomas-Chollier M, Defrance M, van Helden J: Using RSAT to scan genome sequences for transcription factor binding sites and cis-regulatory modules. Nat Protoc. 2008, 3: 1578-1588. 10.1038/nprot.2008.97.
Echave P, Tamarit J, Cabiscol E, Ros J: Novel antioxidant role of alcohol dehydrogenase E from Escherichia coli. J Biol Chem. 2003, 278: 30193-30198. 10.1074/jbc.M304351200.
Janssen R, van der Straaten T, van Diepen A, van Dissel JT: Responses to reactive oxygen intermediates and virulence of Salmonella typhimurium. Microbes Infect. 2003, 5: 527-534. 10.1016/S1286-4579(03)00069-8.
Wright JA, Tötemeyer SS, Hautefort I, Appia-Ayme C, Alston M, Danino V, Paterson GK, Mastroeni P, Ménager N, Rolfe M, Thompson A, Ugrinovic S, Sait L, Humphrey T, Northen H, Peters SE, Maskell DJ, Hinton JC, Bryant CE: Multiple redundant stress resistance mechanisms are induced in Salmonella enterica serovar Typhimurium in response to alteration of the intracellular environment via TLR4 signalling. Microbiology. 2009, 155: 2919-2929. 10.1099/mic.0.030429-0.
Nelson DL, Cox MM: Glycolysis, gluconeogenesis, and the pentose phosphate pathway. Lehninger, Principles of Biochemistry. Edited by: Lehninger AL. 2004, New York: WH Freeman, 521-559. 4
Monje-Casas F, Jurado J, Prieto-Alamo MJ, Holmgren A, Pueyo C: Expression analysis of the nrdHIEF operon from Escherichia coli. Conditions that trigger the transcript level in vivo. J Biol Chem. 2001, 276: 18031-18037. 10.1074/jbc.M011728200.
Martin JE, Imlay JA: The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol Microbiol. 2011, 80: 319-334. 10.1111/j.1365-2958.2011.07593.x.
Carmel-Harel O, Storz G: Roles of glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu Rev Microbiol. 2000, 54: 439-461. 10.1146/annurev.micro.54.1.439.
Imlay JA, Fridovich I: Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991, 266: 6957-6965.
Hébrard M, Viala JP, Méresse S, Barras F, Aussel L: Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J Bacteriol. 2009, 191: 4605-4614. 10.1128/JB.00144-09.
Arenas FA, Díaz WA, Leal CA, Pérez-Donoso JM, Imlay JA, Vásquez CC: The Escherichia coli btuE gene, encodes a glutathione peroxidase that is induced under oxidative stress conditions. Biochem Biophys Res Commun. 2010, 398: 690-694. 10.1016/j.bbrc.2010.07.002.
Golubeva YA, Slauch JM: Salmonella enterica serovar Typhimurium periplasmic superoxide dismutase sodCI is a member of the PhoPQ regulon and is induced in macrophages. J Bacteriol. 2006, 188: 7853-7861. 10.1128/JB.00706-06.
Ibanez-Ruiz M, Robbe-Saule V, Hermant D, Labrude S, Norel F: Identification of RpoS (σS)-regulated genes in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000, 182: 5749-5756. 10.1128/JB.182.20.5749-5756.2000.
Tardat B, Touati D: Two global regulators repress the anaerobic expression of MnSOD in Escherichia coli::Fur (ferric uptake regulation) and Arc (aerobic respiration control). Mol Microbiol. 1991, 5: 455-465. 10.1111/j.1365-2958.1991.tb02129.x.
Alonso-Moraga A, Bocanegra A, Torres JM, López-Barea J, Pueyo C: Glutathione status and sensitivity to GSH-reacting compounds of Escherichia coli strains deficient in glutathione metabolism and/or catalase activity. Mol Cell Biochem. 1987, 73: 61-68.
Takemoto T, Zhang QM, Yonei S: Different mechanisms of thioredoxin in its reduced and oxidized forms in defense against hydrogen peroxide in Escherichia coli. Free Radic Biol Med. 1998, 24: 556-562. 10.1016/S0891-5849(97)00287-6.
Prinz WA, Ǻslund F, Holmgren A, Beckwith J: The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J Biol Chem. 1997, 272: 15661-15667. 10.1074/jbc.272.25.15661.
Henard CA, Bourret TJ, Song M, Vázquez-Torres A: Control of the redox balance by the stringent response regulatory protein promotes antioxidant defenses of Salmonella. J Biol Chem. 2010, 285: 36785-36793. 10.1074/jbc.M110.160960.
Jordan A, Aragall E, Gibert I, Barbe J: Promoter identification and expression analysis of Salmonella typhimurium and Escherichia coli nrdEF operons encoding one of two class I ribonucleotide reductases present in both bacteria. Mol Microbiol. 1996, 19: 777-790. 10.1046/j.1365-2958.1996.424950.x.
Perrenoud A, Sauer U: Impact of global transcriptional regulation by ArcA, ArcB, Cra, Crp, Cya, Fnr, and Mlc on glucose catabolism in Escherichia coli. J Bacteriol. 2005, 187: 3171-3179. 10.1128/JB.187.9.3171-3179.2005.
Messner KR, Imlay J: The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J Biol Chem. 1999, 274: 10119-10128. 10.1074/jbc.274.15.10119.
Korshunov S, Imlay JA: Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol Microbiol. 2010, 75: 1389-1401. 10.1111/j.1365-2958.2010.07059.x.
Uzzau S, Figueroa-Bossi N, Rubino S, Bossi L:Epitope tagging of chromosomal genes inSalmonella. Proc Natl Acad Sci. 2001, 98: 15264-15269. 10.1073/pnas.261348198.
Acknowledgments
This work was supported by grants from FONDECYT #1120384 (to CPS), Universidad Andres Bello DI-34-11/R (to CPS), DI-24-12/I (to EHM) and DI-19-12/I (to BC). EHM and BC received doctoral fellowships by CONICYT and MECESUP UAB0802 additionally to EHM. The authors wish to thank Dr. Claudio Vásquez for critical reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Eduardo H Morales, Bernardo Collao contributed equally to this work.
Electronic supplementary material
12864_2013_5337_MOESM1_ESM.doc
Additional file 1:Probing the ArcA regulon under aerobic/ROS conditions in Salmonella enterica serovar Typhimurium. A) Supplementary methods. B) Figure S1: Characterization of the mechanism of ArcA in response to ROS. Measurement of the transcript and protein levels of arcA by qRT-PCR and Western blot, respectively. Determination of CFU/ml in strains 14028s, ΔarcA, ΔarcA::cat/pBR::arcA, and ΔarcA::cat/pBR::arcA D54A, after H2O2 exposure. C) Table S1: Validation of microarray data using qRT-PCR of randomly selected genes. Fold changes are given for the selected genes in response to hydrogen peroxide in the different genetic backgrounds as determined by qRT-PCR and microarray analysis. D) Supplementary references [60]. (DOC 208 KB)
12864_2013_5337_MOESM2_ESM.xls
Additional file 2: Table S2: Table of genes that showed intensity values over the background. Fold changes are given for every gene in response to H2O2 in the different genetic backgrounds. (XLS 1004 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Morales, E.H., Collao, B., Desai, P.T. et al. Probing the ArcA regulon under aerobic/ROS conditions in Salmonella enterica serovar Typhimurium. BMC Genomics 14, 626 (2013). https://doi.org/10.1186/1471-2164-14-626
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2164-14-626