Abstract
Background
Very little is known about manganese (Mn)-toxicity-responsive genes in citrus plants. Seedlings of ‘Xuegan’ (Citrus sinensis) and ‘Sour pummelo’ (Citrus grandis) were irrigated for 17 weeks with nutrient solution containing 2 μM (control) or 600 μM (Mn-toxicity) MnSO4. The objectives of this study were to understand the mechanisms of citrus Mn-tolerance and to identify differentially expressed genes, which might be involved in Mn-tolerance.
Results
Under Mn-toxicity, the majority of Mn in seedlings was retained in the roots; C. sinensis seedlings accumulated more Mn in roots and less Mn in shoots (leaves) than C. grandis ones and Mn concentration was lower in Mn-toxicity C. sinensis leaves compared to Mn-toxicity C. grandis ones. Mn-toxicity affected C. grandis seedling growth, leaf CO2 assimilation, total soluble concentration, phosphorus (P) and magenisum (Mg) more than C. sinensis. Using cDNA-AFLP, we isolated 42 up-regulated and 80 down-regulated genes in Mn-toxicity C. grandis leaves. They were grouped into the following functional categories: biological regulation and signal transduction, carbohydrate and energy metabolism, nucleic acid metabolism, protein metabolism, lipid metabolism, cell wall metabolism, stress responses and cell transport. However, only 7 up-regulated and 8 down-regulated genes were identified in Mn-toxicity C. sinensis ones. The responses of C. grandis leaves to Mn-toxicity might include following several aspects: (1) accelerating leaf senescence; (2) activating the metabolic pathway related to ATPase synthesis and reducing power production; (3) decreasing cell transport; (4) inhibiting protein and nucleic acid metabolisms; (5) impairing the formation of cell wall; and (6) triggering multiple signal transduction pathways. We also identified many new Mn-toxicity-responsive genes involved in biological and signal transduction, carbohydrate and protein metabolisms, stress responses and cell transport.
Conclusions
Our results demonstrated that C. sinensis was more tolerant to Mn-toxicity than C. grandis, and that Mn-toxicity affected gene expression far less in C. sinensis leaves. This might be associated with more Mn accumulation in roots and less Mn accumulation in leaves of Mn-toxicity C. sinensis seedlings than those of C. grandis seedlings. Our findings increase our understanding of the molecular mechanisms involved in the responses of plants to Mn-toxicity.
Similar content being viewed by others
Background
Manganese (Mn), which is the twelfth abundant element and the third most common element in the Earth’s crust, is absorbed mainly as Mn2+ by plant roots [1]. Mn, an essential trace element for the normal growth and development of higher plant, is involved in many biochemical processes. With decreasing pH, the amount of exchangeable Mn (mainly Mn2+ form) increases in the soil solution [2]. Like other heavy metals, however, Mn is harmful to most of the plants when present in excess [3, 4]. After aluminum (Al), Mn-toxicity is probably the most important factor limiting plant productivity in acidic soils, which comprise up to 50% of the world’s potentially arable lands [5]. Furthermore, the acidity of the soils is gradually increasing due to rapid industrialization, the emission of acidic gases and consequently acid deposition [6].
Disturbance of plant metabolism by Mn-toxicity happens in multiple ways. Toxic effects of Mn on plants include inhibition of growth, transpiration and photosynthesis [4, 7–9], apoplastic deposition of oxidized Mn and phenolics [10, 11], induction of oxidative stress through direct generation of reactive oxygen species (ROS) [3, 12–16], interfering with the absorption, translocation, and use of other mineral elements [4, 15, 17, 18], impairment of leaf structure and chloroplast ultrastructure [18, 19], alteration of hormone balances [18, 20, 21], modification of enzyme (i.e. ribulose-1,5-bisphosphate carboxylase/oxygenase [7, 8], Mn-dependent superoxide dismutase [22], oxalate oxidase [23], indole-acetic acid oxidase [20], glycosy transferase [24], IAA-amino acid hydrolases [25], phenylalanine ammonia-lyase [26], phosphoenol pyruvate carboxykinase [27] and Mn-peroxidase [10]) activities, and affecting carbohydrate, amino acid, protein and nucleic acid metabolisms [4, 8, 10, 13, 14, 17, 18, 28].
To deal with heavy metal stresses, plants have evolved a considerable degree of developmental plasticity, including adaptive responses via cascades of molecular networks [29]. Increasing evidence shows that plant responses to heavy metal stresses is associated with changes in the expression profiles of genes involved in a broad spectrum of physiological, biochemical and cellular processes including carbohydrate and energy metabolism, photosynthesis, protein biosynthesis and degradation, nucleic acid metabolism, signal transduction, transcriptional regulation, cell transport and stress responses [30, 31]. However, limited data are available on the differential expression of genes in response to Mn-toxicity in plants.
Techniques for gene expression analyses in plants have been widely explored. cDNA-amplified fragment length polymorphism (cDNA-AFLP), which does not require prior sequence information, is an efficient, sensitive, and reproducible technology for the discovery and identification of genes based on their polymorphism or differential expression patterns [32]. This technique is a robust and high-throughput tool for analysis of genome-wide gene expression fluctuation induced by a specific stress and is also a useful tool for the isolation of novel genes [30, 31, 33].
Citrus belongs to evergreen subtropical fruit trees and is cultivated in humid and subhumid of tropical, subtropical, and temperate regions of the world mainly on acidic soils. Although the effects of Mn-toxicity on citrus chloroplast ultrastructure, CO2 assimilation, carbohydrates, photosynthetic electron transport and antioxidant systems have been investigated [8, 19], very little is known about Mn-toxicity-responsive genes in citrus plants. In this study, we investigated the effects of Mn-toxicity on growth, leaf CO2 assimilation, leaf concentrations of malondialdehyde (MDA), chlorophyll (Chl) and total soluble protein, root, stem and leaf concentration of Mn, leaf phosphorus (P) and magnesium (Mg) concentrations, and expression of leaf genes revealed by cDNA-AFLP in Citrus grandis and Citrus sinensis seedlings having different Mn-tolerance. The objectives of this study were to understand the mechanisms of citrus Mn-tolerance and to identify differentially expressed genes, which might be involved in Mn-tolerance.
Results
Plant growth, root, stem and leaf Mn concentration, and leaf Mg and P concentrations
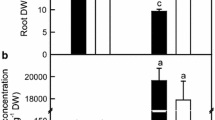
For C. grandis, Mn-toxicity decreased root, shoot and whole plant (root + shoot) dry weight (DW), and increased the ratio of root DW to shoot DW. However, Mn-toxicity did not significantly affect root, shoot and whole plant DW in C. sinensis seedlings except for increased ratio of root DW to shoot DW. Root DW, whole plant DW and the ratio of root DW to shoot DW were higher in C. sinensis seedlings than in C. grandis ones or similar between two species, except that shoot DW was lower in the former at the 2 μM Mn treatment (Figure 1). In addition, a few C. grandis leaves from the minority of Mn-toxicity plants became interveinal chlorosis or necrotic blotching of foliage, while no visible symptoms occurred in Mn-toxicity C. sinensis leaves (Additional file 1).
As shown in Figure 2, Mn-toxicity increased root, stem and leaf Mn concentration, Mn distribution in roots, Mn uptake per plant and per root DW, and decreased Mn distribution in stems and leaves. Under control condition, all these parameters did not significantly differ between C. grandis and C. sinensis seedlings. When exposed to Mn-toxicity, stem Mn concentration, Mn uptake per plant and Mn distribution in roots were higher in C. sinensis seedlings than in C. grandis ones, while leaf Mn concentration and Mn distribution in leaves were lower in the former than in the latter.
Effects of Mn-toxicity on root, stem and leaf Mn concentration, Mn uptake and Mn distribution. (A-C) Root, stem and leaf Mn concentration. (D) Mn uptake per plant. (E-G) Mn distribution in roots, stems and leaves. (H) Mn uptake per root DW. Bars represent means ± SE (n = 4). Different letters above the bars indicate a significant difference at P < 0.05.
Mn-toxicity decreased P and Mg concentrations in C. grandis leaves, but did not significantly affect them in C. sinensis leaves (Figure 3).
Leaf total soluble protein, MDA and Chl concentrations and gas exchange
Total soluble protein concentration was decreased by Mn-toxicity in C. grandis leaves, but was not significantly affected in C. sinensis ones (Figure 4A). As shown in Figure 4B, Mn-toxicity did not significantly affect leaf concentration of MDA.
Mn-toxicity decreased CO2 assimilation (Figure 5A), transpiration (Figure 5B) and stomatal conductance (Figure 5C) in leaves of C. grandis and C. sinensis, especially in the former. Mn-toxicity increased intercellular CO2 concentration in C. grandis leaves, but did not significantly affect it in C. sinensis leaves (Figure 5D). Mn-toxicity did not significantly affect leaf concentrations of Chl a+b, Chl a and Chl b, and the ratio of Chl a to Chl b in the two citrus species (Figure 5E-H).
Effects of Mn-toxicity on gas exchange and Chl of Citrus grandis and C. sinensis leaves. (A-D) Leaf CO2 assimilation, transpiration, stomatal conductance and intercellular CO2 concentration. (E-G) Leaf concentrations of Chl a + b, Chl a and Chl b. (H) Leaf ratio of Chl a/b. Bars represent means ± SE (n = 4 or 5 ). Different letters above the bars indicate a significant difference at P < 0.05.
Identification of differentially expressed genes by cDNA-AFLP
A total of 256 selective primer combinations were used for the cDNA-AFLP analysis in order to identify the genes responsive to Mn-toxicity in the leaves of two citrus species differing in Mn-tolerance (Figure 6). For C. grandis leaves, a total of 16–37 (an average of 21.4) clear and unambiguous transcript-derived fragments (TDFs) were detected with each premier combination, which resulted in approximately 5490 TDFs, ranging from 100–750 bp. A total of 223 differentially expressed and reproducible TDFs were recovered from the silver-stained cDNA-AFLP gels based on their presence, absence or difference in the levels of expression. All these TDFs were re-amplified, ligated and sequenced, and 213 cDNA fragments produced useable sequence data. TDF sequences were compared with those present in the GenBank database (Additional file 2). Of the 213 TDF sequences, 99 TDFs showed significant homology to genes encoding known or putative proteins, and 23 TDFs were homologous to genes encoding uncharacterized proteins, hypothetical proteins or unknown proteins. The remaining 91 TDFs did not show homology to any nucleotide or amino sequence in the public databases. Of these 122 matched TDFs, 42 (34.4%) TDFs increased and 80 (65.6%) decreased in response to Mn-toxicity. According to the biological functional properties, these TDFs were classified into the following functional categories: biological regulation and signal transduction (18.9%), carbohydrate and energy metabolism (10.7%), nucleic acid metabolism (4.1%), protein metabolism (17.2%), lipid metabolism (0.8%), cell wall metabolism (8.2%), stress responses (10.7%), cell transport (6.6%), other and unknown biological processes (23.0%) (Additional file 2, Figure 7B).
cDNA-AFLP profiles using one Eco R I selective primer and eight Mes I selective primers. One Eco R I selective primer: Eco R I-AC; Eight Mes I selective primers: Mes I-AC, AG, AT, AA, CC, CG, CT and CA; 1: Control leaves of Citrus grandis; 2: Mn-toxicity leaves of C. grandis; 3: Control leaves of C. sinensis; 4: Mn-toxicity leaves of C. sinenis; Arrows indicate differentially expressed TDFs.
For C. sinensis leaves, a total of 16–37 (an average of 21.3) clear and unambiguous TDFs were obtained with each premier pair, which yield approximately 5450 TDFs, ranging from 100–750 bp. A total of 20 differentially expressed and reproducible TDFs were isolated from the silver-stained cDNA-AFLP gels. All these TDFs were sequenced, and produced readable sequences (Additional file 3). Of the 20 TDFs, 12 TDFs were homologous to genes encoding known or putative proteins, and three TDFs belonged to genes encoding hypothetical proteins. The remaining five TDFs had no database matches. Among the 15 matched TDFs, seven (46.7%) TDFs were up-regulated and eight (53.3%) was down-regulated by Mn-toxicity. These TDFs were involved in biological regulation and signal transduction (33.3%), carbohydrate and energy metabolism (13.3%), nucleic acid metabolism (13.3%), protein metabolism (6.7%), cell transport (6.7%), other and unknown biological processes (26.7%) (Additional file 3, Figure 7A).
qRT-PCR analysis of some Mn-toxicity-responsive genes
To validate the cDNA-AFLP expression patterns, 15 TDFs from C. grandis leaves and one TDF from C. sinensis ones were selected for qRT-PCR analysis. The expression levels of all these TDFs except for two TDFs (TDFs # 232–1 and 160–6) matched well with the expression profiles observed with cDNA-AFLP (Figure 8). The discrepancy in expression patterns for two TDFs between qRT-PCR and cDNA-AFLP analysis might be due to gene family complexity. Nevertheless, the cDNA-AFLP technique allowed us to isolate the differentially expressed genes under Mn-toxicity.
Relative expression of TDFs from Citrus grandis (A-O) and from C. sinensis (P) leaves. Relative expression of genes encoding ATP synthase subunit alpha (TDF #01-1; A), glycoside hydrolase family 28 protein (TDF #043-1; B), anthranilate phosphoribosyltransferase-like protein (TDF #063-1; C), ATP synthase subunit alpha (Atp 1; TDF #065-1; D), monodehydroascorbate reductase (TDF #098-1; E), xylem cysteine proteinase 2 (TDF #098-2; F), catalase (TDF #103-2; G), peroxidase 42 (TDF #104-1; H), NADP-dependent alkenal double bond reductase P2 (TDF #130-2; I), DNA polymerase phi subunit (TDF #139-1; J), regulator of Vps4 activity in the MVB pathway protein (TDF #160-6; K), glutathione S-transferase Tau2 (TDF #227-1; L), Myb family transcription factor (TDF #232-1; M), cell wall-associated hydrolase (TDF #242-1; N), ABC transporter family protein (TDF #248-1; O) in C. grandis leaves and Atp1 (TDF #065a; P) in C. sinensis leaves. Bars represent means ± SE (n = 3). Different letters above the bars indicate a significant difference at P < 0.05.
Discussion
C. sinensis is more tolerant to Mn-toxicity than C. grandis
As shown in Figures 1 and 2A-C, 600 μM Mn treatment greatly inhibited C. grandis plant growth, especially the shoots and increased the concentration of Mn in roots, stems and leaves, and foliar Mn concentration for 600 μM Mn treatment was far more than the sufficiency range of 25–200 mg kg-1 DW for sweet orange (C. sinensis) leaves [34]. Based on these results, plants that received 600 μM Mn are considered Mn-excess (Mn-toxicity). Mn-toxicity-induced decrease in plant DW and increase in root DW/shoot DW ratio (Figure 1) agree with the previous results obtained on C. grandis[8] and with the view that Mn-toxicity affects plant tops more than root systems [4]. Mn-toxicity, however, had no influence on the ratio of root DW to shoot DW in lucerne (Medicago sativa) plants, despite Mn-toxicity depressed growth of shoots and roots [35].
Our results showed that Mn-toxicity C. grandis plants had decreased root, shoot and root + shoot DW, and increased ratio of root DW to shoot DW, while Mn-toxicity did not significantly affect C. sinensis growth except for increased root DW/shoot DW ratio (Figure 1), meaning that C. sinensis is more tolerant to Mn-toxicity than C. grandis. This is also supported by our data that the gas exchange in C. grandis leaves was affected by Mn-toxicity far more than in C. sinensis ones (Figure 5A-D), and that Mn-toxicity decreased total soluble protein, P and Mg concentrations only in C. grandis leaves (Figures 3 and 4A). Like that of previous workers [8, 36, 37], the observed lower CO2 assimilation in Mn-toxicity leaves of C. grandis and C. sinensi s was primarily caused by non-stomatal factors because the decreases in both CO2 assimilation and stomatal conductance was accompanied by unchanged or increased intercellular CO2 assimilation (Figure 5A, C and D). It is noteworthy that the reduction in CO2 assimilation in leaves of Mn-toxicity plants could not attributed to photo-oxidative damage and decreased Chl, because there were no significant differences in leaf concentrations of MDA (a marker of peroxidative damage), Chl a+b, Chl a and Chl b (Figures 4B and 5E-G) between control and Mn-toxicity leaves. Similar results have been obtained on C. grandis[8].
Under Mn-toxicity, the majority of Mn in C. sinensis and C. grandis plants was retained in the roots (Figure 2E), as previously found for C. grandis[8], lucerne [35] and Douglas fir [38]. However, in rice exposed to Mn-toxicity, Mn was predominantly accumulated in leaves compared with roots [39]. The tolerance of plants to Mn is associated not only with low Mn uptake, but also with relatively little Mn translocation from roots to shoots [40, 41]. Our results showed that under Mn-toxicity, C. sinensis plants accumulated more Mn in roots and less Mn in shoots than C. grandis ones, and that the concentration of Mn was lower in Mn-toxicity C. sinensis leaves than in C. grandis ones (Figure 2). This might contribute to the Mn-tolerance of C. sinensis. We isolated 122 differentially expressed TDFs from Mn-toxicity C. grandis leaves, which belong to different functional categories (Additional file 2 and Figure 7B), meaning that Mn-toxicity affected different physiological and biochemical pathways. In contrast, we only identified 15 differentially expressed TDFs from Mn-toxicity C. sinensis leaves (Additional file 3 and Figure 7A). Obviously, the transcript profile was less affected by Mn-toxicity in C. sinensis leaves compared to C. grandis ones, which may be associated with the less Mn accumulation in Mn-toxicity C. sinensis leaves (Figure 2C and G). These data also support above inference that C. sinensis is more tolerant to Mn-toxicity than C. grandis.
Genes involved in biological regulation and signal transduction
Protein phosphorylation, a versatile post-translational modification (PTM), is involved in almost all plant signal pathways and plays important roles in regulation of abiotic stress responses [42]. Phosphorylation and dephosphorylation of a protein often serve as an “on-and-off” switch in the regulation of cellular activities. For optimal regulation, kinases and phosphatases must strike a balance in any given cell [43]. As shown in Additional file 2, Mn-toxicity decreased the expression levels of genes involved in phosphorylation [leucine-rich receptor-like protein kinase (TDF #066-4), probable receptor-like protein kinase (TDF #104-5), Ser/Thr protein kinase isolog (TDF #170-1), VH1-interacting kinase (TDF #199-1), calcium-dependent protein kinase 1-like (TDF #165-2) and OBP3-responsive gene 1 (TDF #238-1) genes] and dephosphorylation [protein phosphatase 2a, regulatory subunit, putative (TDF #044–1)] except for increased expression of a mitogen-activated protein kinase 1 (MAPK 1, TDF #089–1) gene in C. grandis leaves. This means that the balance between phosphorylation and dephosphorylation was upset and phosphorylation of some proteins might be impaired in Mn-toxicity C. grandis ones. MAPK cascades are important signal modules that convert signals generated at the receptors/sensors to appropriate cellular responses [44]. Increasing evidence demonstrates the role of MAPK signal in different heavy metal stresses for different plant species [45]. The observed higher expression level of MAPK 1 (TDF #089–1) in Mn-toxicity leaves agrees with the previous reports that excess copper (Cu), cadmium (Cd) and mercury (Hg) led to the activation of a novel MAPK gene OsMSRMK2 from Japonica-type rice [46], and that MAPK pathways were activated by excess Cu and Cd in M. sativa seedlings [47]. Thus, MAPK cascade might play a role in the responses of plants to Mn-toxicity. In contrast to C. grandis, VH1-interacting kinase (TDF #199a) gene in C. sinensis leaves was induced by Mn-toxicity (Additional file 3).
MAPKs are able to phosphorylate different substrates in different cellular compartments, including transcription factors (TFs) in the nucleus [29]. Thus, genes related to TFs might be affected in Mn-toxicity C. grandis leaves due to altered expression level of MAPK 1 (TDF #089-1) gene (Additional file 2). As expected, Mn-toxicity increased the expression levels of genes encoding TF jumonji domain-containing protein (TDF #09-2) and Myb family transcription factor (TDF #232-1), and decreased the expression levels of genes encoding TF ILR3 (TDF #105-2), C3H4 type zinc finger protein (TDF #156-2), putative TF (TDF #045-2) and DNA-binding storekeeper protein-related transcriptional regulator (TDF #131-1) in C. grandis leaves (Additional file 2). Jumonji C (jmjC) domain-containing proteins have been shown to function as demethylases and to involve in chromatin structure and gene expression [48]. Recently, Govind et al. [49] showed that two Jumonji TFs (Jumonji like TF and TF jumonji domain-containing protein) and other genes (Lea5, HSP20 and HSP70) were induced in drought-stressed peanut (Arachis hypogaea) plants, which agrees with our results that Mn-toxicity C. grandis leaves had higher mRNA levels of gene encoding TF jumonji domain-containing protein (TDF #09-2, Additional file 2). They also found that silencing of Jumonji (JMJC) made the transgenic tobacco (Nicotiana benthamiana) plants more tolerant to drought, while down-regulation of HSP70 resulted in susceptibility [49]. Rampey et al. [50] reported that ilr3-1 Arabidopsis seedlings were less sensitive than wild type to Mn-toxicity. ILR3 is a basic helix–loop–helix type TF, which seems to regulate metal homeostasis in part through the action of putative Fe/Mn CCC1-like (VIT1-like) transporters. Therefore, the down-regulation of ILR3 in Mn-toxicity C. grandis leaves might be an adaptive response. Like C. grandis, the expression of DNA-binding storekeeper protein-related transcriptional regulator (TDF #131a) was inhibited in C. sinensis leaves. ILR3 (TDF #105b), however, was up-regulated in Mn-toxicity C. sinensis leaves (Additional file 3).
Calcium (Ca) is a secondary messenger and has been suggested to participate in heavy metal signal [29]. Indeed, Ca concentration in runner bean (Phaseolus coccineus) cells greatly increased in response to Cd stress [51]. Ca may interact with calmodulin to propagate the signal and ultimately to regulate downstream genes involved in heavy metal transport, metabolism, and tolerance [52]. In this study, Mn-toxicity decreased the expression levels of Ca-dependent protein kinase 1-like (TDF #165-2), calmodulin-binding transcription activator 5 (TDF# 102) and Ca2+-binding protein (TDF #186-2) genes in C. grandis leaves, and increased the expression levels of Ca-binding EF-hand domain-containing protein (TDF #153-3) gene in C. grandis leaves and calmodulin-binding transcription activator 5 (TDF #100b) in C. sinensis ones (Additional file 2 and Additional file 3). Thus, genes related to Ca2+ signal might be involved in response to Mn-toxicity. Busov et al. [53] characterized a 5NG4 gene from juvenile loblolly pine shoots, which was highly and specifically induced by auxin prior to adventitious root formation. Toxicity of Mn, on the other hand, led to auxin deficiency caused by activation of indole-acetic acid oxidase under excess Mn [20]. This supports our data that auxin-induced protein 5NG4 gene (TDF #233a) was down-regulated in C. sinensis leaves (Additional file 3). Similarly, the transcript abundance of auxin-response factor (TDF #111-2) in C. grandis leaves also decreased in response to Mn toxicity (Additional file 2). However, Mn-toxicity increased 5NG4 mRNA level in C. grandis ones (TDF #233-1, Additional file 2).
Transducin/WD-40 repeat-containing protein is a G protein involved in a wide range of functions that include signal transduction, transcription and stress-tolerant function. It was up-regulated by excess Cu in germinating rice seeds [54, 55], but was down-regulated by Mn-toxicity in C. grandis leaves (TDF #085-3, Additional file 2).
VQ motif-containing proteins play important roles in plant growth, development and defense responses. Loss-of function mutants and/or overexpression lines for the VQ genes are altered in seed size, tolerance to abiotic stress, or resistance to pathogen infection [56, 57]. Liu et al. [56] reported that transgenic Arabidopsis plants overexpressing the AtARVQ1, a gene encoding a plant-specific VQ motif-containing protein, were more resistant to arsenate stress. Recently, Cheng et al. [58] showed that plant VQ motif-containing proteins played critical roles in the network of WRKY-mediated gene expression. We found that under Mn-toxicity, the expression level of VQ genes (TDF #061-2) was decreased in C. grandis, which is in agreement with the previous report that AtARVQ1 expression was strongly down-regulated by arsenate stress [56].
Zhang et al. [59] observed that A. thaliana Fes1A could prevent cytosolic HSP70 degradation. Thus, the observed lower mRNA level of HSP70 nucleotide exchange factor fes1 (TDF #235-2, Additional file 2) in Mn-toxicity C. grandis leaves agrees with the results that the expression levels of HSP70 (TDF #237-6) and stromal 70 kDa heat shock-related protein, chloroplastic-like gene (TDF #156-1) in C. grandis ones decreased in response to Mn-toxicity (Additional file 2). Similar result has been obtained on B-deficient C. sinensis roots [60].
Phosphatidylinositol (PtdIns) 4-kinase catalyzes the phosphorylation of PtdIns in the D-4 position of the inositol ring. This is the committed step in the synthetic pathway leading to PtdIns 4,5-bisphosphate (PtdIns 4,5-P2). Apart from this function in signal transduction, both PtdIns 4-P and PtdIns 4,5-P2 appear to have other cellular functions (such as regulating cytoskeletal architecture, acting as enzyme effectors, or acting as components of vesicular fusion [61]. We found that the expression level of PtdIns 4-kinase type 2-beta (TDF #099-2) in C. grandis leaves decreased in response to Mn-toxicity (Additional file 2), which disagrees with Mn-stimulated PtdIns 4-kinase from Dunaliella parva[62].
Based on these results above mentioned, we conclude that the responses of citrus plants to Mn-toxicity might be regulated in multiple signal pathways.
Genes involved in carbohydrate and energy metabolism
Thirteen genes in C. grandis leaves and two genes in C. sinensis ones related to carbohydrate and energy metabolism were altered under Mn-toxicity (Additional file 2 and Additional file 3, Figure 7). ATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of ATP. Hamilton et al. [63] showed that exposure of wheat to Al increased mitochondrial F1F0-ATPase activity only in the Al-tolerant wheat variety, and concluded that the Al-induced increase in ATP synthase activity was an adaptive response involved in Al tolerance. Similar to Al-toxicity, Mn-toxicity increased the expression levels of synthase subunit alpha (Atp 1) in C. grandis (TDF #065-1) and C. sinensis (TDF #65a) leaves and ATP synthase subunit alpha (TDF #01-1) in C. grandis leaves (Additional file 2 and Additional file 3). Aconitate hydrolyase (AH) involved in glycolysis catalyses the interconversion of citrate and isocitrate, via a cis-aconitate. Our finding that the expression of AH 2 (TDF #116-1, Additional file 2) was induced in Mn-toxicity C. grandis leaves means that its activity was enhanced by Mn-toxicity, which is in agreement with the previous report that AH was up-regulated by Cd stress [64]. In addition, Mn-toxicity also increased the expression levels of NADPH-ferrihemoprotein reductase (TDF #178-1) and cytochrome (Cyt) c biogenesis orf256 (TDF #236-1) genes related to respiration in C. grandis leaves (Additional file 2). Overall, the metabolic pathways related to ATP synthesis and reducing power production might be activated in Mn-stressed leaves to yield more energy needed to meet the high-energy demand of stressed cell. By contrast, the mRNA level of gene encoding 2-phospho-D-glycerate hydrolase (also known as enolase, TDF #244-1), an essential and ubiquitous glycolytic enzyme responsible for the catalysis of the conversion of 2-phosphoglycerate (2-PG) to phosphoenol pyruvate (PEP), was down-regulated in Mn-toxicity C. grandis leaves (Additional file 2). This agrees with the previous report that the abundance of enolase in Agrostis stolonifera leaves was decreased after 10 d of heat stress [65]. Ferreira et al. in Populus euphratica leaves observed that the level of enolase was transiently increased after 30 h of heat stress, followed by a decrease after 54 h of heat stress [66]. However, other studies showed that leaf enolase was induced in various plants by different abiotic stresses, such as water stress in maize [67], cold stress in tobacco [68], salt stress in ice plant (Mesembryanfhemum crysfallinum) [69] and Cd-stress in Phytolacca americana[70]. Like to enolase, the expression level of gene encoding Cyt P450 (TDF #197-1), an enzyme typically catalyzing the reaction: RH + O2 + NADPH + H+ → ROH + H2O + NADP+, was reduced in Mn-toxicity C. grandis leaves (Additional file 2), which might contribute to maintaining NADPH homeostasis by decreasing its utilization. However, the expression of Cyt P450, family 96, subfamily A, polypeptide 9 (TDF #029a) gene in C. sinsnsis leaves was induced by Mn-toxicity (Additional file 3), which is in agreement with the report that at least 5 of the 29 Cyt P450s were induced in Arabidopsis by abiotic and biotic stress including Alternaria brassicicola or Alternaria alternata, paraquat, rose bengal, UV-C stress, heavy metal stress (CuSO4), mechanical wounding, drought, high salinity, low temperature or hormones [71]. Transgenic tobacco and potato plants expressing Cyt P450 tolerated better oxidative stress after herbicide treatment [72]. Therefore, the observed higher mRNA level of Cyt P450, family 96, subfamily A, polypeptide 9 might be advantegous to Mn-toxicity tolerance of C. sinensis plants.
Thioredoxins (Trxs) are small proteins, which are involved in the cell redox regulation. Trx m, a chloroplastic protein, preferentially activates NADP-malate dehydrogenase (NADP-MDH), a key enzyme involved in carbon fixation and sugar biosynthesis during photosynthesis. Historically the m-type Trxs were so named because they were able to activate NADP-MDH [73]. The activity of NADP-MDH might be down-regulated in Mn-toxicity C. grandis leaves due to lower mRNA levels of Trx m (TDF #058-2) and Trx m 4 (TDF #085-2) (Additional file 2). Evidence shows that Trx m 1, m 2, and m 4 could act as antioxidants, i.e. possibly by serving as hydrogen donors for a Trx-dependent peroxidase [74].
Trehalose-6-phosphate synthase (TPS) is a key enzyme for synthesizing trehalose, which plays an important role in abiotic stress tolerance of plants [75]. The observed lower level of TPS (TDF #013-3) in Mn-xicity C. grandis leaves (Additional file 2) indicates that Mn-toxicity might decrease leaf concentration of trehalose, thus lowering the stress tolerance.
ATP sulfurylase (ATPS) activity were enhanced by sulphur (S) deprivation and reduced after resupply of SO42–[76]. Heiss et al. [77] reported that Cd stress strongly increased the expression levels of ATPS and 5’-adenylysulfate reductase in roots and leaves of 6-week-old Brassica juncea, accompanied by enhanced cysteine concentration in roots and leaves, and concluded that roots and leaves responded to Cd stress with a coordinative up-regulation of several S assimilation enzymes to meet an increased demand for cysteine during phytochelatin synthesis. However, the expression of ATPS (TDF #168-1) was down-regulated in Mn-toxicity C. grandis leaves (Additional file 2).
UDP-glycosyltransferases (UGTs), which glycosylate a broad array of aglycones, including plant hormones, all major classes of plant secondary metabolites, and xenobiotics such as herbicides, play an important role for the stabilization, enhancement of water solubility and detoxification of natural products [78]. Cytokinins can be glucosylated to form O-glucosides and N-glucosides. The glycoconjugates are inactive and are considered to play a role in hormonal homeostasis. Hou et al. [79] showed that UGT76C1 and UGT76C2 (two members of group H) recognized all cytokinins and glucosylated them at the N7 and N9 positions. Veselov et al. [21] reported that Cd stress sharply lowered the concentration of cytokinin in wheat roots and shoots caused by elevated activity of cytokinin oxidase. Obviously, the down-regulation of UDP-glycosyltransferase 76F1-like (one member of group H, TDF #185-4) gene in Mn-toxicity C. grandis leaves (Additional file 2) is advantageous to cytokinin homeostasis under Mn-toxicity if leaf concentration of cytokinin decreased in response to Mn-toxicity.
Genes involved in nucleic acid metabolism
Evidence shows that heavy metals inhibit plant nucleic acid metabolism [80, 81]. As expected, the expression levels of genes encoding THO complex, subunit 5 (TDF #134-2), DNA polymerase phi subunit (TDF #139-1), histone H4 (TDF #165-1), DNA (cytosine-5)- methyltransferase DRM2-like (TDF #200-1) and luc7-like protein 3-like (TDF #200-2) in C. grandis leaves (Additional file 2) and genes encoding THO complex, subunit 5 (TDF #134b) and histone H4 (TDF #165a) in C. sinensis ones (Additional file 3) decreased in response to Mn-toxicity, indicating that leaf nucleic acid metabolism might be impaired by Mn-toxicity.
Genes involved in protein metabolism
Mn-toxicity has been demonstrated to affect protein metabolism in plants [12]. We found that the expression levels of three ribosomal genes encoding ribosomal protein S3 (TDF #06-1), ribosomal protein S8 (TDF #097-1) and 60S ribosomal protein L2, mitochondrial-like (TDF #134-1), which are involved in mature ribosome assembly and translation processes, were down-regulated in Mn-toxicity C. grandis leaves (Additional file 2), while only one ribosomal gene encoding 60S ribosomal protein L2, mitochondrial-like (TDF #134a) in C. sinensis leaves was down-regulated by Mn-toxicity (Additional file 3). This indicates that the biosynthesis of protein in Mn-toxicity C. grandis leaves might be impaired more severe than in Mn-toxicity C. sinensis ones, which is in agreement with our data that Mn-toxicity decreased the concentration of total soluble protein in C. grandis leaves, but did not affect its concentration in C. sinensis ones (Figure 4A). However, the expression of 30S ribosomal protein S13 gene (TDF #140-1) was induced in Mn-toxicity C. grandis leaves (Additional file 2).
Translational control of protein synthesis depends on numerous eukaryotic initiation factors (eIFs). Our finding that the expression of gene encoding similar to translation initiation factor IF2 (TDF #011-2) was down-regulated in Mn-toxicity C. grandis leaves (Additional file 2) agrees with our previous report that the abundances of eIF4B1, eIF3 subunit and eIF3G1 in C. sinensis roots decreased in response to B-deficiency [60]. However, salt and heavy metal induced the accumulation of rice eIF5A-1 and eIF5A-2 mRNAs in rice cells [82]. Elongation factors whose expressions have been demonstrated to correlate to the high rate of protein synthesis in developing plant tissues [83] facilitate translational elongation, from the formation of the first peptide bond to the formation of the last one in the ribosome. Guo et al. [84] isolated a translation elongation factor 2-like protein gene from a cold defective Arabidopsis mutant, los 1–1. The los1-1 mutant plants were impaired in protein synthesis under cold stress. We found that the expression level of one gene encoding translation elongation factor-1 alpha, partial (TDF #181-2) in C. grandis leaves decreased in response to Mn-toxicity (Additional file 2), which agrees with the previous results obtained on drought-stressed soybean nodules [85], anoxia maize roots [86], and B-deficient roots of C. sinensis[60], Brassica napus[87] and Lupinus albus[88]. However, the abundances of chloroplast translational elongation factor Tu and elongation factor P in rice leaves increased in response to Cd stress [89]. Thus, it appears that the influence of abiotic stress on translation elongation factors depends on plant species and kinds of stresses. In addition, the expression level of gene encoding tetratricopeptide repeat-containing protein (TDF #090-1) involved in protein synthesis was down-regulated in Mn-toxicity C. grandis leaves (Additional file 2). These results further demonstrate that protein biosynthesis is impaired in Mn-toxicity C. grandis leaves.
Mn-toxicity increased the expression levels of genes encoding ATP-dependent Clp protease (TDF #245-1), cysteine proteinase (TDF #044-2) and xylem cysteine proteinase 2 (TDF #098-2) in C. grandis leaves (Additional file 2). This indicates that the hydrolysis of some proteins might be up-regulated in Mn-toxicity leaves, thus decreasing leaf concentration of total soluble protein. This is also supported by our data that Mn-toxicity leaves had lower concentration of total soluble protein (Figure 4A). However, the expression levels of genes encoding carboxyl-terminal peptidase (TDF #104-6), papain family cysteine protease (TDF #107-1), cathepsin B-like cysteine proteinase like protein (TDF #233-3) and α/β-hydrolase-like protein (TDF #221-1) decreased in response to Mn-toxicity (Additional file 2).
Like other PTMS (such as phosphorylation), ubiquitination, which serves as a versatile PTM, plays a key role in regulating plant response to abiotic stresses [90]. The up-regulation of genes encoding MND1-interacting protein 1 (TDF #148-1) and ubiquitin-conjugating enzyme E2 10 (TDF #240-1) in Mn-toxicity C. grandis leaves (Additional file 2) might be an adaptive response to Mn-toxicity. However, the expression levels of ubiquitin-correlative genes such as ubiquitin-protein ligase (TDF #03-2) and BTB and MATH domain-containing protein (TDF #138-2) in C. grandis leaves decreased in response to Mn-toxicity (Additional file 2), meaning that the ubiquitination of some proteins might be impaired in Mn-toxicity C. grandis leaves.
Three genes [chorismate synthase (TDF #247-1), cystathionine β-synthase (CBS) domain-containing protein (TDF #216-3) and 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase-like protein (TDF #061-3) ] involved in amino acid metabolism was down-regulated in Mn-toxicity C. grandis leaves (Additional file 2). This means that the biosynthesis of amino acids might be impaired in Mn-toxicity C. grandis leaves. Chorismate synthase catalyzes the last of the seven steps in the shikimate pathway which is used in prokaryotes, fungi and plants for the biosynthesis of aromatic amino acids [91]. CBS catalyzes the first step of the transsulfuration pathway from homocysteine to cystathionine. Jung et al. [92] observed that CBSX2 directly modulated Trx in chloroplasts, which affected the level of H2O2 and, consequently, the expression of the genes involved in secondary cell-wall thickening, concluding that CBSX2 protein played a critical role in thickening of the secondary cell walls of the endothecium during anther dehiscence in Arabidopsis. Transgenic tobacco plants overexpressing OsCBSX4 isolated from rice displayed enhanced tolerance to salinity, heavy metal, and oxidative stress [93]. This observed lower expression level of gene encoding CBS domain-containing protein (TDF #216-3) in C. grandis leaves (Additional file 2) disagrees with our previous data that the abundance of CBS family protein in C. sinensis roots increased in response to B-deficiency [60].
Genes involved in lipid metabolism
Lecithin-cholesterol acyltransferase (LCAT) catalyzes the transacylation of acyl groups from phospholipids to sterols in mammals and yeast. Sterol acylation is an essential process of sterol homeostasis in eukaryotic cells. In Arabidopsis, phospholipid sterol acyltransferase 1 (PSAT1), which displays homology with the mammalian LCAT, catalyzes a phospholipid-dependent (acyl-CoA-independent) formation of sterol esters [94]. Recent work with Arabidopsis showed that sterol ester concentration decreased in leaves of psat1-1 or psat1-2 mutants accompanied by an early leaf senescence phenotype, suggesting a major contribution of the PSAT1 in maintaining both free sterol homeostasis in plant cell membranes and leaf viability during developmental aging [95]. The up-regulation of lecithin-cholesterol acyltransferase-like 1 (TDF #153-2, Additional file 2) in Mn-toxicity C. grandis leaves might enhance the leaf concentration of sterol ester, thus preventing leaf senescence.
Genes involved in cell wall metabolism
Our results showed that the expression levels of genes [α-1, 2-fucosyltransferase (TDF #037-3), caffeic acid O-methyltransferase (TDF #080-1), O-fucosyltransferase family protein (TDF #069-8), cellulose synthase-like protein (TDF #044-4), protein SAH7 (TDF #05-1), 4-coumarate-CoA ligase 3 (TDF #151-1) and CBS domain-containing protein (TDF #216-3)] involved in cell wall biosynthesis were down-regulated in Mn-toxicity C. grandis leaves (Additional file 2). In addition, the mRNA levels of gene encoding glycoside hydrolase family 28 protein (TDF #043-1), which catalyze the hydrolytic cleavage of the pectin and gene encoding cell wall-associated hydrolase (TDF #242-1) were up-regulated in Mn-toxicity C. grandis leaves (Additional file 2). Therefore, the formation of cell wall might be impaired in Mn-toxicity leaves. However, the expression of gene encoding protein trichome birefringence-like 39 (TDF #158-3) was induced in Mn-toxicity C. grandis leaves (Additional file 2).
Genes involved in stress responses
Mn-toxicity has been demonstrated to stimulate ROS production in plants [12, 14, 16]. Since Mn-toxicity did not affect the concentration of MDA in citrus leaves (Figure 4B), some protective antioxidant enzymes should be up-regulated to meet the increased requirement for scavenging ROS. Our results showed that the expression of gene encoding catalase (CAT, TDF #103-2), an enzyme involved in scavenging H2O2, was induced in Mn-toxicity C. grandis leaves (Additional file 2), which agrees with the previous report that Mn-toxicity C. grandis leaves had increased specific activity of CAT and similar leaf area-based activity of CAT [8]. Besides ROS scavenger enzymes, this detoxification mechanism also involves “house-keeping” enzymes. The family of Nudix hydrolases (NUDXs), which catalyze the hydrolytic breakdown of nucleoside diphosphates linked to some other moieties such as a phosphate, sugar or nucleoside, are one of these “house-keeping” enzyme families [96]. Ogawa et al. [97] showed that AtNUDX19, a chloroplastic AtNUDX, played an important role in modulation of the NADH and/or NADPH pools through the hydrolysis of NAD(P)H to reduced nicotinamide mononucleotide (NMNH) in Arabidopsis chloroplasts. Transgenic Arabidopsis plants overexpressing AtNUDX2 and AtNUDX7 displayed enhanced tolerance to oxidative stress, resulting from the maintenance of NAD+ and ATP levels by nucleotide recycling of free ADP-ribose molecules [98]. The higher expression level of NUDX19 (TDF #160-1) in C. grandis leaves (Additional file 2) agrees with the previous reports that the abundance of NUDX homolog 3 in C. sinensis roots increased in response to B-deficiency [60] and Chrysanthemum lavandulifolium ClNUDX1, ClNUDX3, ClNUDX7 and ClNUDX8 were induced by salt, drought, heat, and cold stresses [99]. However, the expression levels of genes encoding monodehydroascorbate reductase (MDAR, TDF #098-1), peroxidase 42 (TDF #104-1), glutathione S-transferase (GST) Tau2 (TDF #227-1), NADP-dependent alkenal double bond reductase P2 (TDF #130-2), Trx m (TDF #058-2), TRx m 4, chloroplastic-like (TDF #085-2), and CBS domain-containing protein (TDF #216-3) related to oxidative defense in C. grandis leaves decreased in response to Mn-toxicity (Additional file 2). It is noteworthy that Mn-toxicity C. grandis leaves had higher specific activity of MDAR and leaf area-based activity of MDAR compared with controls [8]. The discrepancy between the expression level of MDAR and the activity of the corresponding enzyme indicates that PTMs might affect MDAR activity.
Senescence is a genetically programmed decline in various cellular processes and involves in the hydrolysis of macromolecules such as proteins and lipids. It is governed by the developmental age and is induced or enhanced by biotic and abiotic stresses. Generally in plants the term senescence and programmed cell death (PCD) denote the processes that initiate the programmed death of individual cell. The senescence can be considered as one of the examples for PCD. As expected, the expression level of one gene encoding for putative senescence-associated protein (TDF #164-4) was up-regulated by Mn-toxicity in the less Mn-tolerant C. grandis leaves (Additional file 2). This agrees with the previous report that the gene was highly differentially expressed only in the heat-sensitive fescue (Festuca sp.) genotype at 44°C [100]. In addition, the expression levels of apoptosis linked genes [xylem cysteine proteinase 2 (TDF #098-2), cysteine proteinase (TDF #044-2) and ALG2-interacting protein X (TDF #054-1) genes] were enhanced in Mn-toxicity C. grandis leaves (Additional file 2). Thus, it is reasonable to assume that the Mn-toxicity sensitive C. grandis plants were under great stress and leaf senescence was accelerated, which might contribute to plant survival by using more metabolites through glycolysis, and protein and lipid degradation. As shown in Figure 9, the Mn-toxicity-induced senescence in C. grandis leaves was a very complicated process.
The potential regulatory network of Mn-toxicity-induced senescene in Citrus grandis leaves. AAM: Amino acid metabolism; ALG2: Apoptosis linked gene 2 (ALG2)-interacting protein X; CBSDP: Cystathionine β-synthase (CBS) domain-containing protein; GST: Glutathione S-transferase; MAPK: mitogen-activated protein kinase; NAM: Nucleic acid metabolism; PCD: Programmed cell death; Pn: Photosynthesis; ROS: Reactive oxygen; SAP: Senescence-associated protein; ↑: Up-regulation; ↓: Down-regulation.
HSPs/chaperones have been demonstrated to play a key role in protecting plants against heavy metal stress [64]. The observed lower expression levels of genes encoding heat shock protein-related, partial (TDF #158-2), heat shock protein 60-3A (TDF #160-5), HSP70 (TDF #237-6) and stromal 70 kDa heat shock-related protein, chloroplastic-likes (TDF #156-1) in Mn-toxicity C. grandis leaves (Additional file 2) indicate decreased protein synthesis in these leaves, as indicated by decreased leaf concentration of total soluble proteins (Figure 4A). This is similar to the previous report that the abundance of HSP70 was enhanced in leaves of high Cd-accumulating soybean cultivar, but was lowered in low Cd-accumulating one [101].
One crucial adaptive mechanism of plants to P-deficiency is the immediate cleavage of phosphate (Pi) from phosphorylated substrates. Phosphoethanolamine/phosphocholine phosphatase (PPsPase) may release Pi from organic to maintain Pi homeostasis of plant cells [102]. The down-regulation of PPsPase, putative gene (TDF #198-1) in C. grandis leaves (Additional file 2) might decrease the release of Pi from phosphorylated substrates, thus lowering leaf P concentration (Figure 3A). This agrees with the previous report that Mn-toxicity decreased P concentration in tea leaves [17].
Genes involved in cell transport
Eight genes in C. grandis leaves and one gene in C. sinensis ones were regulated by Mn-toxicity (Additional file 2 and Additional file 3, Figure 7). We found that the expression of citrus sucrose transporter 1 (TDF #073-1, Additional file 2) was induced in Mn-toxicity C. grandis leaves, which is in agreement with the previous report that the gene strongly expressed in source, sugar exporting organs [103], because Mn-toxicity increased or did not affect the concentrations of non-structural carbohydrates in C. grandis leaves [8]. The up-regulation of citrus sucrose transporter1 might be helpful to decrease sugar accumulation in Mn-toxicity leaves, which might in turn lead to feedback suppression of CO2 assimilation [104].
One of strategies of plants to deal with heavy metals is to transport them out of the cells, thereby removing them from the cytosol. Kim et al. [105] observed that transgenic A. thaliana plants overexpressing the ABC transporter AtPDR8 were more resistant to Cd or lead (Pb) and displayed lower Cd concentration in roots and shoots than wild-type plants, while AtPDR8 RNAi transgenic plants and T-DNA insertion lines were more sensitive to Cd or Pb and had higher Cd concentration, concluding that the ABC transporter AtPDR8 is a Cd extrusion pump conferring heavy metal resistance. The down-regulation of genes encoding ABC-transporter-like protein (TDF #208-1) and ABC transporter family protein (TDF #248-1) in C. grandis leaves (Additional file 2) indicates that the extrusion of Mn from leaves might be lessened in response to Mn-toxicity, which might be one of the causes that Mn-toxicity C. grandis leaves accumulated more Mn than C. sinensis ones (Figure 2G).
Evidence has shown that CorA-like proteins may represent the major transport systems in eukaryotes such as yeast, animals, and plants [106, 107]. The down-regulation of Mg transporter CorA-like protein gene in Mn-toxicity C. grandis (TDF #234-2, Additional file 2) and C. sinensis (TDF #234b, Additional file 3) leaves might reduce Mg transport, hence decreasing leaf Mg concentration. This agrees with the previous report that Mn-toxicity decreased leaf concentration of Mg [17]. Interestingly, Mg concentration was decreased by Mn-toxicity only in C. grandis leaves, but was not significantly affected in C. sinensis leaves (Figure 3B). This means that leaf Mg concentration is also regulated by other factors.
Plant cyclic nucleotide gated channels (CNGCs), which comprise a large gene family in Arabidopsis, have been proposed to be involved in multiple plant physiological processes including plant growth and heavy metal toxicity tolerance [108]. Evidence suggests that CNGCs paly a role in heavy metal homeostasis, for example, in tobacco, overexpression of tobacco CNGC (NtCBP4) led to hypersensitivity to Pb [109]. However, Sunkar et al. [110] showed that transgenic tobacco plants overexpressing a truncated NtCBP4 exhibited improved tolerance to Pb2+ and decreased uptake of this metal ion. We found that the expression of cyclic nucleotide gated channel 9 gene (TDF #216-2) was down-regulated in Mn-toxicity C. grandis leaves (Additional file 2), suggesting that the gene might be involved in the response to Mn-toxicity.
Our results showed that the expression of gene encoding protease inhibitor/seed storage/lipid transfer protein (LTP) family protein (TDF #242-2) was down-regulated in Mn-toxicity C. grandis leaves (Additional file 2), indicating that the exchange of lipids between membranes might be decreased.
Conclusions
Our results clearly demonstrated that C. sinensis was more tolerant to Mn-toxicity than C. grandis. Under Mn-toxicity, C. sinensis plants accumulated more Mn in roots and less Mn in shoots (leaves) than C. grandis ones, and that the leaf concentration of Mn was lower in the former. This might contribute to the Mn-tolerance of C. sinensis. In this study, we first used the cDNA-AFLP technique to compare the mRNA levels of genes from control and Mn-toxicity leaves of two citrus species differing in Mn-tolerance. In Mn-toxicity C. grandis leaves, 42 up-regulated and 80 down-regulated genes were isolated, while only seven up-regulated and eight down-regulated genes were identified in Mn-toxicity C. sinensis ones. Obviously, Mn-toxicity affected gene expression far less in C. sinensis leaves than in C. grandis ones, which might be associated with less leaf Mn concentration in Mn-toxicity C. grandis leaves. cDNA-AFLP analysis suggests that the responses of C. grandis leaves to Mn-toxicity might include following several aspects: (1) accelerating leaf senescence; (2) activating the metabolic pathway related to ATPase synthesis and reducing power production; (3) decreasing cell transport; (4) inhibiting protein and nucleic acid metabolisms; (5) impairing the formation of cell wall; and (6) triggering multiple signal transduction pathways. We also identified many new Mn-toxicity-responsive genes involved in biological and signal transduction (i.e. VH1-interacting kinase, OBP3-responsive gene 1, transcription factor IL), carbohydrate metabolism (i.e. NADPH-ferrihemoprotein reductase, trehalose-6-phosphate synthase), protein metabolism (i.e. MND1-interacting protein 1, chorismate synthase), stress responses (i. e. Nudix hydrolase 19, ALG2-interacting protein X) and cell transport (i.e. cyclic nucleotide gated channel 9). Further studies will elucidate the roles of these genes in response to Mn-toxicity, which will help me to design Mn-tolerant transgenic crops.
Methods
Plant culture, Mn treatments and sampling
The study was conducted from April to December, 2012 at Fujian Agriculture and Forestry University (FAFU). Plant culture, Mn treatments, and sampling were performed according to Li et al. [8]. Briefly, 6-week-old seedlings of ‘Xuegan’ (Citrus sinensis) and ‘Sour pummelo’ (Citrus grandis) were transplanted to 6 L pots containing sand. Seedlings, two per pot, were grown in a greenhouse under natural photoperiod at FAFU. Each pot was supplied with 500 mL of nutrient solution every two day. The nutrient solution contained the following macronutrients (in mM): KNO3, 1.25; Ca(NO3)2, 1; (NH4)H2PO4, 0.25; MgSO4, 0.5; and micronutrients (in μM): H3BO3, 10; ZnCl2, 2; CuSO4, 0.5; (NH4)6Mo7O24, 0.065; MnSO4, 2; Fe-EDTA, 20. Eight weeks after transplanting, each pot was supplied every other day until dripping with nutrient solution (approximately 500 mL) containing 2 μM (control) or 600 μM (Mn-toxicity) MnSO4 for 17 weeks. At the end of the experiment, fully expanded leaves from different replicates and treatments were used for all the measurements. Leaves were collected at noon under full sun and immediately frozen in liquid nitrogen and were stored at −80°C until extraction.
Measurements of root and shoot DW and determination of Mn, Mg and P
Ten plants per treatment from different pots were harvested and divided into their parts (roots, and shoots). The plant parts were then dried at 70°C for 48 h and the DW mesured.
Root, stem and leaf Mn concentration and leaf Mg concentration were assayed by inductively coupled plasma (ICP) emission spectrometry after microwave digestion with HNO3[111]. Leaf P concentration was measured according to Ames [112]. There were four replicates per treatment (one leaf per replicate, one leaf per plant).
Determination of Chl, total soluble protein and MDA in leaves
Leaf Chl a+b, Chl a and Chl b were assayed according to Lichtenthaler [113]. Briefly, 2 frozen leaf discs (0.608 cm2 in size) were extracted with 8 mL of 80% (v/v) acetone for 24 h in the dark. The extracts were determined using Libra S22 ultraviolet–visible spectrophotometer (Biochrom Ltd., Cambridge, UK). Leaf total soluble protein was extracted with 50 mM Na2HPO4-KH2PO4 (pH 7.0) and 5% (w/v) insoluble polyvinylpolypyrrolidone (PVPP), and determined according to Bradford [114] using bovine serum albumin (BSA) as standard. Extraction and determination of leaf MDA was performed according to Hodges et al. [115]. There were four replicates per treatment (one leaf per replicate, one leaf per plant).
Leaf gas exchange measurements
Measurements were made with a CIARS-2 portable photosynthesis system (PP systems, Herts, UK) at ambient CO2 concentration under a controlled light intensity of 1000 μmol m-2 s-1 between 9:30 and 10:30 on a clear day. During measurements, leaf temperature and vapor pressure deficit (VPD) were 26.9 ± 1.1°C and 2.0 ± 0.1 kPa, respectively. There were five replicates per treatment (one leaf per replicate, one leaf per plant).
RNA preparation and cDNA synthesis
Total RNA was extracted from 200–300 mg of the frozen leaves using Recalcirtant Plant Total RNA Extraction Kit (Centrifugal column type, Bioteke Corporation, China) according to manufacturer’s instructions. The integrity and quantity of total RNA was detected by 1% (w/v) agarose gel electrophoresis and spectrophotometer at 260 nm. First-strand cDNA was synthesized from 2 μg of total RNA using RevertAid™ First Strand cDNA Synthesis Kit (Thermo Scientific, Massachusetts, USA). Second cDNA strand was performed with Escherichia coli RNase H, E. coli DNA Ploymerase I and T4 DNA Polymerase (TaKaRa, China), and stoped with 0.25 M EDTA (pH 8.0) and 10% sodium dodecyl sulfate (SDS). The resulting double-stranded cDNA was purified using equal volume of phenol : chloroform : isoamyl alcohol (25 : 24 : 1). Five μL was checked using agarose gel electrophoresis in order to observe an expected smear between 100 bp and 1000 bp.
cDNA-AFLP analysis
The cDNA-AFLP-based transcript profiling procedure was performed as described by Cao et al. [116] with some modifications. Double-stranded cDNA (600 ng) was digested with restriction enzymes: 5 U each of Eco R I (Thermo Scientific, Massachusetts, USA; 3 h at 37°C) and Mse I (Tru 1I, Thermo Scientific, Massachusetts, USA; 3 h at 65°C). The resulting restricted fragments were ligated to adaptors (EcoR I, 0.2 μM forward primer: 5’- CTCGTAGACTGCGTACC-3’ and reverse primer: 3’- CATCTGACGCATGGTTAAP −5’; Mse I, 2 μM forward primer: 5’-GACGATGAGTCCTGAG-3’ and reverse primer: 3’-TACTCAGGACTCATP-5’) with T4-DNA ligase (Thermo Scientific, Massachusetts, USA) for 10–16 h at 16°C. Prior to ligation, the two adaptors were heated at 94°C for 3 min, followed by 65°C for 10 min, 37°C for 10 min and 25°C for 10 min. The resulting ligated products were pre-amplified with the corresponding preamplification premiers: Eco R I, 5’-GACTGCGATCCAATTC-3’ and Mse I, 5’-GATGAGTCCTGAGTAA-3’. From a 100-fold dilution of the pre-amplified samples, a 5 μL diluted sample was used for the selective amplification using 256 combinations of the following primers: 16 derivatives of Eco R I primers 5’-GACTGCGATCCAATTCEE-3’ and 16 derivatives of Mse I primers 5’-GATGAGTCCTGAGTAAMM-3’; where EE and MM represent AA, AT, AC, AG, TA, TC, TT, TG, CA, CT, CG, CC, GA, GC, GT and GG. The selective amplification products were separated on a 6% (w/v) polyacrylamide gel run at 50 W for 2.5 h. The gels were silver stained as described by Bassam et al. [117] to visualize the cDNA bands. Samples for cDNA-AFLP analysis were run in two replicates at least.
The TDFs of interests were selected based on their presence, absence or differential intensity and cut out with a scalpel, and incubated in 50 μL of dd H2O for 30 min in a boiling water bath, then centrifuged at 10000 revolutions per min at room temperature (Eppendorf 5418R, Hamburg, Germany). The supernatant was stored at −20°C for re-amplification. The eluted DNA was re-amplified by PCR using the same primer combinations. The re-amplified products representing the Mn-toxicity-responsive TDFs were checked on 1% (w/v) agarose gels, each band is isolated and eluted using DNA Agarose Gel Recovery Kit (Solarbio, China). Before being sequenced by BGI Technology Corporation (Shenzhen, China), these TDFs fragments were ligated to pGEM-T EASY vector according to usage information of pGEM®-T Easy Vector System I (Promega, USA), then transduced into E. coli (DH5α) competent cells using Ampicillin as the selecting agent. All sequences were input into the VecScreen (http://www.ncbi.nlm.nih.gov/tools/vecscreen/) to identify and remove all of the vector sequence. Homology of TDFs’ sequences was analyzed using the BLASTX and BLASTN searching engines (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Their functional categories were assigned based on the analysis of information reported for each sequence by The Gene Ontology (http://www.geneontology.org/) and Uniprot (http://www.uniprot.org/).
Quantitative RT-PCR (qRT-PCR) analysis
Total RNA was isolated from the frozen leaves of control and Mn-excess plants by TRIzol reagent (Invitrogen, Carlsbad, CA, USA). About 2.0 μg total RNA was used for first-strand cDNA synthesis using the RevertAid™ First-Strand cDNA Synthesis Kit (Thermo Scientific, Massachusetts, USA) following the manufacturer’s instructions. The resulting cDNA was diluted to 100 μL using Tris-EDTA buffer (10 mM Tris, 50 mM NaCl, 1 mM EDTA, pH 7.8). Specific primers were designed from the sequences of 15 singleton TDFs using Primer Primier Version 5.0 (PREMIER Biosoft International, CA, USA). The sequences of the F and R primers used are given in Additional file 4. qRT-PCR was performed using a SYBR® Premix Ex TaqTM (Tli RNaseH Plus, Takara Bio, Inc, Otsu, Shiga, Japan) with the Step One Plus Real-Time System (Applied Biosystems, California, USA) in an Eco Real-Time PCR System (Illumina, USA ). The cycling conditions were 30 s at 95°C, followed by 40 cycles of 95°C for 5 s, 60°C for 15 s. Samples for qRT-PCR were run in 3 biological replicates with 3 technical replicates. Relative gene expression was calculated using ddCt algorithm. For the normalization of gene expression, citrus actin (GU911361.1) gene was used as an internal standard and the leaves from control plants were used as reference sample, which was set to 1.
Experimental design and statistical analysis
There were 20 pots (40 seedlings) per treatment in a completely randomized design. Experiments were performed with 2–10 replicates. Results represented the mean ± SE. Statistical analyses of data were carried out by ANOVA tests. Means were separated by the least significant difference (LSD) test at P < 0.05 level.
References
Armstrong FA: Why did nature choose manganese to make oxygen?. Phil Trans R Soc B. 2008, 363: 1263-1270. 10.1098/rstb.2007.2223.
Marschner H: Mineral Nutrition of Higher Plants. 1995, London: Academic Press
González A, Steffen KL, Lynch JP: Light and excess manganese implications for oxidative stress in common bean. Plant Physiol. 1998, 118: 493-504. 10.1104/pp.118.2.493.
Mukhopadhyay MJ, Sharma A: Manganese in cell metabolism of higher plants. Bot Rev. 1991, 57: 117-149. 10.1007/BF02858767.
Foy CD: Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soils. Soil Acidity and Liming. Edited by: Adams F. 1984, Madison: American Society of Agronomy, 57-97. 2
Michopoulos P, Cresser MS: Effects of simulated acid precipitation on the cycling of manganese under stika spruce (Picea sitchensis). Biogeochemistry. 2002, 61: 323-325. 10.1023/A:1020236016344.
Kitao M, Lei TT, Koike T: Effects of manganese toxicity on photosynthesis of white birch (Betula platyphylla var. japonica) seedlings. Physiol Plant. 1997, 101: 249-256. 10.1111/j.1399-3054.1997.tb00994.x.
Li Q, Chen LS, Jiang HX, Tang N, Yang LT, Lin ZH, Yang GH: Effects of manganese-excess on CO2 assimilation, ribulose-1, 5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport of leaves, and antioxidant systems of leaves and roots in Citrus grandis seedlings. BMC Plant Biol. 2010, 10: 42-10.1186/1471-2229-10-42.
Millaleo R, Reyes-Díaz M, Alberdi M, Ivanov AG, Krol M, Hüner NPA: Excess manganese differentially inhibits photosystem I versus II in Arabidopsis thaliana. J Exp Bot. 2013, 64: 343-354. 10.1093/jxb/ers339.
Fecht-Christoffers MM, Braun HP, Lemaitre-Guillier C, VanDorsselaer A, Horst WJ: Effect of manganese toxicity on the proteome of the leaf apoplast in cowpea. Plant Physiol. 2003, 133: 1935-1946. 10.1104/pp.103.029215.
Dučić TD, Polle A: Transport and detoxification of manganese and copper in plants. Braz J Plant Physiol. 2005, 17: 103-112. 10.1590/S1677-04202005000100009.
Demirevska-Kepova K, Simova-Stoilova L, Stoyanova Z, Holzer R, Feller U: Biochemical changes in barley plants after excessive supply of copper and manganese. Environ Exp Bot. 2004, 52: 253-266. 10.1016/j.envexpbot.2004.02.004.
Führs H, Behrens C, Gallien S, Heintz D, Van Dorsselaer A, Braun HP, Horst WJ: Physiological and proteomic characterization of manganese sensitivity and tolerance in rice (Oryza sativa) in comparison with barley (Hordeum vulgare). Ann Bot. 2010, 105: 1129-1140. 10.1093/aob/mcq046.
Gangwar S, Singh VP, Prasad SM, Maurya JN: Modulation of manganese toxicity in Pisum sativum L. seedlings by kinetin. Sci Hort. 2010, 126: 467-474. 10.1016/j.scienta.2010.08.013.
Shi Q, Zhu Z, Xu M, Qian Q, Yu J: Effect of excess manganese on the antioxidant system in Cucumis sativus L. under two light intensities. Environ Exp Bot. 2006, 58: 197-205. 10.1016/j.envexpbot.2005.08.005.
Maksimović JD, Mojović M, Maksimović V, Römheld V, Nikolic M: Silicon ameliorates manganese toxicity in cucumber by decreasing hydroxyl radical accumulation in the leaf apoplast. J Exp Bot. 2012, 63: 2411-2420. 10.1093/jxb/err359.
Venkatesan S, Hemalatha KV, Jayaganesh S: Characterization of manganese toxicity and its influence on nutrient uptake, antioxidant enzymes and biochemical parameters in tea. Res J Phytochem. 2007, 1: 52-60.
Yao Y, Xu G, Mou D, Wang J, Ma J: Subcellular Mn compartation, anatomic and biochemical changes of two grape varieties in response to excess manganese. Chemosphere. 2012, 89: 150-157. 10.1016/j.chemosphere.2012.05.030.
Papadakis IE, Giannakoula A, Therios IN, Bosabalidis AM, Moustakas M, Nastou A: Mn-induced changes in leaf structure and chloroplast ultrastructure of Citrus volkameriana (L.) plants. J Plant Physiol. 2007, 164: 100-103. 10.1016/j.jplph.2006.04.011.
Srivastava AK, Singh S: Biochemical markers and nutrient constraints diagnosis in citrus: a perspective. J Plant Nutr. 2006, 29: 827-855.
Veselov D, Kudoyarova G, Symonyan M, Veselov S: Effect of cadmium on ion uptake, transpiration and cytokinin content in wheat seedlings. Bulg J Plant Physiol. 2003, 29: 353-359.
Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, van Montagu M, Inze D: Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J. 1991, 10: 723-1732.
Woo EJ, Dunwell JM, Goodenough PW, Marvier AC, Pickersgill RW: Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat Struct Biol. 2000, 7: 1036-1040. 10.1038/80954.
Zeng W, Chatterjee M, Faik A: UDP-xylose-stimulated glucuronyltransferase activity in wheat microsomal membranes: characterization and role in glucurono (arabino) xylan biosynthesis. Plant Physiol. 2008, 147: 78-91. 10.1104/pp.107.115576.
LeClere S, Tellez R, Rampey RA, Matsuda SPT, Bartel B: Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J Biol Chem. 2002, 277: 20446-20452. 10.1074/jbc.M111955200.
Durst F: The correlation of phenylalanine ammonia-lyase and cinnamic acid hydroxylase activity changes in Jerusalem artichoke tuber tissues. Planta. 1976, 132: 221-227. 10.1007/BF00399721.
Chen ZH, Walker RP, Acheson RM, Leegood RC: Phosphoenol pyruvate carboxykinase assayed at physiological concentrations of metal ions has a high affinity for CO2. Plant Physiol. 2002, 128: 160-164. 10.1104/pp.010431.
Führs H, Specht A, Erban A, Kopka J, Horst WJ: Functional associations between the metabolome and manganese tolerance in Vigna unguiculata. J Exp Bot. 2012, 63: 329-340. 10.1093/jxb/err276.
DalCorso G, Farinati S, Furini A: Regulatory networks of cadmium stress in plants. Plant Signal Behav. 2010, 5: 663-667. 10.4161/psb.5.6.11425.
Craciun AR, Courbot M, Bourgis F, Salis P, Saumitou-Laprade P, Verbruggen N: Comparative cDNA-AFLP analysis of Cd-tolerant and -sensitive genotypes derived from crosses between the Cd hyperaccumulator Arabidopsis halleri and Arabidopsis lyrata ssp. petraea. J Exp Bot. 2006, 57: 2967-2983. 10.1093/jxb/erl062.
Fusco N, Micheletto L, DalCorso G, Borgato L, Furini A: Identification of cadmium-regulated genes by cDNA-AFLP in the heavy metal accumulator Brassica juncea L. J Exp Bot. 2005, 56: 3017-3027. 10.1093/jxb/eri299.
Bachem CWB, Hoeven RS, Bruijn SM, Vreugdenhil D, Zabeau M, Visser RGF: Visualization of differential gene expression using a novel method of RNA fingerprinting based on AFLP: analysis of gene expression during potato tuber development. Plant J. 1996, 9: 745-753. 10.1046/j.1365-313X.1996.9050745.x.
Ditt RF, Nester EW, Comai L: Plant gene expression response to Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 2001, 98: 10954-10959. 10.1073/pnas.191383498.
Mills HA, Jones JB: Plant Analysis Handbook II. 1996, Georgia: MicroMacro Publishing
Gherardi MJ, Rengel Z: Genotypes of lucerne (Medicago sativa L.) show differential tolerance to manganese deficiency and toxicity when grown in bauxite residue sand. Plant Soil. 2003, 249: 287-296. 10.1023/A:1022872524844.
Lidon FC, Barreiro MG, Ramalho JC: Manganese accumulation in rice: implications for photosynthetic functioning. J Plant Physiol. 2004, 161: 1235-1244. 10.1016/j.jplph.2004.02.003.
Nable RO, Houtz RL, Cheniae GM: Early inhibition of photosynthesis during development of Mn toxicity in tobacco. Plant Physiol. 1988, 86: 1136-1142. 10.1104/pp.86.4.1136.
Dučić TD, Leinemann L, Finkeldey R, Polle A: Uptake and translocation of manganese in seedlings of two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca). New Phytol. 2006, 170: 11-20. 10.1111/j.1469-8137.2006.01666.x.
Lidon F: Tolerance of rice to excess manganese in the early stages of vegetative growth. Characterization of manganese accumulation. J Plant Physiol. 2001, 158: 1341-1348. 10.1078/0176-1617-00507.
Mora ML, Rosas A, Ribera A, Rengel Z: Differential tolerance to Mn toxicity in perennial ryegrass genotypes: involvement of antioxidative enzymes and root exudation of carboxylates. Plant Soil. 2009, 320: 79-89. 10.1007/s11104-008-9872-1.
Vose PB, Randall PJ: Resistance to aluminum and manganese toxicities in plants related to variety and cation exchange capacity. Nature. 1962, 196: 85-86. 10.1038/196085a0.
Mazzucotellin E, Mastrangelo AM, Crosatti C, Guerra D, Stanca AM, Cattivelli L: Abiotic stress response in plants: when post-transcriptional and post-translational regulations control transcription. Plant Sci. 2008, 174: 420-431. 10.1016/j.plantsci.2008.02.005.
Luan S: Protein phosphatase in plants. Annu Rev Plant Biol. 2003, 54: 63-92. 10.1146/annurev.arplant.54.031902.134743.
Kim CY, Liu Y, Thorne ET, Yang H, Fukushige H, Gassmann W, Hildebrand D, Sharp RE, Zhang S: Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell. 2003, 15: 2707-2718. 10.1105/tpc.011411.
Opdenakker K, Remans T, Vangronsveld J, Cuypers A: Mitogen-activated protein (MAP) kinases in plant metal stress: regulation and responses in comparison to other biotic and abiotic stresses. Int J Mol Sci. 2012, 13: 7828-7853. 10.3390/ijms13067828.
Agrawal GK, Rakwal R, Iwahashi H: Isolation of novel rice (Oryza sativa L.) multiple stress responsive MAP kinase gene, OsMSRMK2, whose mRNA accumulates rapidly in response to environmental cues. Biochem Biophys Res Commun. 2002, 294: 1009-1016. 10.1016/S0006-291X(02)00571-5.
Jonak C, Nakagami H, Hirt H: Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004, 136: 3276-3283. 10.1104/pp.104.045724.
Chen Z, Zang J, Whetstine J, Hong X, Davrazou F, Kutateladze TG, Simpson M, Mao Q, Pan CH, Dai S, Hagman J, Hansen K, Shi Y, Zhang G: Structural insights into histone demethylation by JMJD2 family members. Cell. 2006, 125: 691-702. 10.1016/j.cell.2006.04.024.
Govind G, Vokkaliga H, Gowda T, Kalaiarasi PJ, Iyer DR, Muthappa SK, Nese S, Makarla UK: Identification and functional validation of a unique set of drought induced genes preferentially expressed in response to gradual water stress in peanut. Mol Genet Genomics. 2009, 281: 591-605. 10.1007/s00438-009-0432-z.
Rampey RA, Woodward AW, Hobbs BN, Tierney MP, Lahner B, Salt DE, Bartel B: An Arabidopsis basic helix-loop-helix leucine zipper protein modulates metal homeostasis and auxin conjugate responsiveness. Genetics. 2006, 174: 1841-1857. 10.1534/genetics.106.061044.
Skórzyńska-Polit E, Tukendorf A, Selstam E, Baszyński T: Calcium modifies Cd effect on runner bean plants. Environ Exp Bot. 1998, 40: 275-286. 10.1016/S0098-8472(98)00045-8.
Yang T, Poovaiah BW: Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003, 8: 505-512. 10.1016/j.tplants.2003.09.004.
Busov VB, Johannes E, Whetten RW, Sederoff RR, Spiker SL, Lanz-Garcia C, Goldfarb B: An auxin-inducible gene from loblolly pine (Pinus taeda L.) is differentially expressed in mature and juvenile-phase shoots and encodes a putative transmembrane protein. Planta. 2004, 218: 916-927. 10.1007/s00425-003-1175-4.
Ahsan N, Lee DG, Lee SH, Kang KY, Lee JJ, Kim PJ, Yoon HS, Kim JS, Lee BH: Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere. 2007, 67: 1182-1193. 10.1016/j.chemosphere.2006.10.075.
Mishra AK, Puranik S, Bahadur RP, Prasad M: The DNA-binding activity of an AP2 protein is involved in transcriptional regulation of a stress-responsive gene, SiWD40, in foxtail millet. Genomics. 2012, 100: 252-263. 10.1016/j.ygeno.2012.06.012.
Liu AN, Teng Y, Xu WZ, Ma M: AtARVQ1 encodes a novel VQ motif-containing protein involved in arsenate stress response regulation in Arabidopsis. Chin Sci Bull. 2011, 56: 1891-1898. 10.1360/972011-494.
Wang A, Garcia D, Zhang H, Feng K, Chaudhury A, Berger F, Peacock WJ, Dennis ES, Luo M: The VQ motif protein IKU1 regulates endosperm growth and seed size in Arabidopsis. Plant J. 2010, 63: 670-679. 10.1111/j.1365-313X.2010.04271.x.
Cheng Y, Zhou Y, Yang Y, Chi YJ, Zhou J, Chen JY, Wang F, Fan B, Shi K, Zhou YH, Yu JQ, Chen Z: Structural and functional analysis of VQ motif-containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiol. 2012, 159: 810-825. 10.1104/pp.112.196816.
Zhang JX, Wang C, Yang CY, Wang JY, Chen L, Bao XM, Zhao YX, Zhang H, Liu J: The role of Arabidopsis AtFes1A in cytosolic Hsp70 stability and abiotic stress tolerance. Plant J. 2010, 62: 539-548. 10.1111/j.1365-313X.2010.04173.x.
Yang LT, Qi YP, Lu YB, Guo P, Sang W, Feng H, Zhang HX, Chen LS: iTRAQ protein profile analysis of Citrus sinensis roots in response to long-term boron-deficiency. J Proteomics. http://dx.doi.org/10.1016/j.jprot.2013.04.025 (on line)
Westergren T, Ekblad L, Jergil B, Sommarin M: Phosphatidylinositol 4-kinase associated with spinach plasma membranes. Isolation and characterization of two distinct forms. Plant Physiol. 1999, 121: 507-516. 10.1104/pp.121.2.507.
Steinert P, Wissing JB, Wagner KG: Manganese-stimulated phosphatidylinositol 4-kinase from Dunaliella parva: purification and characterization. Plant Sci. 1994, 101: 105-114. 10.1016/0168-9452(94)90246-1.
Hamilton CA, Good AG, Taylor GJ: Induction of vacuolar ATPase and mitochondrial ATP synthase by aluminum in an aluminum-resistant cultivar of wheat. Plant Physiol. 2001, 125: 2068-2077. 10.1104/pp.125.4.2068.
Hossain Z, Komatsu S: Contribution of proteomic studies towards understanding plant heavy metal stress response. Front Plant Sci. 2013, 3: 310-
Xu Y, Gianfagna T, Huang B: Proteomic changes associated with expression of a gene (ipt) controlling cytokinin synthesis for improving heat tolerance in a perennial grass species. J Exp Bot. 2010, 61: 3273-3289. 10.1093/jxb/erq149.
Ferreira S, Hjernø K, Larsen M, Wingsle G, Larsen P, Fey S, Roepstorff P, Salomé Pais M: Proteome profiling of Populus euphratica Oliv. upon heat stress. Ann Bot. 2006, 98: 361-377. 10.1093/aob/mcl106.
Riccardi F, Gazeau P, de Vienne D, Zivy M: Protein changes in response to progressive water deficit in maize. Quantitative variation and polypeptide identification. Plant Physiol. 1998, 117: 1253-1263. 10.1104/pp.117.4.1253.
Jin Y, Zhang C, Yang H, Yang Y, Huang C, Tian Y, Lu X: Proteomic analysis of cold stress responses in tobacco seedlings. Afr J Biotech. 2011, 10: 18991-19004.
Forsthoefel NR, Cushman MA, Cushman JC: Posttranscriptional and posttranslational control of enolase expression in the facultative crassulacean acid metabolism plant Mesembryanthemum crystallinum L. Plant Physiol. 1995, 108: 1185-1195. 10.1104/pp.108.3.1185.
Zhao L, Sun YL, Cui SX, Chen M, Yang HM, Liu HM, Chai TY, Huang F: Cd-induced changes in leaf proteome of the hyperaccumulator plant Phytolacca americana. Chemospher. 2011, 85: 56-66. 10.1016/j.chemosphere.2011.06.029.
Narusaka Y, Narusaka M, Seki M, Umezawa T, Ishida J, Nakajima M, Enju M, Shinozaki K: Crosstalk in the responses to abiotic and biotic stresses in Arabidopsis: analysis of gene expression in cytochrome P450 gene superfamily by cDNA microarray. Plant Mol Biol. 2004, 55: 327-342. 10.1007/s11103-004-0685-1.
Gorinova N, Nedkovska M, Atanassov A: Cytochrome P450 monooxygenases as a tool for metabolization herbicides in plants. Biotechnol Biotechnol Eq. 2005, 19: 105-115.
Bassham JA, Krause GH: Free energy changes and metabolic regulation in steady-state photosynthetic carbon reduction. Biochim Biophys Acta. 1969, 189: 207-221. 10.1016/0005-2728(69)90048-6.
Collin V, Issakidis-Bourguet E, Marchand C, Hirasawa M, Lancelin JM, Knaff DB, Miginiac-Maslow M: The Arabidopsis plastidial thioredoxins: new functions and new insights into specificity. J Biol Chem. 2003, 278: 23747-23752. 10.1074/jbc.M302077200.
Yuan L, Xiao Y, Christopher AM: Plant cohesins, common themes and unique roles. Curr Protein Pept Sci. 2011, 12: 93-104. 10.2174/138920311795684904.
Lappartient GA, Vidmar JJ, Leustek T, Glass ADM, Touraine B: Inter-organ signaling in plants: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound. Plant J. 1999, 18: 89-95. 10.1046/j.1365-313X.1999.00416.x.
Heiss S, Schäfer HJ, Haag-Kerwer A, Rausch T: Cloning sulfur assimilation genes of Brassica juncea L.: cadmium differentially affects the expression of a putative low-affinity sulfate transporter and isoforms of ATP sulfurylase and APS reductase. Plant Mol Biol. 1999, 39: 847-857. 10.1023/A:1006169717355.
Ross J, Li Y, Lim E-K, Bowles DJ: Higher plant glycosyltransferases. Genome Biol. 2001, 2: 3004.1-3004.6.
Hou B, Lim EK, Higgins GS, Bowles DJ: N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. J Biol Chem. 2004, 279: 47822-47832. 10.1074/jbc.M409569200.
Cheng S: Effects of heavy metals on plants and resistance mechanisms. Environ Sci Pollut Res. 2003, 10: 256-264. 10.1065/espr2002.11.141.2.
Gichner T, Patková Z, Száková J, Demnerová K: Toxicity and DNA damage in tobacco and potato plants growing on soil polluted with heavy metals. Ecotox Environ Safe. 2006, 65: 420-426. 10.1016/j.ecoenv.2005.08.006.
Chou WC, Huang YW, Tsay WS, Chiang TY, Huang DD, Huang HJ: Expression of genes encoding the rice translation initiation factor, eIF5A, is involved in developmental and environmental responses. Physiol Plant. 2004, 121: 50-57. 10.1111/j.0031-9317.2004.00292.x.
Xu WL, Wang XL, Wang H, Li XB: Molecular characterization and expression analysis of nine cotton GhEF1A genes encoding translation elongation factor 1A. Gene. 2007, 389: 27-35. 10.1016/j.gene.2006.09.014.
Guo Y, Xiong L, Ishitani M, Zhu JK: An Arabidopsis mutation in translation elongation factor 2 causes superinduction of CBF/DREB1 transcription factor genes but blocks the induction of their downstream targets under low temperatures. Proc Natl Acad Sci U S A. 2002, 99: 7786-7791. 10.1073/pnas.112040099.
Gil-Quintana E, Larrainzar E, Seminario A, Díaz-Leal JL, Alamillo JM, Pineda M, Arrese-Igor C, Wienkoop S, González EM: Local inhibition of nitrogen fixation and nodule metabolism in drought-stressed soybean. J Exp Bot. 2013, 64: 2171-2182. 10.1093/jxb/ert074.
Chang WWP, Huang L, Shen M, Webster C, Burlingame AL, Roberts JKM: Patterns of protein synthesis and tolerance to anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry. Plant Physiol. 2000, 122: 295-317. 10.1104/pp.122.2.295.
Wang ZH, Wang ZF, Chen SS, Shi L, Xu FS: Proteomics reveals the adaptability mechanism of Brassica napus to short-term boron deprivation. Plant Soil. 2011, 347: 195-210. 10.1007/s11104-011-0838-3.
Alves M, Moes S, Jenö P, Pinheiro C, Passarinho J, Ricardo CP: The analysis of Lupinus albus root proteome revealed cytoskeleton altered features due to long-term boron deficiency. J Proteomics. 2011, 74: 1351-1363. 10.1016/j.jprot.2011.03.002.
Lee K, Bae DW, Kim SH, Han HJ, Liu X, Park HC, Lim CO, Lee SY, Chung WS: Comparative proteomic analysis of the short-term responses of rice roots and leaves to cadmium. J Plant Physiol. 2010, 167: 161-168. 10.1016/j.jplph.2009.09.006.
Lyzenga WJ, Stone SL: Abiotic stress tolerance mediated by protein ubiquitination. J Exp Bot. 2012, 63: 599-616. 10.1093/jxb/err310.
Macheroux P, Schmid J, Amrhein N, Schaller A: A unique reaction in a common pathway: mechanism and function of chorismate synthase in the shikimate pathway. Planta. 1999, 207: 325-334. 10.1007/s004250050489.
Jung KW, Kim YY, Yoo KS, Ok SH, Cui MH, Jeong BC, Yoo SD, Jeung JU, Shin JS: A cystathionine-β-synthase domain-containing protein, CBSX2, regulates endothecial secondary cell wall thickening in anther development. Plant Cell Physiol. 2012, 54: 195-208.
Singh AK, Kumar R, Pareek A, Sopory SK, Singla-Pareek SL: Overexpression of rice CBS domain containing protein improves salinity, oxidative, and heavy metal tolerance in transgenic tobacco. Mol Biotechnol. 2012, 52: 205-216. 10.1007/s12033-011-9487-2.
Banas A, Carlsson AS, Huang B, Lenman M, Banas W, Lee M, Noiriel A, Benveniste P, Schaller H, Bouvier-Navé P, Stymne S: Cellular sterol ester synthesis in plants is performed by an enzyme (phospholipid: sterol acyltransferase) different from the yeast and mammalian acyl-CoA:sterol acyltransferase. J Biol Chem. 2005, 280: 34626-34634. 10.1074/jbc.M504459200.
Bouvier-Navé P, Berna A, Noiriel A, Compagnon V, Carlsson AS, Banas A, Stymne S, Schaller H: Involvement of the phospholipid sterol acyltransferase1in plant sterol homeostasis and leaf senescence. Plant Physiol. 2010, 152: 107-119. 10.1104/pp.109.145672.
Jambunhathan N, Mahalingam R: Analysis of Arabidopsis growth factor gene 1 (GGF1) encoding a nudix hydrolase during oxidative signaling. Planta. 2006, 224: 1-11. 10.1007/s00425-005-0183-y.
Ogawa T, Yoshimura K, Miyake H, Ishikawa K, Ito D, Tanabe N, Shigeru Shigeoka S: Molecular characterization of organelle-type Nudix hydrolases in Arabidopsis. Plant Physiol. 2008, 148: 1412-1424. 10.1104/pp.108.128413.
Ogawa T, Ishikawa K, Harada K, Fukusaki E, Yoshimura K, Shigeoka S: Overexpression of an ADP-ribose pyrophosphatase, AtNUDX2, confers enhanced tolerance to oxidative stress in Arabidopsis plants. Plant J. 2009, 57: 289-301. 10.1111/j.1365-313X.2008.03686.x.
Huang H, Cao H, Niu Y, Dai S: Expression analysis of Nudix hydrolase genes in Chrysanthemum lavandulifolium. Plant Mol Biol Rep. 2012, 30: 973-982. 10.1007/s11105-011-0401-7.
Zhang Y, Mian MAR, Chekhovskiy K, So S, Kupfer D, Lai H, Roe BA: Differential gene expression in Festuca under heat stress conditions. J Exp Bot. 2005, 56: 897-907. 10.1093/jxb/eri082.
Hossain Z, Hajika M, Komatsu S: Comparative proteome analysis of high and low cadmium accumulating soybeans under cadmium stress. Amino Acids. 2012, 43: 2393-2416. 10.1007/s00726-012-1319-6.
May A, Berger S, Hertel T, Köck M: The Arabidopsis thaliana phosphate starvation responsive gene AtPPsPase1 encodes a novel type of inorganic pyrophosphatase. Biochim Biophys Acta. 1810, 2011: 178-185.
Li YC, Shi JX, Weiss D, Goldschmidt EE: Sugars regulate sucrose transporter gene expression in citrus. Biochem Biophys Res Commun. 2003, 306: 402-407. 10.1016/S0006-291X(03)00978-1.
Stitt M, von Schaewen A, Willmitzer L: “Sink” regulation of photosynthetic metabolism in transgenic tobacco plants expressing yeast invertase in their cell wall involves a decrease of the Calvin-cycle enzymes and an increase of glycolytic enzymes. Planta. 1991, 183: 40-50.
Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y: The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50: 207-218. 10.1111/j.1365-313X.2007.03044.x.
Deng W, Luo K, Li D, Zheng XL, Wei XY, Smith W, Thammina C, Lu LT, Li Y, Pei Y: Overexpression of an Arabidopsis magnesium transport gene, AtMGT1, in Nicotiana benthamiana confers Al tolerance. J Exp Bot. 2006, 57: 4235-4243. 10.1093/jxb/erl201.
Warren MA, Kucharski LM, Veenstra A, Shi L, Grulich PF, Maguire ME: The CorA Mg2+ transporter is a homotetramer. J Bacteriol. 2004, 186: 4605-4612. 10.1128/JB.186.14.4605-4612.2004.
Ma W, Smigel A, Verma R, Berkowitz GA: Cyclic nucleotide gated channels and related signaling components in plant innate immunity. Plant Signal Behav. 2009, 4: 277-282. 10.4161/psb.4.4.8103.
Arazi T, Sunkar R, Kaplan B, Fromm H: A tobacco plasma membrane calmodulin-binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J. 1999, 20: 171-182. 10.1046/j.1365-313x.1999.00588.x.
Sunkar R, Kaplan B, Bouche N, Arazi T, Dolev D, Talke I, Maathuis FJM, Sanders D, Bouchez D, Fromm H: Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous Arabidopsis CNGC1 gene confer Pb2+ tolerance. Plant J. 2000, 24: 533-542. 10.1046/j.1365-313x.2000.00901.x.
Wang J, Nakazato T, Kinya Sakanishi K, Yamada O, Tao H, Saito I: Single-step microwave digestion with HNO3 alone for determination of trace elements in coal by ICP spectrometry. Talanta. 2006, 68: 1584-1590. 10.1016/j.talanta.2005.08.034.
Ames BN: Assay of inorganic phosphate, total phosphate and phosphatase. Methods Enzymol. 1966, 8: 115-118.
Lichtenthaler HK: Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148: 350-382.
Bradford MM: A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72: 248-254. 10.1016/0003-2697(76)90527-3.
Hodges DM, DeLong JM, Forney CF, Prange RK: Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999, 207: 604-611. 10.1007/s004250050524.
Cao YY, Zhang Q, Chen YH, Zhao H, Lang YZ, Yu CM, Yang JC: Identification of differential expression genes in leaves of rice (Oryza sativa L.) in response to heat stress by cDNA-AFLP analysis. BioMed Res Int. 2013, 2013: 576189-
Bassam BJ, Caetano-Anolles G, Gresshoff PM: Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Boichem. 1991, 196: 80-83. 10.1016/0003-2697(91)90120-I.
Acknowledgement
This study was financially supported by the earmarked fund for China Agriculture Research System.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CPZ carried out most of the experiments and drafted the manuscript. YPQ participated in the design of the study. XY participated in the sequence alignment. LTY participated in the design of the study and coordination. PG performed the statistical analysis. XXZ carried out the measurement of Mn and Chl. FJK carried out the cultivation of seedlings. LSC designed and directed the study and revised the manuscript. All authors have read and approved the final manuscript.
Electronic supplementary material
12864_2013_5333_MOESM1_ESM.doc
Additional file 1:Manganese (Mn)-toxicity symptoms on leaves of Citrus grandis and C. sinensis . A: Control leaves of C. grandis; B: Mn-toxicity leaves of C. grandis; C: Control leaves of C. sinensis; D: Mn-toxicity leaves of C. sinenis. (DOC 4 MB)
12864_2013_5333_MOESM2_ESM.docx
Additional file 2:Homology of differentially expressed cDNA-AFLP fragments with known gene sequences in database using BLASTN algorithm along their expression patterns in Mn-toxicity leaves of Citrus grandis .(DOCX 34 KB)
12864_2013_5333_MOESM3_ESM.docx
Additional file 3:Homology of differentially expressed cDNA-AFLP fragments with known gene sequences in database using BLASTN algorithm along their expression patterns in Mn-toxicity leaves of Citrus sinensis .(DOCX 20 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhou, CP., Qi, YP., You, X. et al. Leaf cDNA-AFLP analysis of two citrus species differing in manganese tolerance in response to long-term manganese-toxicity. BMC Genomics 14, 621 (2013). https://doi.org/10.1186/1471-2164-14-621
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2164-14-621