Abstract
Background
Polyploidization is an important mechanism in plant evolution. By analyzing the leaf transcriptomes taken from the allotetraploid Nicotiana tabacum (tobacco) and parental genome donors, N. sylvesteris (S-Genome) and N. tomentosiformis (T-Genome), a phylogenomic approach was taken to map the fate of homeologous gene pairs in this plant.
Results
A comparison between the genes present in the leaf transcriptomes of N. tabacum and modern day representatives of its progenitor species demonstrated that only 33% of assembled transcripts could be distinguished based on their sequences. A large majority of the genes (83.6% of the non parent distinguishable and 87.2% of the phylogenetic topology analyzed clusters) expressed above background level (more than 5 reads) showed similar overall expression levels. Homeologous sequences could be identified for 968 gene clusters, and 90% (6% of all genes) of the set maintained expression of only one of the tobacco homeologs. When both homeologs were expressed, only 15% (0.5% of the total) showed evidence of differential expression, providing limited evidence of subfunctionalization. Comparing the rate of synonymous nucleotide substitution (Ks) and non-synonymous nucleotide substitution (Kn) provided limited evidence for positive selection during the evolution of tobacco since the polyploidization event took place.
Conclusions
Polyploidization is a powerful mechanism for plant speciation that can occur during one generation; however millions of generations may be necessary for duplicate genes to acquire a new function. Analysis of the tobacco leaf transcriptome reveals that polyploidization, even in a young tetraploid such as tobacco, can lead to complex changes in gene expression. Gene loss and gene silencing, or subfunctionalization may explain why both homeologs are not expressed by the associated genes. With Whole Genome Duplication (WGD) events, polyploid genomes usually maintain a high percentage of gene duplicates. The data provided little evidence of preferential maintenance of gene expression from either the T- or S-genome. Additionally there was little evidence of neofunctionalization in Nicotiana tabacum suggesting it occurs at a low frequency in young polyploidy.
Similar content being viewed by others
Background
Polyploidy occurs either through the combination of two or more genomes from different parents (alloploidization), or the multiplication of an endogenous genome (autoploidization). The majority of flowering plants have undergone polyploidization (whole genome duplication events [WGD]) during their evolutionary history, suggesting that it provides a mechanism that can increase the fitness of an organism [1], possibly through heterosis [2]. Two WGD events are dated to have occurred before the diversification of extant seed plants and extant angiosperms [3]. Analysis of the Arabidopsis thaliana genome supports three more recent WGD events (named γ, β and α). Evidence from investigations on the genome sequences of Vitis vinifera and Medicago truncatula[4–6], suggests that the first, or γ event, extends to all the core-eudicots and many other plant species. Polyploidization is relatively common in agricultural and commercial species, such as wheat (Triticum aestivum), potato (Solanum tuberosum), coffee (Coffea arabica) and cotton (Gossypium hirsutum), indicating that this evolutionary mechanism may be important in plant domestication.
Polyploidization involves complex genetic and epigenetic process and genome duplication is often followed by changes in gene expression and gene loss [7–14]. Complementary hypotheses that explain this phenomena suggest that either selection is based on absolute gene dosage, or relative gene dosage (dosage balance) [15]. The absolute gene dosage hypothesis states that gene networks have balanced states of interaction that are critical for proper function and any disturbances on the network’s stoichiometry of interaction are not optimal for plant survival. The relative dosage hypothesis argues that a gene product can have multiple interactions that may assist in the survival of the plant, upon which selection is based.
Duplicated genes generated by polyploidization events are referred to as homeologs. The fate of homeologous genes can be divided in four general categories; conservation or redundancy, nonfunctionalization (gene function loss for one copy), subfunctionalization (partitioning ancestral functions/expression patterns between duplicated genes) and neofunctionalization (evolution of a gene copy to a new function) [16, 17]. The relative ratio of these gene fates may differ between species.
Nicotiana species are excellent models for investigating plant polyploidization. Approximately 40% of Nicotiana genera are allotetraploids [18, 19]. With an estimated age of 0.2 Myr [20], Nicotiana tabacum is a relatively young allotetraploid originating through the hybridization of Nicotiana sylvesteris (maternal, S-genome donor) [21, 22] and Nicotiana tomentosiformis (paternal, T-genome donor) [21, 23]. Extensive studies within the Nicotiana genus, and specifically within N. tabacum including the first generation of a synthetic N. tabacum, have revealed a complex landscape for polyploid genome evolution [24]. Many evolutionary changes in the tobacco genome have been elucidated. They include evidence for an early genomic shock [25], a great increase in the frequency of heterozygosity and T-genome repeat losses leading to genome size reduction [26, 27]. Other evolutionary events, such as intergenomic translocations [24, 28, 29] and epigenetic patterns of 45S rDNA expression have been characterized as well [30]. In addition, gene expression studies of N. tabacum have been performed using microarrays [31], although the technology may have limited ability to distinguish between homeologs.
This study presents a characterization of the N. tabacum transcriptome constructed from an evolutionary perspective by combining a Next Generation Sequencing (NGS) and expression analysis with a phylogenetic approach applied on a genomic scale.
Results
Transcriptome assembly and annotation for a polyploid species
A set of expressed sequence tags (ESTs) was generated from leaves of N. tabacum and modern day representatives of its progenitor species N. sylvesteris and N. tomentosiformis. Test assemblies of the N. sylvesteris ESTs were generated using several programs (see Materials and Methods). GsAssembler produced longer contigs and was significantly faster than the other assembly programs (data not shown) so it was selected for further optimization.
An assembly strategy was adopted to maximize contig length while attempting to separate homeologous genes within N. tabacum. To identify optimal parameters, a set of assemblies were conducted using four EST datasets generated with 454 sequencing chemistry: i- N. tabacum ESTs, ii- N. tomentosiformis ESTs, iii- N. sylvesteris ESTs and iv- a combined dataset of the N. sylvesteris and N. tomentosiformis ESTs (to represent a synthetic polyploid transcriptome). The contigs generated from the four data sets were analyzed for a minimum overlap identity parameter set to a range of values between 75% and 99% (Figure 1). The N. tabacum ESTs and the synthetic polyploid data set produced a similar profile. An increase in the number of contigs was observed using a 97% identity setting (Figure 1). Unlike the N. tabacum and combined assemblies, the number of contigs in the individual N. sylvesteris and N. tomentosiformis assemblies did not increase at this level (Figure 1). The result suggested that 97% was the optimal identity threshold that could be used to separate homeologous sequences in the N. tabacum data set and homologous sequences in the combined data set without having a detrimental impact on the N. sylvesteris and N. tomentosiformis assemblies.
Nicotiana EST assemblies. Chart showing the number of contigs in EST assemblies generated with GsAssembler using minimum overlap identity levels between 75 and 99% (see Methods). Assemblies were carried out from four data sets; N. sylvesteris (green), N. tomentosiformis (blue), N. tabacum (red) and a hybrid set of N. sylvesteris and N. tomentosiformis (brown) sequences.
All four data sets showed an increase in the number of contigs when an identity setting of 99% was used. This level was considered too stringent as it was likely to be separating sequences based on sequencing errors (Figure 1). The assemblies based on an identity of 97% therefore provided the best data sets for subsequent analysis. This was further supported by manual inspection of contigs from the N. tabacum assemblies using the Tablet assembly viewer [32]. Manual inspection confirmed that contigs with more than 3 SNPs per 100 bp generated in the 95% identity assembly had correctly been separated into two contigs in the 97% identity assembly (data not shown).
Relative to the number of contigs in either individual assembly, the total number of contigs in the combined N. sylvesteris and N. tomentosiformis assembly was reduced, suggesting the collapse of orthologous sequences in the combined assembly. The lower number of contigs for the N. tabacum assembly compared with the N. sylvesteris and N. tomentosiformis assemblies may be partially explained by the higher number of sequences included in these assemblies. Increasing the number of N. tabacum reads with additional sequencing libraries, not included in this study, did indeed increase the number of contigs in the assembly (data not shown). However, a more likely explanation for the lower number of contigs in the N. tabacum and combined assemblies was there being no, or very low sequence polymorphisms between the orthologous genes of the ancestral parents, making it impossible to separate them during assembly at 97%.
To investigate the percentage of homeologs that were collapsed during the assembly process, reads from N. tabacum, N. sylvesteris and N. tomentosiformis were mapped onto the N. tabacum assembly produced with the 97% identity setting. Sequence polymorphisms could not be detected between the reads from the three species for 67% of the N. tabacum contigs (9718 contigs), indicating that sequences for a large portion of the assembly, were likely to have collapsed. This also meant that these sequences were not amenable to subsequent phylogenetic analysis. The remaining 33% of the sequences showed SNPs between the N. tomentosiformis and N. sylvesteris orthologs. When mapped against the N. tabacum assembly, a low number of N. tabacum sequences (3.4%) showed SNPs either supporting the possibility of collapsing of homeologous sequences in the assembly, or sequencing errors.
The three separate assemblies for N. tabacum, N. sylvesteris and N. tomentosiformis transcripts were further assembled using GsAssembler. In order to cluster the homeologous and homologous sequences across all three assemblies, the identity parameter of this combined Nicotiana assembly was set to the lower stringency level of 95%. PhygOmicss, a custom data processing pipeline was developed in order to carry out a phylogenetic analysis of the sequences for the entire transcriptome. The 17,220 clusters generated from the combined Nicotiana assembly were processed through the pipeline which selected 7974 clusters containing at least one contig of each individual species for further analysis. Alignments were then extracted and filtered by the length of the sequence overlap (minimum of 100 bp) and the average alignment percentage identity (minimum of 75%).
The consensus sequences for each of the clusters were annotated based on homology using BlastX [33]. Searches were conducted against four datasets and annotation results are summarized in Table 1. As expected, the Nicotiana clusters demonstrated the highest number of matches against the tomato gene model dataset (ITAG2). InterProScan [34] was used to perform a protein domain analysis on 13,504 of the clusters, 6913 of which had been annotated using the BlastX method previously described.
Topology analysis of Nicotiana genes
The combined Nicotiana assembly was used to construct a set of phylogenetic trees for each cluster of sequences. Phylogenetic trees were constructed for 14,344 Nicotiana clusters that also contained at least one possible Solanum lycopersicum homolog as an out group. Bootstrapping analysis and filtering of these clusters (see Materials and Methods) identified 968 as containing either a single N. tabacum sequence, or two N. tabacum sequences along with the N. tomentosiformis, N. sylvesteris and S. lycopersicum sequence members.
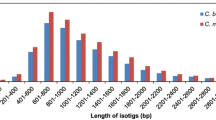
Neighbor Join (NJ) and Maximum Likelihood (ML) methods were used to build phylogenetic trees for each of the 968 clusters (Additional files 1 and 2). The topologies of these trees were grouped into 11 categories. The distribution of the results in these 11 categories was similar between the NJ and ML methods (Figure 2). Approximately 10% of the clusters contained two N. tabacum sequences, each of which could be associated with the respective N. sylvesteris and N. tomentosiformis sequences. This topology would be expected if both the T and S homeologs had been maintained and expressed by the plant following polyploidization (AB_AC; Figure 2). The majority (approximately 90%) of clusters, however, contained only a single N. tabacum sequence the majority of which could be associated with either the N. sylvesteris (AB_C) or N. tomentosiformis (AC_B) sequence (Figure 2). Given that these clusters contained genes where SNPs existed between the parental homeologs, reducing the likelihood of collapse of the sequences during assembly, the abundance of this latter topology is most likely explained by either gene subfunctionalization (when the absent gene is not expressed in the tissue analyzed), or gene loss/nonfunctionalization.
Phylogenomic analysis of Nicotiana gene clusters. Bar chart showing the number of Nicotiana genes that were present in a set of pre-defined phylogenetic tree topologies. Genes from the N. tabacum, N. sylvesteris and N. tomentosiformis assemblies were clustered and phylogenetic trees for each cluster were generated by Maximum Likelihood (ML; black bars) and Neighbour Joining (NJ; open bars) methods using S. lycopersicum as an out-group. The different tree topologies are shown along the x-axis with N. tabacum (A; Ntab) N. sylvesteris (B; Nsyl), N. tomentosiformis (C; Ntom) and S. lycopersicum (Slyc) genes represented in text and/or dendrogram form.
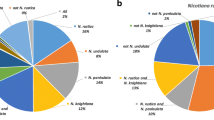
Gene Ontology (GO) analysis of the most abundant topologies from the Nicotiana data (AB_AC, AB_C, AC_B and BC_A) was performed [38]. Figure 3 shows the representation of GO Biological process terms (levels 2 and 3) for these topologies. No significant differences between AB_AC, AB_C and AC_B were observed relative to the biological process categories. The same was true when comparing AB_C and AC_B topologies for cellular component and molecular function categories compared to the global list of the combined Nicotiana consensus sequences. Three percent of the trees showed an unexpected topology with the N. sylvesteris and N. tomentosiformis sequences being more closely related to each other than to the N. tabacum sequences (BC_A; Figure 2). While false clustering of an N. tabacum paralog with the N. sylvesteris and N. tomentosiformis sequences is the most likely explanation for the anomaly, GO analysis showed significant overrepresentation (P < 0.05) in some categories, such as pathogenesis signaling (see Additional file 1), for these clusters, suggesting that they may contain genes with an interesting evolutionary history. However, it should be noted that this set of clusters contained fewer than 50 members.
Gene Ontology analysis of Nicotiana gene clusters. Plot showing the percentage of Nicotiana gene clusters annotated with level 2 (A) and level 3 (B) Biological Process Gene Ontology terms for all gene clusters and each of the main phylogenetic tree topologies (AB_AC, AB_C, AC_B and BC_A). Bars are coloured according to topology group (see inset key for identification).
Identification and expression analysis of Nicotiana tabacum homeologs
Estimations of gene expression levels were calculated based on the number of sequence reads and used to compare gene expression levels for the three different Nicotiana species. For the 9718 N. tabacum transcript clusters (67% of total number of clusters) where there were no reliable inter-specific SNPs that could be used to identify the ancestral origin, only 2171 transcript clusters contained five or more reads for each of the three Nicotiana species. The same expression levels (R < 7, see Materials and Methods) across all three species were observed for 83.6% of these genes. Among the remaining differentially expressed genes, the most frequent category was N. tabacum genes (with no distinction between homeologs) overexpressed in comparison with N. sylvesteris and N. tomentosiformis (4.7%), followed by N. tabacum genes with similar expression to the N. sylvesteris homolog (4.5%) and N. tomentosiformis homolog (4.2%). Only 0.7% of the N. tabacum genes were expressed at a lower level when compared with both parental sequences. The rest of the transcripts (2.3%) showed variable trends relative to differential expression between all the transcripts (for example, over-expressed compared to one of the parents and the contrary when compared to the other parent).
741 of the 968 gene clusters described above contained 975 N. tabacum consensus sequences (5.3% of the total N. tabacum sequences) that could be assigned ancestral origin based on the phylogenetic trees. 482 of these sequences were assigned to N. sylvesteris (S) origin and 493 sequences were assigned to N. tomentosiformis (T) origin. A total of 103 gene clusters (10.6% of the topology analyzed clusters, 0.6% of the total) showed differential expression (R > = 7): 51 gene clusters (0.3% of the total) for N. tabacum S-homeologs (ie, AB_C), of which 22 clusters (0.1% of the all clusters) were overexpressed in comparison with N. sylvesteris homeologs. Similar results were observed for N. tabacum T-homeologs (from topology AC_B); 52 clusters (0.3%) showed differential expression (R > 7) and of them 8 N. tabacum clusters (0.05%) were overexpressed in comparison with N. tomentosiformis.
Of the 274 AB_AC gene clusters containing two N. tabacum sequences, 77 clusters were selected where the consensus sequences were built with at least 5 reads. Figure 4 shows scatter plots comparing expression levels of the homeologous and homologous gene pairs for these gene clusters.
Expression level of Nicotiana gene pairs. Scatter plot showing the expression level (RPKM) for the N. sylvesteris / S genome gene (x-axis) versus N. tomentosiformis / T genome gene (y-axis) for homologous gene pairs (open red circles) and homeologous N. tabacum gene pairs (closed black circles). Solid black line across diagonal represents no difference in gene expression level between species.
Differential expression (R > = 7, see Materials and Methods) was observed for 27.3% of the N. tabacum homeologous gene pairs (21 of the 77 gene clusters, 2.2% of the topology analyzed clusters, 0.1% of the total transcribed genes). In comparison to the parental homeologs, 3.9% of the T-genes (3 clusters) and 11.7% of the S-gene (9 clusters) were over-expressed. Only 3.9% of the genes demonstrated differential expression when comparing T-genes with S-genes in N. tabacum (3 clusters, in one of which S-gene expression was higher than the T-gene). In comparison, 22.7% of the homologous genes in this set were differentially expressed between the N. tomentosiformis and N. sylvesteris samples. A more consistent level of gene expression between N. tabacum homeologs was also indicated by the Pearson correlation coefficient, which was higher between these genes than between the N. sylvesteris and N. tomentosiformis homeologs (R2 values of 0.93 and 0.82, respectively). The increased level of differential expression between the homologous genes pairs may simply reflect that the comparison was conducted using independent samples and may be due to experimental/biological variation. The homeologs comparison was conducted between genes from the same N. tabacum sample. However, the data clearly suggests that in the vast majority of cases when both N. tabacum homeologs are expressed in the same tissue (such as the leaf tissue analyzed here) there is little difference in expression on a transcriptional level.
Given the small number of homeologous gene pairs showing differential expression, the function of these genes were analyzed. Genes with higher expression of the S homeolog showed over-representation of GO terms associated with the biological processes for proteolysis, protein folding and aldehyde metabolism. Genes with higher expression of the T homeolog showed over-representation of GO terms associated with the biological processes for oligopeptide transport and translation.
Non-synonymous and synonymous site substitution rates between N. tabacum, N. sylvesteris and N. tomentosiformis species
Comparison of expression in only a single tissue/organ is too limited to differentiate between redundancy and subfunctionalization. A more extensive expression study might provide the ability to distinguish between these two evolutionary processes. Cases of neofunctionalization, however, could be distinguished by a comparison of the gene sequences in the current data set.
Changes to a gene sequence resulting in an altered protein sequence potentially alters the function of that gene. A comparison of the rate of synonymous (Ks) and non-synonymous (Kn) nucleotide substitutions provides insights into the evolutionary history of a gene [39, 40]. Genes showing a low rate of non-synonymous substitutions are likely to have undergone strong selective pressure to be conserved more faithfully and thus their function maintained. Genes showing a relatively high level of non-synonymous substitutions are likely to have undergone positive selection and possible neofunctionalization [41].
To estimate the rate of synonymous (Ks) and non-synonymous (Kn) nucleotide substitutions, an analysis was carried out using clusters of genes selected when topology analysis suggested that either one or both N. tabacum homeologs were maintained and could be assigned to T or S origin, (mainly topologies AB_AC, AB_C, AC_B and BC_A, Figure 2; 787 clusters, 3251 sequences). The ratio between Kn and Ks (ω) for each pair of sequences was also calculated. Figure 5 shows the distribution of Ks for sequence pair comparisons between the Nicotiana species. For reference, a comparison between N. tabacum and S. lycopersicum genes is also shown (Figure 5A). This older divergence event showed a higher rate of Ks relative to the comparisons between the Nicotiana species. Peak distribution of Ks values were approximately 0.27, compared to approximately 0.09 for the N. sylvesteris and N. tomentosiformis. Ks values were even lower for the N. tabacum to N. sylvesteris and N. tomentosiformis comparisons (Figure 5). The high number of sequence pairs with a Ks value < 0.01 in the comparison between N. tabacum and N. sylvesteris (397), or N. tomentosiformis (390) as compared to the number between N. tabacum and S. lycopersicum (39) or between N. sylvesteris and N. tomentosiformis (96) suggests that a high percentage of the N. tabacum genes have not diverged from their ancestral sequences (Figure 5).
Nucleotide substitution rates in Nicotiana genes. Frequency histograms showing the rate of synonymous nucleotide substitutions (Ks) in orthologous genes between N. tabacum and S. lycopersicum (A), N. sylvesteris and N. tomentosiformis (B), N. tabacum and N. sylvesteris (C) and N. tabacum and N. tomentosiformis (D).
Analysis of the Kn/Ks ratio demonstrated that the majority of genes had an ω value lower than 1, suggesting that few genes had undergone positive selection during the evolution of N. tabacum (assuming the limitations of Kn/Ks for the gene positive selection studies [42]). Only 3% of the clusters showed positive selection associated to N. sylvesteris and N. tomentosiformis homeologs and the correspondent homeologs (N. tabacum S- and T-genome), but no positive selection between the N. tabacum and the parental homeologs pairs (N. tabacum S-genome/N. sylvesteris or N. tabacum T-genome/N. tomentosiformis). The GO annotations associated with the small number of genes that were undergoing positive selection showed a similar distribution across the three species and corresponded to the more representative GO term categories such as metabolic process, cellular process, cell, catalytic activity and binding (Figure 6). Genes with an ω > 1 for the N. sylvesteris and N. tabacum (T-genome) homolog pairs (22 clusters) showed overrepresentation of the level 2 biological process ontologies: biological regulation, cellular component organization and regulation of a biological process. Examples of these genes include cluster 00509 (similar to Arabidopsis thaliana APL transcription factor involved in biological regulation) and 02926 (NAC domain protein involved in biological regulation). The 23 genes with an ω > 1 for the N. tomentosiformis and N. tabacum (S-genome) homolog pairs showed over-representation of the GO terms cellular process, developmental process and metabolic process. This included clusters 04302 (similar to Arabidopsis thaliana E3 ubiquitin-protein ligase SINAT2 involved in some developmental process), 04210 (Tyrosyl-tRNA synthetase) and 02320 (similar to cell division protein ftsy).
Evolutionary rates in Nicotiana genes from different Gene Ontology groups. Non-synonymous: synonymous nucleotide subtraction ratio (ω) values for Nicotiana genes separated according to their Level 2 (A) and Level 3 (B) Biological Process, Level 2 (C) and Level 4 (D) Cellular Component and Level 2 (E) and Level 3 (F) Molecular Function Gene Ontology annotations. Omega values for comparisons between homologous N. sylvesteris and N. tomentosiformis gene pairs (black circles), N. tabacum and N. sylvesteris gene pairs (green circles) and N. tabacum and N. tomentosiformis gene pairs (red circles) are shown.
Within the set of genes analyzed, there were no instances of homeologous genes from N. tabacum demonstrating positive selection (ω > 1). A comparison between the respective N. tomentosiformis and N. sylvesteris homeologs also did not show any instances of positive selection. This absence of positive selection suggests that the majority of positive selection represented in the gene set analyzed occurred following the divergence of the two ancestral species rather than since the formation of N. tabacum. It also suggests that the rate of neofunctionalization in N. tabacum has been relatively low.
Discussion
Polyploid species sequence assembly
Using a next generation sequencing approach, leaf transcriptome sequence data was generated for the allotetraploid N. tabacum and its progenitor species N. sylvesteris and N. tomentosiformis. These sequences were assembled into species specific sets of unigenes and then further combined into a consensus set of clusters for the three species. The process of assembly revealed that default parameters of sequence assemblers were probably not stringent enough when working with sequences originating from polyploid species. Sequencing errors, such as homopolymer length issues associated with pyrosequencing, can further confound this problem by potentially masking low polymorphism content between homeologs. Other sequencing technologies, such as Illumina, may not be impacted by this homopolymer problem, but read length may be a limiting factor given the requirement that a single read must contain at least one polymorphism per overlapping region. These factors should be taken into consideration for any future assembly attempts on polyploid species and the methodology applied for the assembly of an allopolyploid transcriptome in this study could be useful for guiding future genome assembly work in polyploidy.
Additionally, the number of collapsed homeologs was estimated in N. tabacum assembled transcripts (using the 97% identity assembly) based on SNPs shared with N. sylvesteris or N. tomentosiformis reads. In this analysis, only 3.4% of N. tabacum transcripts were polymorphic and shared SNPs with the parental transcripts. This methodology cannot be applied for transcripts lacking SNPs in the transcript fragment analyzed (67% of the transcripts). More information could be obtained by deeper transcriptomic sequencing (more mapped sequences and more reliable SNP calling), use of longer sequences (increasing the possibility to find a parent relative SNP) or genomic DNA sequencing (where the intron sequencing, being more diverge region, could increase the number of parent relative SNPs).
Homeologous gene fate in Nicotiana tabacum
Based on the leaf transcriptome data for the Nicotiana species generated in this study, a pipeline was developed to carry out a phylogenetic analysis on a genomic scale. The PhygOmicss pipeline works on a single transcriptome set, but can be applied to transcriptomic data from multiple tissues/organs, or gene models from genomic sequence data.
The majority of the N. tabacum transcripts (69%) did not show any polymorphisms with the parental sequences, making it impossible to distinguish the homeologous genes and excluding the possibility of neofunctionalization in these genes. Additionally the expression analysis of clusters with genes expressed above background level (more than 5 reads), revealed that the expression of a majority of these genes was not changed (83.6% of genes in clusters; 57.7% of the total transcribed genes) between these three species. With this level of conserved expression, the possibility of subfunctionalization is low.
A more specific topology analysis with the newly developed PhygOmicss pipeline revealed that in N. tabacum transcripts where homeologous genes can be differentiated there was evidence for the presence of only a single homeolog (90% of gene clusters, 6% of the total transcribed genes). Given that the data is transcriptomic, it is not possible to distinguish between gene loss and subfunctionalizaton. Tissue-specific gene silencing [14] provides one possible mechanism of gene subfunctionalization and may partially explain the pattern observed in the Nicotiana topologies. An analysis of a broader set of tissues might resolve the question and increase the chance of detecting expression differences in any individual genes. However, studies in other polyploid plants suggest that only a small number of genes display tissue specific gene silencing. For example, a similarly low level of gene silencing (around 1-5%) was estimated in both synthetic allotetraploid wheat [8] and synthetic cotton [10], and results from gene expression analysis of Tragopogon miscellus showed a similar trend (3.4%) [43]. Even lower estimates of silencing were suggested from experiments with an early allotetraploid formed by the hybridization of Arabidopsis thaliana and Cardaminopsis arenosa (> 0.4%) [7].
Based on the distribution of topologies and the relative expression level of homeologous genes, there was little evidence to suggest preferential loss, or transcriptional silencing of genes from one or other progenitor genomes from the sub-set of Nicotiana sequences that this analysis could be completed on. This is in contrast to the apparent preferential loss of repetitive sequences from the T genome in N. tabacum, as shown in a recent study also using 454 sequencing in these Nicotiana species [27]. Previous studies in other allotetraploids have shown preferential expression of homeologous genes. For example, there is evidence of preferential expression of the D genome in cotton [44]. Differential expression was shown for 22% of homeologous genes pairs in the 40 generation-old allotetraploid T. miscellus[13], similar to the 27% of N. tabacum genes observed in this study. It should also be noted that genes expressed in the leaf tissue at a very low level may have been missed in the transcriptome sets, particularly since clusters with less than 5 sequence members were removed from the analysis. As such, increasing the sequence depth might reveal more differentially expressed homeologous genes, but it is unlikely that this will increase the contribution of subfunctionalization extensively.
With the caveat that this study was based on a subset of genes identified in the leaf transcriptomes of Nicotiana species, the data would suggest the expression of homeologous genes is mostly conserved between N. tabacum and its parent relatives and supporting the hypothesis of gene dosage compensation [15, 45] reported previously in other species [46]. This level may be over-estimated as the transcriptome was sampled in only one tissue type, thus reducing the possibility of observing subfunctionalization. However, based on the levels observed in other species [7, 8, 10, 43] subfunctionalization is unlikely to account for a large proportion of genes.
There is also limited evidence of neofunctionalization having occurred in N. tabacum, based on comparison of the homeologous and homologous gene sequences. Indeed, no genes could be identified as undergoing positive selection in N. tabacum that did not also show the same response between N. sylvesteris and N. tomentosiformis. This suggests that these differences may have predated the formation of tobacco. Again, the apparent low level of neofunctionalization may be explained by only having sampled the leaf transcriptome. Sequencing transcripts from other tissues, perhaps more specifically involved in secondary metabolite synthesis, may increase the likelihood of identifying genes showing positive selection in tobacco; two such examples are trichomes [47] or roots, where alkaloids, including nicotine, are synthesized [48].
In addition to an increased spatial and temporal coverage of the transcriptome for the Nicotiana species covered in this study, it would be interesting to compare the proportion of subfunctionalization and neofunctionalization in tobacco with an older Nicotiana allotetraploid species, such as Nicotiana nesophila (dated approx. 4.5 Myr old), or Nicotiana benthamiana (dated > 10 Myr old) [20]. Similarly, a comparative analysis of allele selection between wild and cultivated N. tabacum varieties might provide insight into the role of homologous genes in the species’ domestication process. Gene duplication plays an important role in the successful transition of a wild species into its cultivated relatives, as shown for several wheat loci [49]. Indeed there are also examples for duplicated genes from diploid species playing an important role in domestication, including GRAIN INCOMPLETE FILLING 1 (GIF1) and the cell wall invertase OsCIN1 in [50].
Conclusions
This study represents the first time that a phylogenetic analysis of the tobacco genes has been carried out on a genome scale to further elucidate the complex evolutionary history of the species. Transcriptome assembly for polyploid species possesses the intrinsic difficulty of homeolog collapse. 67% of the N. tabacum assembled transcripts lack any polymorphism that can be used to elucidate the sequence origin. Read depth, read length and use of more variable regions such as introns will be critical to dissecting these genes.
There was evidence of a general maintenance of the expression levels between N. tabacum, N. sylvesteris and N. tomentosiformis homeologs. Despite the conservation of transcriptomic levels in tobacco, there was little evidence for the occurrence of neofunctionalization, suggesting that, at 0.2 Myr old, tobacco may be too young evolutionarily and that this is a more common fate for duplicated genes in older polyploidy species. There may, however, be particular interest in comparing cultivated with more primitive varieties using the method developed here in order to identify the genes selected during the domestication of tobacco. The low level of neofunctionalization may make such an analysis easier.
Methods
Plant material
Nicotiana tabacum (cv. K326), N. sylvesteris and N. tomentosiformis plants (in-house accessions available at ATC) were grown in a glass house on soil (Levingtons M2) under 16 h: 8 h light dark cycles. Fully expanded green leaves were harvested from 3–4 month old plants, snap frozen in liquid nitrogen and stored at −80°C.
Transcriptome sequencing
Leaf samples were ground under liquid nitrogen and total RNA was extracted using Trizol (Invitrogen, Paisley) and purified with RNeasy spin columns (Qiagen, Crawley, UK) according to the manufacturer’s instructions. mRNA was isolated with a Dynabead kit and quantified using a RiboGreen assay (Invitrogen, Paisley, UK).
Sequencing libraries were generated from 200 ng mRNA using the cDNA Rapid Library preparation method and sequenced on the GS FLX Ti according to manufacturer’s instructions (Roche, Burgess Hill, UK).
Sequence assembly and annotation
Test assemblies of the N. sylvesteris ESTs were carried out with default parameters using the assemblers GsAssembler (version 2.5.3; Roche), MIRA (version 3.0.5) and CAP3 (version 10/15/07).
Sequence assembly was performed on an SGN server (Red Hat Enterprise Linux Server release 5.4, CPUs: 48 cores, RAM: 256 Gb). GsAssembler version 2.5.3 was used with the option cDNA enabled. Seven different assemblies were created with identity values of 75, 80, 85, 90, 95, 97 and 99 in a minimum overlap length of 40 bp for each of the samples plus an extra sample with half of a 454 run of N. sylvesteris and half of a run of N. tomentosiformis. Contigs were selected using a perl script with a length cutoff value of 40 bp. The reassembly of the contigs for each sample was performed with the same software using an identity percentage value of 95. Contigs with a length of 2000 bp or longer were reassembled with CAP3 [51].
The collapse of homeologous sequences was evaluated by remapping all the Nicotiana reads with Bowtie [52] using the N. tabacum transcriptome assembly with 97% minimum identity as reference sequence. The mapping file in sam format was filtered and SNPs were called using Samtools and Bcftools [53]. SNP files were loaded into a generic Postgres database to perform a simple full outer join search.
Sequences were annotated using the basic local alignment search tool (blastx [35]) with the databases: GenBank nr [33], Swissprot [35] and TAIR9 [36] and 1e-20 e-value cutoff. Proteins were predicted using EstScan [54] and domain annotation was performed using InterProScan [34]. Gene Annotations were analyzed with the Bioconductor module goProfile [38].
Tree topology analysis
Tree topology analyses were performed with the PhygOmicss pipeline (manuscript in preparation). Sequence alignments were extracted from the assembly ace file with a Perl script integrated into the PhygOmicss pipeline. Solanum lycopersicum sequence homeologs were assigned based on the BLAST [37] results of the consensus sequence of the Nicotiana alignments with the Tomato Gene Model ITAG2 dataset. Only matches with alignment lengths of more than 100 bp and a nucleotide identity percentage of at least 70% were selected as homeologs. They were aligned with the Nicotiana sequences using ClustalW [55], Mafft [56] and Muscle [57] programs as an integrated part of the PhygOmicss pipeline. Mis-alignments were quantified using the global alignment length and the identity percentage average of each alignment, discarding the alignments with lengths shorter than 100 bp and identity percentages lower than 75%. ClustalW was run with non-default parameters (see Additional file 2 for the configuration parameters for the pipeline) as the preferred alignment program based on a minimum number of mis-alignments (data not shown). A pruning Perl script that is part of the pipeline was used to select closely related sequences for each alignment, based on a maximum alignment score (manuscript in preparation). Alignments that did not include members of one of the selected species (N.tabacum, N.sylvesteris, N.tomentosiformis and S. lycopersicum) were discarded. Phylogenetic trees using S.lycopersicum sequence as out-group were constructed with Phylip [58] following two methods: Neighbor-joining [59] and Maximum Likelihood [60]. A bootstrapping with 1000 replicates was also performed for each tree. Trees containing nodes with low bootstrap support (under 60%) were discarded. 968 isotigs (5.6% of the total isotigs produced in the assembly) produced trees that were able to be analysed based on these parameters.
Topology comparisons were performed using a Perl module Bio::Tree::Topology as an element of the pipeline (see additional file 3 for the PhygOmicss configuration file). This module compares if the tree obtained is the same after the replacement of the tree leaves (contig ids) with the sample source (species) and the branch length with an equivalence value (0.01 as length cutoff value).
Homeolog identification
Homeolog identification was performed by leaf species identity in a neighbor comparison using the PhygOmicss pipeline. Homeolog assignment was performed based in the closest parental homolog in the tree structure. A cutoff value of 90% identity and 60 bp length in the alignment between the candidate homeologs and the reference sequence were used.
Expression analysis
Expression analysis was performed parsing the assembly .ace files using a Perl script. Read count and RPKM calculation [61] was performed with Perl scripts available at the Solgenomics GitHub page (https://github.com/solgenomics/sgn-home/tree/master/aure/scripts/phylo/PhygOmicss). The R statistical differential expression value was calculated as described by Stekel [62].
Non synonymous-synonymous analysis of N. tabacum homeologs
Non-synonymous to synonymous analysis was performed using Codeml from the PAML software package [63] through the PhygOmic pipeline. CDS sequences used with Codeml were predicted with GsAssembler (longest 6 frame method). The results were parsed using a Perl script. Clusters with pairs with omega values = 99 were removed from the analysis. A close inspection of majority of them revealed sequences with an identity of 100% where the Kn/Ks ratio was 0/0.
Abbreviations
- Myr:
-
Million years
- RPKM:
-
Reads per kilobase per million of reads
- NGS:
-
Next generation sequencing.
References
Proost S, Pattyn P, Gerats T, Van de Peer Y: Journey through the past: 150 million years of plant genome evolution. Plant J. 2011, 66 (1): 58-65. 10.1111/j.1365-313X.2011.04521.x.
Chen ZJ: Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 2010, 15 (2): 57-71. 10.1016/j.tplants.2009.12.003.
Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, de Pamphilis CW: Ancestral polyploidy in seed plants and angiosperms. Nature. 2011, 473 (7345): 97-100. 10.1038/nature09916.
Bowers JE, Chapman BA, Rong J, Paterson AH: Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 2003, 422 (6930): 433-438. 10.1038/nature01521.
Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, Vezzi A, Legeai F, Hugueney P, Dasilva C, Horner D, Mica E, Jublot D, Poulain J, Bruyere C, Billault A, Segurens B, Gouyvenoux M, Ugarte E, Cattonaro F, Anthouard V, Vico V, Del Fabbro C, Alaux M, Di Gaspero G, Dumas V, French-Italian Public Consortium for Grapevine Genome Characterization: The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007, 449 (7161): 463-467. 10.1038/nature06148.
Blanc G, Wolfe KH: Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell. 2004, 16 (7): 1667-1678. 10.1105/tpc.021345.
Comai L, Tyagi AP, Winter K, Holmes-Davis R, Reynolds SH, Stevens Y, Byers B: Phenotypic instability and rapid gene silencing in newly formed arabidopsis allotetraploids. Plant Cell. 2000, 12 (9): 1551-1568.
Kashkush K, Feldman M, Levy AA: Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics. 2002, 160 (4): 1651-1659.
Wang J, Tian L, Lee HS, Wei NE, Jiang H, Watson B, Madlung A, Osborn TC, Doerge RW, Comai L, Chen ZJ: Genomewide nonadditive gene regulation in Arabidopsis allotetraploids. Genetics. 2006, 172 (1): 507-517.
Adams KL, Percifield R, Wendel JF: Organ-specific silencing of duplicated genes in a newly synthesized cotton allotetraploid. Genetics. 2004, 168 (4): 2217-2226. 10.1534/genetics.104.033522.
Thomas BC, Pedersen B, Freeling M: Following tetraploidy in an Arabidopsis ancestor, genes were removed preferentially from one homeolog leaving clusters enriched in dose-sensitive genes. Genome Res. 2006, 16 (7): 934-946. 10.1101/gr.4708406.
Stupar RM, Bhaskar PB, Yandell BS, Rensink WA, Hart AL, Ouyang S, Veilleux RE, Busse JS, Erhardt RJ, Buell CR, Jiang J: Phenotypic and transcriptomic changes associated with potato autopolyploidization. Genetics. 2007, 176 (4): 2055-2067. 10.1534/genetics.107.074286.
Buggs RJ, Chamala S, Wu W, Gao L, May GD, Schnable PS, Soltis DE, Soltis PS, Barbazuk WB: Characterization of duplicate gene evolution in the recent natural allopolyploid Tragopogon miscellus by next-generation sequencing and Sequenom iPLEX MassARRAY genotyping. Mol Ecol. 2010, 19 (Suppl 1): 132-146.
Buggs RJ, Zhang L, Miles N, Tate JA, Gao L, Wei W, Schnable PS, Barbazuk WB, Soltis PS, Soltis DE: Transcriptomic shock generates evolutionary novelty in a newly formed, natural allopolyploid plant. Curr Biol. 2011, 21 (7): 551-556. 10.1016/j.cub.2011.02.016.
Freeling M, Thomas BC: Gene-balanced duplications, like tetraploidy, provide predictable drive to increase morphological complexity. Genome Res. 2006, 16 (7): 805-814. 10.1101/gr.3681406.
Rastogi S, Liberles DA: Subfunctionalization of duplicated genes as a transition state to neofunctionalization. BMC Evol Biol. 2005, 5: 28-10.1186/1471-2148-5-28.
Hahn MW: Distinguishing among evolutionary models for the maintenance of gene duplicates. J Hered. 2009, 100 (5): 605-617. 10.1093/jhered/esp047.
Goodspeed TH: On the Evolution of the Genus Nicotiana. Proc Natl Acad Sci USA. 1947, 33 (6): 158-171. 10.1073/pnas.33.6.158.
Leitch IJ, Hanson L, Lim KY, Kovarik A, Chase MW, Clarkson JJ, Leitch AR: The ups and downs of genome size evolution in polyploid species of Nicotiana (Solanaceae). Ann Bot. 2008, 101 (6): 805-814. 10.1093/aob/mcm326.
Clarkson JJ, Lim KY, Kovarik A, Chase MW, Knapp S, Leitch AR: Long-term genome diploidization in allopolyploid Nicotiana section Repandae (Solanaceae). New Phytol. 2005, 168 (1): 241-252. 10.1111/j.1469-8137.2005.01480.x.
Goodspeed TH: The genus Nicotiana. 1954, Waltham, Mass: Chronica Botanica Co.
Kenton A, Parokonny AS, Gleba YY, Bennett MD: Characterization of the Nicotiana tabacum L. genome by molecular cytogenetics. Mol Gen Genet. 1993, 240 (2): 159-169. 10.1007/BF00277053.
Gazdova B, Siroky J, Fajkus J, Brzobohaty B, Kenton A, Parokonny A, Heslop-Harrison JS, Palme K, Bezdek M: Characterization of a new family of tobacco highly repetitive DNA, GRS, specific for the Nicotiana tomentosiformis genomic component. Chromosome Res. 1995, 3 (4): 245-254. 10.1007/BF00713050.
Lim KY, Kovarik A, Matyasek R, Chase MW, Clarkson JJ, Grandbastien MA, Leitch AR: Sequence of events leading to near-complete genome turnover in allopolyploid Nicotiana within five million years. New Phytol. 2007, 175 (4): 756-763. 10.1111/j.1469-8137.2007.02121.x.
Melayah D, Lim KY, Bonnivard E, Chalhoub B, De Borne FD, Mhiri C, Leitch AR, Grandbastien MA: Distribution of the Tnt1 retrotransposon family in the amphidiploid tobacco (Nicotiana tabacum) and its wild Nicotiana relatives. Biol J Linn Soc. 2004, 82 (4): 639-649. 10.1111/j.1095-8312.2004.00348.x.
Skalicka K, Lim KY, Matyasek R, Matzke M, Leitch AR, Kovarik A: Preferential elimination of repeated DNA sequences from the paternal, Nicotiana tomentosiformis genome donor of a synthetic, allotetraploid tobacco. New Phytol. 2005, 166 (1): 291-303. 10.1111/j.1469-8137.2004.01297.x.
Renny-Byfield S, Chester M, Kovarik A, Le Comber SC, Grandbastien MA, Deloger M, Nichols R, Macas J, Novak P, Chase MW, Leitch AR: Next generation sequencing reveals genome downsizing in allotetraploid Nicotiana tabacum, predominantly through the elimination of paternally derived repetitive DNAs. Mol Biol Evol. 2011, 28 (10): 2843-2854. 10.1093/molbev/msr112.
Leitch IJ, Bennett MD: Polyploidy in angiosperms. Trends Plant Sci. 1997, 2 (12): 470-476. 10.1016/S1360-1385(97)01154-0.
Lim KY, Matyasek R, Kovarik A, Leitch AR: Genome evolution in allotetraploid Nicotiana. Biol J Linn Soc. 2004, 82 (4): 599-606. 10.1111/j.1095-8312.2004.00344.x.
Dadejova M, Lim KY, Souckova-Skalicka K, Matyasek R, Grandbastien MA, Leitch A, Kovarik A: Transcription activity of rRNA genes correlates with a tendency towards intergenomic homogenization in Nicotiana allotetraploids. New Phytol. 2007, 174 (3): 658-668. 10.1111/j.1469-8137.2007.02034.x.
Edwards KD, Bombarely A, Story GW, Allen F, Mueller LA, Coates SA, Jones L: TobEA: an atlas of tobacco gene expression from seed to senescence. BMC Genomics. 2010, 11: 142-10.1186/1471-2164-11-142.
Milne I, Bayer M, Cardle L, Shaw P, Stephen G, Wright F, Marshall D: Tablet–next generation sequence assembly visualization. Bioinformatics. 2010, 26 (3): 401-402. 10.1093/bioinformatics/btp666.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ: Basic local alignment search tool. J Mol Biol. 1990, 215 (3): 403-410.
Zdobnov EM, Apweiler R: InterProScan–an integration platform for the signature-recognition methods in InterPro. Bioinformatics. 2001, 17 (9): 847-848. 10.1093/bioinformatics/17.9.847.
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW: GenBank. Nucleic Acids Res. 2010, 38 (Database issue): D46-D51.
Suzek BE, Huang H, McGarvey P, Mazumder R, Wu CH: UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics. 2007, 23 (10): 1282-1288. 10.1093/bioinformatics/btm098.
Swarbreck D, Wilks C, Lamesch P, Berardini TZ, Garcia-Hernandez M, Foerster H, Li D, Meyer T, Muller R, Ploetz L, Radenbaugh A, Singh S, Swing V, Tissier C, Zhang P, Huala E: The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucleic Acids Res. 2008, 36 (Database issue): D1009-D1014.
Sánchez A, Salicrú M, Ocaña J: Statistical methods for the analysis of high-throughput data based on functional profiles derived from the Gene Ontology. Journal of Statistical Planning and Inference. 2007, 137 (12): 3975-3989. 10.1016/j.jspi.2007.04.015.
Muse SV: Estimating synonymous and nonsynonymous substitution rates. Mol Biol Evol. 1996, 13 (1): 105-114. 10.1093/oxfordjournals.molbev.a025549.
Li WH: Unbiased estimation of the rates of synonymous and nonsynonymous substitution. J Mol Evol. 1993, 36 (1): 96-99. 10.1007/BF02407308.
Kroymann J: Natural diversity and adaptation in plant secondary metabolism. Curr Opin Plant Biol. 2011, 14 (3): 246-251. 10.1016/j.pbi.2011.03.021.
Crandall KA, Kelsey CR, Imamichi H, Lane HC, Salzman NP: Parallel evolution of drug resistance in HIV: failure of nonsynonymous/synonymous substitution rate ratio to detect selection. Mol Biol Evol. 1999, 16 (3): 372-382. 10.1093/oxfordjournals.molbev.a026118.
Buggs RJ, Doust AN, Tate JA, Koh J, Soltis K, Feltus FA, Paterson AH, Soltis PS, Soltis DE: Gene loss and silencing in Tragopogon miscellus (Asteraceae): comparison of natural and synthetic allotetraploids. Heredity. 2009, 103 (1): 73-81. 10.1038/hdy.2009.24.
Hovav R, Udall JA, Chaudhary B, Rapp R, Flagel L, Wendel JF: Partitioned expression of duplicated genes during development and evolution of a single cell in a polyploid plant. Proc Natl Acad Sci USA. 2008, 105 (16): 6191-6195. 10.1073/pnas.0711569105.
Birchler JA, Veitia RA: The gene balance hypothesis: implications for gene regulation, quantitative traits and evolution. New Phytol. 186: 54-62.
Coate JE, Doyle JJ: Quantifying whole transcriptome size, a prerequisite for understanding transcriptome evolution across species: an example from plant allopolyploid. Genome Biol Evol. 2: 534-546.
Wagner GJ: Secreting glandular trichomes: more than just hairs. Plant Physiol. 1991, 96 (3): 675-679. 10.1104/pp.96.3.675.
Dawson RF, Solt ML: Estimated Contributions of Root and Shoot to the Nicotine Content of the Tobacco Plant. Plant Physiol. 1959, 34 (6): 656-661. 10.1104/pp.34.6.656.
Dubcovsky J, Dvorak J: Genome plasticity a key factor in the success of polyploid wheat under domestication. Science. 2007, 316 (5833): 1862-1866. 10.1126/science.1143986.
Wang E, Xu X, Zhang L, Zhang H, Lin L, Wang Q, Li Q, Ge S, Lu BR, Wang W, He Z: Duplication and independent selection of cell-wall invertase genes GIF1 and OsCIN1 during rice evolution and domestication. BMC Evol Biol. 2010, 10: 108-10.1186/1471-2148-10-108.
Huang X, Madan A: CAP3: a DNA sequence assembly program. Genome Res. 1999, 9 (9): 868-877. 10.1101/gr.9.9.868.
Langmead B, Trapnell C, Pop M, Salzberg SL: Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10: R25-10.1186/gb-2009-10-3-r25.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup: The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009, 25 (16): 2078-2079. 10.1093/bioinformatics/btp352.
Iseli C, Jongeneel CV, Bucher P: ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc Int Conf Intell Syst Mol Biol. 1999, 138-148.
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG: Clustal W and Clustal X version 2.0. Bioinformatics. 2007, 23 (21): 2947-2948. 10.1093/bioinformatics/btm404.
Katoh K, Toh H: Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008, 9 (4): 286-298. 10.1093/bib/bbn013.
Edgar RC: MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004, 5: 113-10.1186/1471-2105-5-113.
Felsenstein J: Notices. Cladistics. 1989, 5 (2): 163-166.
Saitou N, Nei M: The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evo. 1987, 4 (4): 406-425.
Felsenstein J: Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981, 17 (6): 368-376. 10.1007/BF01734359.
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B: Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008, 5 (7): 621-628. 10.1038/nmeth.1226.
Stekel DJ, Git Y, Falciani F: The comparison of gene expression from multiple cDNA libraries. Genome Res. 2000, 10 (12): 2055-2061. 10.1101/gr.GR-1325RR.
Yang Z: PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evo. 2007, 24 (8): 1586-1591. 10.1093/molbev/msm088.
Acknowledgments
The authors would like to thank Matthew Humphry, Robert Hurst and Fraser Allen for sampling, library preparation and sequencing, Robert Lister for Plant Husbandry and MH and Barbara Nasto for editorial assistance. The authors would like to thank the anonymous reviewer for the constructive criticism that made the paper much stronger. Funding was provided by Advanced Technologies Cambridge Ltd., a wholly owned subsidiary of BAT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors have no competing interests.
Authors' contributions
AB, KDE and LAM conceived of the study. All authors were involved in the writing and editing of paper. Sequencing was carried out in the laboratory of KDE. PhygOmics pipeline development and bioinformatics analysis was carried out by AB. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bombarely, A., Edwards, K.D., Sanchez-Tamburrino, J. et al. Deciphering the complex leaf transcriptome of the allotetraploid species Nicotiana tabacum: a phylogenomic perspective. BMC Genomics 13, 406 (2012). https://doi.org/10.1186/1471-2164-13-406
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2164-13-406