Abstract
Background
Pathogen-associated molecular patterns (PAMPs) are structural components of pathogens such as lipopolysaccharide (LPS) and peptidoglycan (PGN) from bacterial cell walls. PAMP-recognition by the host results in an induction of defence-related genes and often the generation of an inflammatory response. We evaluated both the transcriptomic and inflammatory response in trout (O. mykiss) macrophages in primary cell culture stimulated with DAP-PGN (DAP; meso-diaminopimelic acid, PGN; peptidoglycan) from two strains of Escherichia coli (PGN-K12 and PGN-O111:B4) over time.
Results
Transcript profiling was assessed using function-targeted cDNA microarray hybridisation (n = 36) and results show differential responses to both PGNs that are both time and treatment dependent. Wild type E. coli (K12) generated an increase in transcript number/diversity over time whereas PGN-O111:B4 stimulation resulted in a more specific and intense response. In line with this, Gene Ontology analysis (GO) highlights a specific transcriptomic remodelling for PGN-O111:B4 whereas results obtained for PGN-K12 show a high similarity to a generalised inflammatory priming response where multiple functional classes are related to ribosome biogenesis or cellular metabolism. Prostaglandin release was induced by both PGNs and macrophages were significantly more sensitive to PGN-O111:B4 as suggested from microarray data.
Conclusion
Responses at the level of the transcriptome and the inflammatory outcome (prostaglandin synthesis) highlight the different sensitivity of the macrophage to slight differences (serotype) in peptidoglycan structure. Such divergent responses are likely to involve differential receptor sensitivity to ligands or indeed different receptor types. Such changes in biological response will likely reflect upon pathogenicity of certain serotypes and the development of disease.
Similar content being viewed by others
Background
Detection of pathogens by host organisms requires direct contact between host PRRs (pattern recognition receptors) and pathogen-associated molecular patterns (PAMPs) where PAMP-PRR interactions subsequently dictate the development of the host immune response [1, 2]. PAMPs such as the lipopolysaccharides (LPS) and peptidoglycans (PGN), both bacterial cell wall components, have been directly implicated in the induction of the host immune response across the vertebrata [3–9]. Peptidoglycan and related fragments are recognised by the host and induce diverse biological effects, including inflammation, leukocytosis, or enhanced immune responses [10–13]. Like LPS, peptidoglycan, including its minimal immunomodulatory subunit, muramyl dipeptide, can bind to the CD14 receptor of target cells in mammals [14–16] although peptidoglycan does not bind to LBP or BPI [17, 18]. PGN's do not activate TLR4-mediated signal transduction but do activate both the TLR2 and NOD pathways [19–24].
In Drosophila, PGN recognition is achieved by the Toll or Immune deficiency (Imd) pathways, at least in part, through peptidoglycan recognition proteins (PGRPs) [25, 26]. Both pathways share common features with mammalian Toll-like receptor (TLR) and tumour necrosis factor-α (TNF-α) receptor signalling cascades that regulate NF-κB activation [27–29]. In vivo studies in the zebrafish have shown that the PGRP response is essential for successful responses to bacterial infection [30]. Recently, PGN in trout macrophages has been shown to be the major stimulatory component in crude LPS preparations characterised by an increase in cytokine mRNAs, IL-1β and IL-6, and release of inflammatory products as prostaglandin E2 (PGE2) [9]. However, studies addressing different responses to serotype-specific PGNs are scarce throughout the vertebrata including mammals.
PGN may account for approximately one-half of the cell wall mass in gram-positive bacteria whereas in gram-negative bacteria only a relatively thin PGN layer in the periplasmic space is present [31, 32]. Gram-negative peptidoglycan contains meso-diaminopimelic acid (DAP) as the major peptide group that is directly cross-linked whereas most gram-positive bacteria have L-lysine as the third amino acid (Lys-type). These Lys-type peptides are cross-linked through an inter-peptide bridge that varies in length and amino acid composition in different bacteria [32–34].
As the structure and composition of the microbial motif has an important role in host sensing and minor modifications in structure can influence the immune response [35–38] we explored the response of differentiated trout macrophages in cell culture to different PGNs from E. coli of different strains (K12 and O111:B4). Our results show that trout macrophages differentially respond to different PGNs at the level of the transcriptome by either differentially activating RNA transcripts related to prostaglandin synthesis resulting in the liberation of prostaglandin's (PGN-O111:B4) or by generating a non-defined inflammatory response,(PGN-K12).
Methods
Animals and Materials
Healthy adult specimens (160 g mean weight) of rainbow trout (O. mykiss) were purchased from a commercial hatchery (Piscifactoria Andrés, St Privat, Girona) and held in recirculating freshwater stock tanks (300 L) in the aquarium facilities at the Universitat Autònoma de Barcelona. Fish were kept at 15°C with a 12 h light/12 h dark photoperiod cycle, and were fed with a maintenance ratio of about 0.5% body weight per day. Water quality indicators (dissolved oxygen, ammonia, nitrite, pH) were analysed periodically.
DMEM and FBS were purchased from PAA Laboratories (Spain). Poly-D-lysine was purchased from Sigma (Tres Cantos, Madrid). Primocin, and PGN preparations (PGN E. coli K12, O111:B4) were purchased from Invivogen (Nucliber, Spain). Cell strainers and plasticware were purchased from BD Biosciences (Madrid, Spain). Gel Green was purchased from Biotium (Labnet, Spain). Prostaglandin E2 and D2 enzyme immunoassay (EIA) kit was from Cayman (Scharlab, Spain).
Cell culture and stimulation
The experimental protocols used for head kidney isolation have been reviewed and approved by the Ethics and Animal Welfare Committee of the Universitat Autonoma de Barcelona, Spain. After anaesthetising the fish in 3-aminobenzoic acid ethyl ester (0.1 g/L), animals were sacrificed and the head kidney was dissected out. Trout macrophages were isolated as previously described [39]. Before stimulation, differentiated macrophages were incubated in serum free medium for 3 h. For stimulation, the medium of each well was removed and fresh medium containing the indicated concentrations of PGN were added and the cultures were incubated for the indicated times.
RNA extraction and complementary DNA (cDNA) synthesis
Total RNA was extracted from cell cultures using 1 mL of TriReagent (Molecular Research Center) per well cell culture, following the manufacturer's instructions. Quantification was carried out with a Nanodrop1000 (Thermo Scientific) and the quality of the RNA was checked with a Bioanalyzer (Agilent technologies). All RNA samples had a RIN value >7. Total RNA (2 μg) was used to synthesise cDNA with SuperScript III Transcriptase (Invitrogen) and oligo-dT primer (Promega).
Measurement of PGE2 and PGD2 levels
Supernatants from stimulated cell cultures (triplicates) from 3 different fish were recovered, centrifuged and stored at -80°C until use. Measurement of PGE2 and PGD2 levels was completed with a monoclonal EIA according to the manufacturer's instructions. The prostaglandin kit detection limit was 8 pg/mL. Prior to prostaglandin determination supernatants were diluted five times in EIA assay buffer. The same macrophage cells were used to obtain total RNA for the determination of COX-2 and Prostaglandin D synthase gene expression as well as the supernatants for PGE2-PGD2 determination.
Microarray analysis
The design of the microarray is described in detail elsewhere [40, 41] and a full description of the platform and data presented in this manuscript are accessible through the public GEO depositories (accession number GPL6154 and GSE22330). The genes were selected by functional classes; random clones from common and subtracted cDNA libraries 1800 genes printed in six replicates each were compared with the known vertebrate proteins using BlastX; overall, the platform was enriched in a number of functional classes, such as immune response (236 genes), signal transduction (245 genes), receptor activity (126 genes), apoptosis (120 genes), cell cycle (70 genes), protein catabolism (90 genes), folding (70 genes), response to oxidative stress (39 genes), stress and defence response (145 and 105 genes, respectively), and chaperone activity (41 genes). Total RNA was extracted from cell cultures using 1 mL of TriReagent (Molecular Research Centre) per well, following the manufacturer's instructions, the quantity and integrity was analysed by Experion RNA StdSens Analysis Kit (Bio-Rad). Microarray analyses were conducted in pooled samples (see experimental design of microarray assay). A dye-swap design of hybridisation was applied. In analyses of infected immune cells, the non-infected cells were used as a control. Each sample was analysed with two slides. Scanning was performed with Alphascan (High Performance Dual-Laser Scanner for Microarray Slides from Alpha Innotech and images were processed with VisionLite (ThermoSpectronic). The measurements in spots were filtered by criteria I/B ≥ 3 and (I-B)/(SI + SB) ≥ 0.6, where I and B are the mean signal and background intensities and SI, SB are the standard deviations. After subtraction of mean background, locally weighted non-linear regression (Lowess) normalisation [42] was performed separately for each slide. To assess differential expression of genes, the normalised log intensity ratios were analysed with Student's t-test (p < 0.01). The Bayesian modification to the false discovery rate (FDR) was used to correct for multiple comparison tests, estimating the q-value for the set of differentially expressed genes [43]. The functional categories of Gene Ontology [44] were compared with regulated genes (p < 0.01) by the sums of ranks (Student t-test p < 0.05). The statistical significance of over-represented functional categories, showing the differential expression in the experiment grouped by functional classes compared with all genes an GO categories from the chip, was assessed using the Chi square test with Yates correction (p < 0.05).
Real-Time quantitative PCR and validation
In order to verify microarray results, real-time PCR (qRT-PCR) was carried out. Two micrograms of the individuals RNA was used to synthesise cDNA with SuperScript III RNase Transcriptase (Invitrogen) and oligo-dT primer (Promega). As a house-keeping gene, 18S was amplified from the same cDNA samples. For different gene expression analysis specific primers were used (Additional file 1). Real-time PCR reactions were carried out in a 25 μL reaction with SYBR Green I (Stratagene) using a 1:25 dilution of the cDNA and 250 nM of primers. Quantitative qRT-PCR was performed using a Mx 3000P System (Stratagene) and quantification was done according to the Pfaffl method corrected for efficiency for each primer set [45]. Values for each sample were expressed as "fold differences", calculated relative to controls group and normalised for each gene against those obtained for the house keeping gene 18S.
Experimental design
Microarray analysis
macrophage cell cultures isolated from 84 animals were stimulated with PGNs from E.coli O111:B4 and K12 strains and compared to parallel control cultures (without stimulation). Cell cultures were individually stimulated with both peptidoglycans for 1, 6 and 12 h (12 by PGN and time, n = 72), and 12 control cultures (total; n = 84). Individuals RNAs were grouped into three pools from 4 cell cultures for each time point (1, 6, and 12 h). The transcriptomic response was analysed by microarray assay, and divided in three experimental time points named early (1 h), median (6 h) and late stage (12 h). The analysis was carried out with common genes expressed within three replicate pools over the control (GDE one way ANOVA p > 0.01). The qRT-PCR validation assay was conducted with total RNA from late stage cell cultures.
Time Course
macrophage cell cultures isolated from 9 animals were stimulated with PGN O111:B4 and K12 during 0, 30 min, 1, 3, 6, and 12 h (10 μg/mL). The mRNA abundance of COX-2 (or prostaglandin endoperoxide synthase 2) and PTGDS was measured by qRT-PCR, prostaglandin release (PGE2-PGD2) were measured using a prostaglandin EIA assay (Cayman). Three individual replicates were made for each peptidoglycan stimulation. The control group was non-stimulated cell cultures (n = 3).
Dose-Response
macrophage cell cultures isolated from 9 animals were stimulated with PGN from the E. coli strains 0111:B4 and K12. The treatment was conducted overnight (12 h) with different concentrations, 0, 0.1 and 10 μg/mL, of PGNs. Expression of COX-2 and PTGDS mRNAs was measured by qRT-PCR, prostaglandin release (PGE2-PGD2) were measured using a prostaglandin EIA assay (Cayman). Three individual replicates were made for each peptidoglycan stimulation. The control group was non-stimulated cell cultures (n = 3).
Statistical analysis
All statistical analysis was conducted with the software SPSS Statistic 17.0. The relationship between intensity of expression and time was examined and tested for significant differences between the PGNs with covariance analysis (ANCOVA) using the transcriptomic magnitude as co-variable, followed by one-way ANOVA analysis for up- or down regulated transcripts. The Student t-test was made to explore the difference between the expression registered in the microarray assay and the qRT-PCR (Additional file 2). Two-way ANOVAs were made to compare the differences between COX-2 and PTGDS expression and prostaglandin release in the time-course and dose-response assay.
Results
Global comparisons of the transcriptomic response to PGN (microarray analysis at 1, 6, and 12 hours)
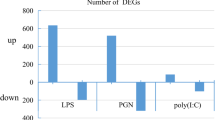
Microarray analyses were evaluated using a salmonid-specific targeted cDNA microarray containing 1800 cDNAs enriched with immune system related genes (SFA 2.0). Gene expression profiles obtained highlighted a marked contrast in the macrophage response to PGN purified from E. coli (PGN-O111:B4 and K12). Samples were taken over time early (1 h), median (6 h) and late stage (12 h) and separate one-way ANOVAs (p > 0.01) were conducted to identify differentially expressed transcripts over the control (GDE). Transcripts expressed within all three biological replicates were used to analyse changes for both treatment (PGN) and time stage (Additional files 3, 4, 5, 6, 7, 8). The kinetics of the response obtained from peptidoglycans derived from K12 or O111:B4 were significantly different in both transcript number (total number of differentially expressed transcript over the control, one-way ANOVA p < 0.01) and intensity (fold change FC >2) (Figure 1). In total 819 transcripts were differentially expressed (GDE) in both treatments over the control (all cDNAs expressed on the array), with 270, 221 and 328 in the early stage, median and late stages respectively (Figure 1, and Additional file 3, 4, 5, 6, 7, 8, 9). Stimulation with PGN-O111:B4 revealed a significant peak in intensity at the median stage (130 transcripts one-way ANOVA p < 0.01 and FC >2; 92) and a strong and intense response was maintained throughout (FC >2; 51, 92 and 72 at 1, 6 and 12 h respectively). In contrast PGN-K12 induced a significant diversity of transcripts (magnitude) over time, note a decrease at 6 h, where the response intensity although high at 1 h (FC >2; 134 transcripts) significantly decreased through time where late stage transcripts with FC >2 represent only 17% of the early stage total (Figure 1, Additional file 9). Regression analysis (up regulated genes ANCOVA, F5, 68 = 1.178 p > 0.05, followed by two-way ANOVA, F2, 68 = 27.124: p < 0.05; down regulated genes ANCOVA, F5, 68 = 2.303: p > 0.05, followed by two-way ANOVA, F2, 68 = 37.124: p < 0.05) (Additional file 10, and 11) highlights that a stronger induction of gene expression and likely more directed response is obtained with PGN-O111:B4 challenge.
Characterisation of the transcriptomic response. A; Venn diagram representing mRNA transcripts differentially expressed over control during PGN-O111:B4 and PGN-K12 challenges throughout the time (early, median and late stage). The area of the circles is scaled to the number of transcripts (one way ANOVA p < 0.01) and the fold change (FC >2) expressed in each stage. Black circles: 69, 130, 86 number of transcripts differentially expressed under PGN-O111:B4 treatment. White circles: 173, 64, 219 number of transcripts differentially expressed under PGN-K12 challenge.
Qualitative comparisons of the transcriptomic response to PGN: Differentially expressed transcripts in early, median, and late stages of activation
Early stage
A higher number of induced transcripts were observed with PGN-K12 treatment in respect to PGN-B4 highlighting a common down-regulation of inflammatory processes (Table 1 and 2). Major differences could also be identified in ligand recognition where macrophages stimulated with PGN-K12 up-regulated BPI binding protein (BPI). In fish, BPI has been suggested to be involved in LPS binding and recognition [46] whereas PGN-B4 stimulation led to up-regulation of antigen-processing including MHC I, and MARCO. The alternative spliced form of MARCO, Cysteine-rich protein 1, that also recognises bacterial cell wall PAMP's was co-ordinately down-regulated [47]. Transcripts related to the inflammatory response were down regulated under both PGN challenges including for PGN-B4; N-acetylmuramoyl-L-alanine amidase (bactericidal activity), PGLYPR6 and peroxiredoxin (Table 1) and for PGN-K12; NF-κB inhibitor alpha-1 and arachidonate 5-lipoxygenase (Table 2). Microsomal glutathione S-transferase, a precursor for leukotriene and prostaglandin production [48] was down-regulated by both treatments. Interestingly, annexin A1-1 was strongly up-regulated (FC; 9.8) in response to PGN-K12. This transcript has been suggested to have anti-inflammatory activity due to its phospholipase A2 (essential for inflammatory prostaglandin production) inhibitory activity [49] (Table 2 and Additional file 6).
Median stage
Of note at the median stage is that PGN-B4 induces a co-ordinated increase in pro-inflammatory and cellular defence activity with increased intensity (Table 1 and 2). Mediators of inflammatory prostaglandin production are up-regulated highlighted by increased arachidonate 5 lipoxygenase mRNA synthesis. In parallel, cathepsin transcripts (protease activity) (n = 6), PGLYPR6 (amidase) and the interleukin enhancer 3 mRNA (regulates interleukin production during infectious processes (e.g., [50]), were also up-regulated. PGN-K12 stimulation at this point is highlighted by a strong down-regulation of transcript diversity, including cell adhesion, defence response, cell homeostasis and metabolism, with almost all observed early stage transcripts returning to base-line conditions (Table 1 and 2). Potentially of importance is the up-regulation of the transcription factor CCAAT/enhancer binding protein β (C/EBP-β) mRNA by PGN-K12. C/EBP-β has been shown to be intimately linked to immune and inflammatory processes and regulates the transcription of the pro-inflammatory cytokine, interleukin-6. On the other hand, the tumour necrosis factor (TNF) decoy receptor, which inhibits apoptosis, and NF-κB inhibitor alpha-3 were strongly up-regulated in addition to an abrupt increase in BPI with PGN-B4.
Late stage
For PGN-B4 a defined response was observed after 12 h of stimulation where the prostaglandin endoperoxide synthase-2 (COX-2), and prostaglandin D synthase, both linked to the synthesis of inflammatory prostaglandins, were strongly up-regulated (Table 1). COX-2 (prostaglandin endoperoxide synthase-2) catalyses the conversion of arachidonic acid to prostaglandin (PGH2) [51, 52], and prostaglandin D synthase (PTGDS) catalyses the conversion of PGH2 to prostaglandin D2 (PGD2) [53, 54]. Signalling components for TLR pathways are also up-regulated by PGN-B4 including the MAPK pathways and myeloid differentiation primary response (MyD88) mRNA, an adapter protein between TLR and the transcription factor NF-κB. Interestingly these components plus the serine/threonine-protein kinase 2 are required to respond to microbial ligands [55]. TNF decoy receptor is maintained up-regulated highlighting the anti-apoptotic response of PGN-B4 activated macrophages. TNF-α is secreted into the culture medium as soon as 1 h after PGN treatment [MacKenzie et al, unpublished results]. In contrast to the strong inflammatory profile obtained for PGN-B4 the PGN-K12, response at 12 h appears related to biological themes associated with energy, protein metabolism and cellular homeostasis at a low level of intensity (Table 2 and Additional file 8). These results imply close similarities with those previously obtained for trout macrophages activated with crude LPS [56–59] suggesting a common recognition mechanism distinct to that observed for PGN-B4.
From transcripts identified as differentially expressed and significantly up- or down-regulated (one-way ANOVA p < 0.01) we selected sixteen transcripts from the late stage for qRT-PCR validation. All sixteen transcripts were significantly expressed between the two PGNs and significantly correlated when tested by qRT-PCR and Students-T test (p < 0.05); thereby confirming the microarray results. FC values obtain by microarray and qRT-PCR analyses are listed in the additional file 2 (Student T tests p > 0.05).
Functional categories are associated with combinations of PGN and time parameters
Analysis of function using GO annotations revealed that most over-expressed transcripts were related to the immune response and GO functional categories are specifically influenced by a combinatorial PGN-Time effect (Chi-square with Yates correction, p < 0.01, Figure 2). In the early stage, different GO categories expressed were PGN-dependent and include MHC class I receptor, lysozome, NF-κB cascade, peptidase activity, cell adhesion, ribosome, or chromatin assembly or disassembly (Figure 2). At the median stage the intensity of the PGN-B4 response is highlighted by a set of biological processes specifically associated to the immune response whereas only two GO classes, cell adhesion and negative regulation of cell proliferation, were represented with PGN-K12 (Figure 2). At the late stage an inverse correlation was observed where peptidase activity, complement activation, cell homeostasis, and mitochondrial electron transport were highly represented with PGN-K12 and NF-κB cascade, protein-MAPK cascade, and ribosome related to the PGN-O111:B4 response (Figure 2). Remarkably, cell wall catabolism was only observed with PGN-K12 and not during PGN-O111:B4 challenge (Figure 2).
Qualitative and quantitative representations of biological processes (GO) over represented during challenges. Qualitative and quantitative representations of over expressed GO categories (Chi-square with Yates correction p < 0.05). The corners of the spider-web maps represent biological processes identified in the GO analysis. Different numbers of transcripts were grouped in each biological process. The continuous mark lines (black or grey) represent the different number of transcripts in each biological process. The differences in the shape of the GO pattern (continuous mark line) are due to divergence in the number of transcripts grouped to each Gene Class (biological process) under both PGN challenges; the black line shows the GO pattern for PGN-O111:B4 and the grey line shows the GO pattern for PGN-K12 treatment.
Characterisation of the prostaglandin response (time course and dose response of PGN challenges)
Both COX-2 and PTGDS were identified by microarray analyses as differentially expressed between the two PGNs, therefore we measured both PGE2 and PGD2 release into the culture supernatant and in parallel COX-2 and PTGDS mRNA abundance by qRT-PCR. Analyses were done both in respect to response to PGN-B4 and PGN-K12 over time (30 min, 1, 3, 6 and 12 h; Figure 3) and subsequently as a dose response (0.1, 1 and 10 μg/mL; Figure 4).
Temporal characterisation of the prostaglandin response. Time course response to macrophages stimulated during 0, 30 min, 1, 3, 6, and 12 h with 10 μg/mL of PGN O111:B4 and K12. Experiments were performed in independent groups of PGN-stimulated (n = 3) or control macrophage cultures (n = 9). a) COX-2 and PTGDS mRNA abundance over time in response to PGN-B4 (black bar) or PGN-K12 (white bar). Were observed significative differences in the mRNA abundance between the times and treatments (PGNs) in both genes (two way ANOVA p < 0.01). b) PGE2 and PGD2 release (pg/mL) stimulated by PGN-O111:B4 (black bars), PGN-K12 (white bars) and control (grey bars) into the culture medium (n = 3/treatment). Were observed significative differences in the release between the times and treatments (PGNs or control) by both prostaglandin (two way ANOVAs p < 0,01). The results are presents as fold change relative to 18S abundance and ± std deviation.
Concentration dependence of the prostaglandin response. Dose response (0.1, 1, and 10 μg/mL) of trout macrophages to PGN O111:B4 and K12 challenge. Experiments were performed overnight in independent macrophage cultures (n = 3). a) COX-2 and PTGDS mRNA abundance (black bar) in response to different doses of PGN-O111:B4 or PGN-K12 (0.1, 1, 10 μg/mL). Were observed significative differences in the mRNA abundance between different doses and treatments (PGNs) in both genes (two way ANOVAs p < 0.01). b) PGE2 and PGD2 release (pg/mL) into the culture medium (grey bars). Were observed significative differences in the release between doses and treatments (PGNs or control) by both prostaglandins (two way ANOVAs p < 0.01). The results are presented as fold change relative to 18S abundance and mean ± std deviation.
Time course response assay (0, 30 min, 1, 3, 6, and 12 hrs of PGN challenges)
COX-2 mRNA expression is strongly regulated by PGN-B4 over time followed by a significant increase in PGE2 secretion into the culture medium. Stimulation with PGN-K12 results in an increase of mRNA abundance at 1 h (two way ANOVA, F5, 35 = 8.678, p < 0.05, Figure 3a, Additional file 12) and a more gradual accumulation of PGE2 in the culture medium in comparison with PGN-B4. The dynamics of PTGDS mRNA expression was time dependent (two way ANOVA, F5, 35 = 4.584, p < 0.05, Figure 3a, Additional file 12) showing changes a few minutes after stimulation with both PGNs (30 min) and a strong increase 1 h post-treatment (Figure 3a). The release of PGD2 was significantly different (increasing) in PGN-B4 treated macrophages 6 h after stimulation. Differences observed between PGE2 and PGD2 release are correlated to both time and treatment (two way ANOVA, F10, 54 = 4.553, p < 0.05, Figure 3b, Additional file 12) where PGD2 has a low response, concentrations in the range of 1-14 pg/mL, when compared with the PGE2 secretion, >200 pg/mL. PGE2 and PGD2 liberation patterns were strongly influenced by the interaction between PGN and time (two way ANOVA, F10, 54 = 2.522, p < 0.05, Figure 3b, Additional file 12).
Dose response assay (0.1, 1, and 10 μg/mL of PGN O111:B4 and K12)
In dose response assays the expression pattern of COX-2 mRNA induction was both dose and PGN-dependent (two way ANOVA, F5, 18 = 5.824, p < 0.05, Figure 4a, Additional file 12). In figure 4a, a peak of COX-2 expression was registered at 10 μg/mL of PG-B4. Interestingly, PGN-K12 stimulation generated a lower expression of COX-2 mRNA (10 μg/mL; >50 fold) when compared to PGN-B4 although at a dose of 1 μg/mL fold changes are similar for both PGNs (Figure 4a). This is reflected in PGE2 liberation where 10 μg/mL of PGN-B4 generated a strong response (>600 fold increase; PGN-interaction, two way ANOVA, F2, 48 = 182.588, p < 0.05, Additional file 12) that correlated to increased COX-2 mRNA abundance and all other concentrations for both PGNs induced similar responses (>50 fold). The liberation pattern of PGD2 was significantly dependent upon PGN type, and showed a single increase at 10 μg/mL with PGN-B4 (Two way ANOVA, F2, 48 = 4.588, p < 0.05, Additional file 12). Surprisingly this is not mirrored in PTGDS mRNA abundance levels where PTGDS mRNA is significantly up-regulated by PGN-K12 at 0.1 and 1 μg/mL and PGN-B4 at 1 μg/mL (Figure 4b, Additional file 12).
Discussion
In recent studies on trout macrophages, peptidoglycan (PGN-B4) was identified as a major pro-inflammatory component of crude LPS preparations in which TLR4 and canonical TLR2 signalling pathways were discarded as potential recognition systems for peptidoglycans [9]. As structural differences in PGN peptide moieties from different bacterial-strains have been shown to modulate host responses in both Drosophila and mammals [32, 36, 38] we investigated, a priori with targeted microarray analysis, the effects of two different PGNs from different strains of E. coli, O111:B4 and K12. These different serological features have been shown to affect the host immune response [60]. A systematic dissection of the impact of (combinations of) culture parameters (time and treatment) revealed a significant re-modelling of the trout macrophage transcriptome highlighting the divergence of the response to the two different PGNs (PGN-B4 vs. PGN-K12). As there were no other known variables, the differences in the transcriptomic profile are assumed to be solely due to the structure of the different PGNs and therefore differential recognition of those by the macrophages. This assumption is supported by the variation in transcript number (Figure 1a, 2), their intensities (Figure 1a,b), and diversity (Table 1 and 2). In fish, modifications in the transcriptomic profile have been observed in response to environmental changes, stress and maintenance of the steady state of transcriptional activity [61, 62], or bidirectional transcriptomic remodelling to inflammatory stimuli [56, 63–67]; however, our data emphasises that macrophages respond differentially to highly similar bacterial PGNs resulting in a directed response i.e. prostaglandin release or a more generalised 'state of activation'.
In fish, the shift from a steady state to a functional inflammatory state, i.e. secretion of pro-inflammatory cytokines or PGE2, in trout macrophages stimulated with crude LPS preparations has been shown to be driven mainly by gram negative PGN, where DNA and RNA and ultra-pure LPS preparations are unable to induce mRNA expression of pro-inflammatory cytokines [9, 68]. Our microarray analysis identified differential regulation of both prostaglandin D-synthase (PTGDS) and prostaglandin endoperoxide synthase-2 (COX-2) that are directly involved in eicosanoid production; PGD2 and PGE2 respectively [51, 53] (Table 1 and 2). COX-2 is regulated in macrophage/monocyte cell types and is responsible for inflammatory prostaglandin, PGE2, synthesis from arachidonic acid, and is involved in cellular or tissue damage generated in acute and/or chronic inflammatory states [69]. PTGDS metabolises PGH2 to PGD2, [53, 54, 70, 71] where PGD2 plays a role during the injury process as vasodilator/constrictor or as potent inflammatory mediator [72, 73]. However, the action of PGD2 in fish as a mediator of the immune response is undefined. Downstream analyses, qRT-PCR and prostaglandin release, of both COX-2 and PTGDS mRNA regulation and PGH2 and PGD2 concentration in supernatants reveals a strong correlation, both time and dose-dependent, between PGN-type (B4 vs K12), mRNA abundance and inflammatory outcome (Figure 4). PGN-B4 is clearly a more potent regulator of the COX-2 mRNA/PGE2 pathway where the activation threshold for de novo synthesis of COX-2 is 10 μg/mL of PGN-B4. Interestingly this threshold concentration has also been observed on numerous occasions for pro-inflammatory cytokine mRNA synthesis in trout macrophages [9, 39, 46, 68]. On the other hand, PTGDS mRNA synthesis appears as more dose sensitive for both PGNs with a similar temporal expression pattern suggestive of a different signal transduction mechanism. However PGN-B4 stimulation at 10 μg/mL results in higher PGD2 secretion. The regulation and biological effects of PGE2 and PGD2 secretion in inflammatory responses in fish clearly warrant more investigation.
In Drosophila the biological activity of a large panel of natural and synthetic DAP-PGN showed significant variability in their stimulatory capacity and immune response [74] and PGRP (peptidoglycan recognition protein) deficient Drosophila are more susceptible to bacterial infections [75]. In human monocytes exposed to synthetic muropeptides (peptide moiety of PGNs), TNF-α mRNA expression and release was highly dependent upon structural modifications between peptides [38]. Thus inflammatory outcomes are modified in accordance to sensitivity to peptidoglycan structure. Such sensitivity is likely conferred by the participation of different PRRs, PAMP-PRR interactions or the accumulative signalling intensity (i.e. threshold) of the group of PRRs involved in recognition.
Peptidoglycan recognition in mammals is mainly facilitated by three different PRR families; TLR2 (gram positive peptidoglycan), NOD2 and PGRPs all of which can bind peptidoglycans [35, 75, 76]. TLR2 has been described in fish species [77] although stimulation with lipoprotein (Pam3CSK4), a classical TLR2-ligand, does not stimulate an inflammatory response in our macrophage model [9]. However MyD88, an adaptor molecule involved in the classical Drosophila or mammalian Toll signalling cascades, which together with the receptor associated kinase (IRAK) and TNF activated factor (TRAF6) allow NF-κB translocation to the nucleus (promoting expression of inducible inflammatory cytokines such as TNF-α) during gram-negative bacterial infection [46, 78–80] was specifically up-regulated during PGN-B4 stimulation. This suggests TLR involvement in the PGN-mediated inflammatory response in trout macrophages. Concerning PGRPs, PGRP-2, -5 and -6, have been shown in the zebrafish to play an essential role in defence during bacterial infections [30] and in the trout PGRP-2 responds to PGN-B4 [9]. In this study we also identified PGLYRP-6 (up-regulated; PGN-B4) suggesting that the PGRPs also play a role in specific-PGN recognition and this may be conserved throughout the fishes.
In contrast to the specific directed response obtained from PGN-B4 stimulation, PGN-K12 did not elicit a clear functional response at the level of the macrophage transcriptome or release of inflammatory mediators. A wide diversity of transcripts were activated although at a relatively low level. These results are similar to those previously observed for stimulation with crude LPS preparations in trout macrophages [[56], Boltaña et al., unpublished data] where both preparations can stimulate the release of TNF-α into the culture medium [[68], Roher et al., unpublished data]. Interestingly, TNF receptor associated factor 1 was specifically induced by PGN-K12. This transcript encodes a receptor-protein involved in the activity of apoptotic pathways mediated by TNF-α [80–82], however, we did not detect apoptosis during the experimental period [MacKenzie et al, unpublished data]. Moreover, the gene ontology category cell wall catabolism was consistently over-expressed throughout PGN-K12 treatment (Figure 2a,b) supporting the existence of a strong transduction signal generated by PGN-K12.
Conclusions
Our data highlights the significant differences observed in macrophages responding to two PGNs derived from different serotypes of the same bacteria. Responses at the level of the transcriptome and the inflammatory outcome (prostaglandin synthesis) highlight the different sensitivity of the macrophage to slight differences (serotype) in peptidoglycan structure. Such divergent responses are likely to involve differential receptor sensitivity to ligands or indeed different receptor types. Such changes in biological response will likely reflect upon pathogenicity of certain serotypes and the development of disease.
Abbreviations
- PAMPs:
-
pathogen-associated molecular patterns
- PGN:
-
peptidoglycans
- PGRPs:
-
peptidoglycan recognition proteins
- GDE:
-
differentially expressed genes
- COX-2:
-
Cyclooxygenase 2
- PTGDS:
-
prostaglandin D synthase.
References
Kopp E, Medzhitov R: Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003, 15: 396-401. 10.1016/S0952-7915(03)00080-3.
Kabelitz D, Medzhitov R: Innate immunity cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr Opin Immunol. 2007, 19: 1-3. 10.1016/j.coi.2006.11.018.
Gupta D, Kirkland TN, Viriyakosol S, Dziarski R: CD14 Is a Cell-activating Receptor for Bacterial Peptidoglycan. J Biol Chem. 1996, 271 (38): 23310-23316. 10.1074/jbc.271.38.23310.
Dziarski R, Tapping RI, Tobias PS: Binding of Bacterial Peptidoglycan to CD14. J Biol Chem. 1998, 273 (15): 8680-8690. 10.1074/jbc.273.15.8680.
Dziarski R, Viriyakosol S, Kirkland TN, Gupta D: Soluble CD14 Enhances Membrane CD14-Mediated Responses to Peptidoglycan: Structural Requirements Differ from those for Responses to Lipopolysaccharide. Infect Immun. 2000, 68 (9): 5254-5260. 10.1128/IAI.68.9.5254-5260.2000.
Schrijver IA, Melief MJ, Eulderink F, Hazenberg MP, Laman JD: Bacterial Peptidoglycan Polysaccharides in Sterile Human Spleen Induce Proinflammatory Cytokine Production by Human Blood Cells. J Infect Dis. 1999, 179: 1459-1468. 10.1086/314761.
Rabehi L, Irinopoulou T, Cholley B, Heaffner-Cavaillon N, Carreno MP: Gram-Positive and Gram-Negative Bacteria Do Not Trigger Monocytic Cytokine Production through Similar Intracellular Pathways. Infect Immun. 2001, 69 (7): 4590-4599. 10.1128/IAI.69.7.4590-4599.2001.
Iliev DB, Liarte CQ, Mackenzie S, Goetz FW: Activation of rainbow trout (Oncorhynchus mykiss) mononuclear phagocytes by different pathogen associated molecular pattern (PAMP) bearing agents. Mol Immunol. 2005, 42: 1215-1223. 10.1016/j.molimm.2004.11.023.
Mackenzie S, Roher N, Boltaña S, Goetz FW: Peptidoglycan, not endotoxin, is the key mediator of cytokine gene expression induced in rainbow trout macrophages by crude LPS. Mol Immunol. 2010, 47: 1450-1457. 10.1016/j.molimm.2010.02.009.
Dziarski R, Gupta D: Role of MD-2 in TLR2-and TLR4-mediated recognition of Gram-negative and Gram-positive bacteria and activation of chemokine genes. Innate Immun. 2000, 6: 401-405.
Dziarski R, Gupta D: Staphylococcus aureus Peptidoglycan Is a Toll-Like Receptor 2 Activator: a Reevaluation. Infect Imm. 2005, 78 (8): 5212-5216. 10.1128/IAI.73.8.5212-5216.2005.
Wang JE, Dahle MK, McDonald M, Foster SJ, Aasen AO, Thiemermann C: Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and sinergismo. Shock. 2003, 20 (5): 402-414. 10.1097/01.shk.0000092268.01859.0d.
Xu Z, Dziarski R, Wang Q, Swartz K, Sakamoto KM, Gupta D: Bacterial Peptidoglycan-Induced tnf- Transcription Is Mediated Through the Transcription Factors Egr-1, Elk-1, and NF-. J Immunol. 2001, 167 (12): 6975-
Weidemann B, Schletter J, Dziarski R, Kusumoto S, Stelter F, Rietschel ET, Flad HD, Ulmer AJ: Specific Binding of Soluble Peptidoglycan and Muramyldipeptide to CD14 on Human Monocytes. Infect Imm. 1997, 65 (3): 858-864.
Dziarski R, Tapping RI, Tobias PS: Binding of Bacterial Peptidoglycan to CD14. J Biol Chem. 1998, 273 (15): 8680-8690. 10.1074/jbc.273.15.8680.
Gupta D, Wang Q, Vinson CH, Dziarski R: Bacterial Peptidoglycan Induces CD14-dependent Activation of Transcription Factors CREB/ATF and AP-1. J Biol Chem. 1999, 274 (20): 14012-14020. 10.1074/jbc.274.20.14012.
Mathison JC, Tobias PS, Wolfson E, Ulvevitch RJ: Plasma lipopolysaccharide (LPS)-Binding protein. A Key Component in macropophage Recognition of Gram-Negative LPS. J Immunol. 1992, 149 (1): 200-206.
Dziarski R: Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol Life Sci. 2003, 60: 1793-1804. 10.1007/s00018-003-3019-6.
Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ: Peptidoglycan-and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999, 274 (25): 17406-17419. 10.1074/jbc.274.25.17406.
Schröder N, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel U, Weber J, Schumann Ralf: Lipoteichoic Acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus Activates Immune Cells via Toll-like Receptor (TLR)-2, Lipopolysaccharide-binding Protein (LBP), and CD14, whereas TLR-4 and MD-2 Are Not Involved. J Biol Chem. 2003, 278 (18): 15587-15594.
Chamaillard M, Girardin S, Viala J, Philpott D: NODs, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol. 2003, 5: 581-592. 10.1046/j.1462-5822.2003.00304.x.
Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ: Nod2 Is a General Sensor of Peptidoglycan through Muramyl Dipeptide (MDP) Detection. J Biol Chem. 2003, 278 (11): 8869-8872. 10.1074/jbc.C200651200.
Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zaehringer U, Coyl AJ, Di Stefano PS, Bertin J, Sansonetti PJ, Philpott DJ: Nod1 Detects a Unique Muropeptide from Gram-Negative Bacterial Peptidoglycan. Science. 2003, 300: 1584-1587. 10.1126/science.1084677. 2003
Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JS, Nuñez G: Host Recognition of Bacterial Muramyl Dipeptide Mediated through NOD2. J Biol Chem. 2003, 278: 5509-5512. 10.1074/jbc.C200673200.
Kang D, Liu G, Lundstrom A, Gelius E, Steiner H: A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc Natl Acad Sci USA. 1998, 95: 10078-10082. 10.1073/pnas.95.17.10078.
Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D: A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci USA. 2000, 97: 13772-13777. 10.1073/pnas.97.25.13772.
De Gregorio E, Spellman PT, Rubin GM, Lemaitre B: Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA. 2001, 98: 12590-12595. 10.1073/pnas.221458698.
De Gregorio E, Spellman P, Tzou P, Rubin GM, Lematrie B: The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO Journal. 2002, 21 (11): 2568-2579. 10.1093/emboj/21.11.2568.
Boutros M, Agaisse H, Perrimon N: Sequential Activation of Signaling Pathways during Innate Immune Responses in Drosophila. Dev Cell. 2002, 3: 711-722. 10.1016/S1534-5807(02)00325-8.
Li X, Wang S, Qi J, Echtenkamp SF, Chatterjee R, Wang M, Boons G, Dziarski R, Gupta D: Zebrafish Peptidoglycan Recognition Proteins Are Bactericidal Amidases. Nature Rev Immunol. 2007, 2: 725-734. 10.1038/nri910.
Ghuysen JM: Use of Bacteriolytic Enzymes in Determination of Wall Structure and Their Role in Cell Metabolism. Bacteriol Rev. 1968, 32 (4): 425-464.
Schleifer KH, Kandler O: Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972, 36: 407-77.
Jenni R, Berger-Bächi B: Teichoic acid content in different lineages of Staphylococcus aureus NCTC832. Arch Microbiol. 1998, 170: 171-178. 10.1007/s002030050630.
Deininger S, Stadelmaier A, Von Aulock S, Morath S, Schmidt RR, Hartung T: Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J Immunol. 2003, 170: 4134-4138.
Boneca IG: The role of peptidoglycan in pathogenesis. Curr Opin Microbiol. 2005, 8: 46-53. 10.1016/j.mib.2004.12.008.
Kumar S, Roychowdhury A, Ember B, Wang Q, Guan R, Mariuzza RA, Boons GJ: Selective recognition of synthetic lysine and meso- diaminopimelic acid-type peptidoglycan fragments by human peptidoglycan recognition proteins. J Biol Chem. 2005, 280: 37005-37012. 10.1074/jbc.M506385200.
Wolfert MA, Murray TF, Boons GJ, Moore JN: The Origin of the Synergistic Effect of Muramyl Dipeptide with Endotoxin and Peptidoglycan. J Biol Chem. 2002, 277 (42): 39179-39186. 10.1074/jbc.M204885200.
Wolfert MA, Roychowdhury A, Boons GJ: Modification of the Structure of Peptidoglycan Is a Strategy To Avoid Detection by Nucleotide-Binding Oligomerization Domain Protein 1. Infect Immun. 2007, 75 (2): 706-713. 10.1128/IAI.01597-06.
Mackenzie S, Planas JV, Goetz FW: LPS-stimulated expression of a tumor necrosis factor-alpha mRNA in primary trout monocytes and in vitro differentiated macrophages. Dev Comp Immunol. 2003, 27: 393-400. 10.1016/S0145-305X(02)00135-0.
Koskinen H, Pehkonen P, Vehniainen E, Krasnov A, Rexroad C, Afanasyev S, Molsa H, Oikari A: Response of rainbow trout transcriptome to model chemical contaminants. Biochem Biophys Res Commun. 2004, 320: 745-753. 10.1016/j.bbrc.2004.06.024.
Krasnov A, Koskinen H, Pehkonen P, Rexroad CE, Afanasyev S, Molsa H: Gene expression in the brain and kidney of rainbow trout in response to handling stress. BMC Genomics. 2005, 6 (1): 3-18. 10.1186/1471-2164-6-3.
Cleveland WS, Grosse E, Shyu WM: Local regression models. Statistical Models. Edited by: Chambers JM, S TJHE. 1999, Pacific Grove, Wadsworth and rooks/Cole, 309-376.
Storey JD, Tibshirani R: Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences of the United States of America. 2003, 100 (16): 9440-9445. 10.1073/pnas.1530509100.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G: Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000, 25 (1): 25-29. 10.1038/75556.
Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29: 45-53. 10.1093/nar/29.9.e45.
Iliev DB, Roach KC, Mackenzie S, Planas JV, Goetz FW: Endotoxin recognition: In fish or not in fish?. FEBS Letters. 2005, 579: 6519-6528. 10.1016/j.febslet.2005.10.061.
Kraal G, Luc JW, Laan V, Eloma O, Tryggvason K: The macrophage receptor MARCO. Microbes Infect. 2000, 2: 313-316. 10.1016/S1286-4579(00)00296-3.
Hebert H, Jegerschöld C: The structure of membrane associated proteins in eicosanoid and glutathione metabolism as determined by electron crystallography. Curr Opin Struc Biol. 2007, 17 (4): 396-404. 10.1016/j.sbi.2007.07.008.
Wallner BP, Mattaliano RJ, Hession C, Cate RL, Tizard R, Sinclair LK, Foeller C, Chow EP, Browning JL, Ramachandran KL, Pepinsky B: Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986, 320: 77-81. 10.1038/320077a0.
Saunders L, Perkins R, Balachandran D, Michaels S, Ford R, Mayeda R, Barber A: Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR. J. Biological Chem. 2001, 276: 32300-32312. 10.1074/jbc.M104207200.
Hla T, Neilson K: Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992, 89: 7384-7388. 10.1073/pnas.89.16.7384.
Tazawa R, Xu XM, Wu KK, Wang LH: Characterization of the Genomic Structure, Chromosomal Location and Promoter of Human Prostaglandin H Synthase-2 Gene. Biochem Bioph Res Co. 1994, 203 (1): 190-199. 10.1006/bbrc.1994.2167.
Shimizu T, Yamamoto S, Hayaishi O: Purification and properties of prostaglandin D synthetase from rat brain. J Biol Chem. 1979, 254: 5222-5228.
Nagata A, Suzuki Y, Igarashi M, Eguchi N, Toho H, Urade Y, Hayaishi O: Human brain prostaglandin D synthase has been evolutionarily differentiated from lipophilic-ligand carrier proteins. Proc. Nati. Acad. Sci. USA. 1991, 88: 4020-4024. 10.1073/pnas.88.9.4020.
Kambris Z, Jang BS, Nam IH, Romeo HJ, Takahashi Y, Lee K, Ueda WJ, Lemaitre B: Drosophila immunity: a large-scale in vivo RNAi screen identifies five serine proteases required for Toll activation. Curr Biol. 2006, 16: 808-813. 10.1016/j.cub.2006.03.020.
Mackenzie S, Balasch JC, Novoa B, Ribas L, Roher N, Krasnov A, Figueras A: Comparative analysis of the acute response of the trout, O. mykiss, head kidney to in vivo challenge with virulent and attenuated infectious hematopoietic necrosis virus and LPS-induced inflammation. BMC Genomics. 2008, 9: 141-156. 10.1186/1471-2164-9-141.
Goetz FW, Planas JV, Mackenzie S: Tumor necrosis factors. Dev Comp Immunol. 2004, 28: 487-497. 10.1016/j.dci.2003.09.008.
Mackenzie S, Montserrat N, Mas M, Acerete L, Tort L, Krasnov A, Goetz FW, Planas JV: Bacterial lipopolysaccharide induces apoptosis in the trout ovary. Reprod Biol Endocrin. 2006, 4: 46-58. 10.1186/1477-7827-4-46.
Mackenzie S, Iliev D, Liarte C, Koskinen H, Planas JV, Goetz FW, Molsä H, Krasnov A, Tort L: Transcriptional analysis of LPS-stimulated activation of trout (Oncorhynchus mykiss) monocyte/macrophage cells in primary culture treated with cortisol. Molec Immun. 2006, 43 (9): 1340-1348. 10.1016/j.molimm.2005.09.005.
Barclay GR, Scott BB: Serological Relationships between Escherichia coli and Salmonella smooth- and rough-mutant lipopolysaccharides as revealed by enzyme-linked immunosorbent assay for human immunoglobulin G Antiendotoxin Antibodies. Innate Immun. 1987, 55 (11): 2706-2714.
Bayne CJ, Gerwick L, Wheeler PA, Thorgaard GH: Transcriptome profiles of livers and kidneys from three rainbow trout (Oncorhynchus mykiss) clonal lines distinguish stocks from three allopatric populations. Com Biochem Physiol. 2006, 1: 396-403.
Roberge C, Einum S, Guderley H: Bernatchez Rapid parallel evolutionary changes of gene transcription profiles in farmed Atlantic salmon. Mol Ecol. 2006, 15: 9-20. 10.1111/j.1365-294X.2005.02807.x.
Doñate , Balasch JC, Callol A, Bobe J, Tort L, Mackenzie S: The effects of immunostimulation through dietary manipulation in the rainbow trout; evaluation of mucosal immunity. Mar Biotechnol. 2010, 12 (1): 88-99.
Byon JY, Ohira T, Hirono I, Aoki T: Use of a cDNA microarray to study immunity against viral hemorrhagic septicemia (VHS) in Japanese flounder (Paralichthys olivaceus) following DNA vaccination. Fish Shellfish Immunol. 2005, 18: 135-147. 10.1016/j.fsi.2004.06.008.
Martin S, Blaney SC, Houlihan DF, Secombes CJ: Transcriptome response following administration of a live bacterial vaccine in Atlantic salmon (Salmo salar). Mol Immunol. 2006, 43: 1900-1911. 10.1016/j.molimm.2005.10.007.
Jorgensen SM, Afanasyev S, Krasnov A: Gene expression analyses in Atlantic salmon challenged with infectious salmon anemia virus reveal differences between individuals with early, intermediate and late mortality. BMC Genomics. 2008, 9: 179-186. 10.1186/1471-2164-9-179.
Aluru N, Vijayan MM: Stress transcriptomics in fish: A role for genomic cortisol signaling. Gen Com Endoc. 2009, 164: 142-150. 10.1016/j.ygcen.2009.03.020.
Roher N, Callol A, Planas J, Goetz F, Mackenzie S: Endotoxin recognition in fish results in inflammatory cytokine secretion not gene expresión. Innate Immun. 2010, 10: 245-267.
Hinz B, Brune K: Cyclooxygenase-2-10 years later. J Pharmacol Exp Ther. 2002, 300 (2): 367-375. 10.1124/jpet.300.2.367.
Chen BC, Liao CC, Hsu MJ, Liao YT, Lin CC, Sheu JR, Lin CH: Peptidoglycan-induced IL-6 production in RAW 264.7 macrophages is mediated by cyclooxygenase-2, PGE2/PGE4 receptors, protein kinase A, I kappa B kinase, and NF-kappa B. J. Immunol. 2006, 177: 681-693.
Chen BC, Kang JC, Lu YT, Hsu MJ, Liao CC, Chiu WT, Yeh FL, Lin CH: Rac1 regulates peptidoglycan-induced nuclear factor-kappaB activation and cyclooxygenase-2 expression in RAW 264.7 macrophages by activat- ing the phosphatidylinositol 3-kinase/Akt pathway. Mol. Immunol. 2009, 46: 1179-1188. 10.1016/j.molimm.2008.11.006.
Urade Y, Hayaishi O: Prostaglandin D synthase: Structure and function. Vitam Horm. 2000, 58: 89-120. full_text.
Kanaoka Y, Urade Y: Hematopoietic prostaglandin D synthase. Prostaglandins Leukot Essent Fat Acids. 2003, 69 (3): 163-167. 10.1016/S0952-3278(03)00077-2.
Stenbak C, Ryu J, Leulier F, Pili-Floury S, Parquet C, Herve M, Chaput C, Boneca I, Lee W, Lemaitre B, Mengin-Lecreulx D: Peptidoglycan molecular requirements allowing detection by the Drosophila immune deficiency pathway. J. Immunol. 2004, 173: 7339-7348.
Royet J, Dziarski R: Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nature Rev Microbiol. 2007, 5 (4): 267-277. 10.1038/nrmicro1620.
McDonald C, Inohara N, Nuñez G: Peptidoglycan Signaling in Innate Immunity and Inflammatory Disease. J Biol Chem. 2005, 280 (21): 20177-20180. 10.1074/jbc.R500001200.
Baoprasertkul P, Peatman E, Abernathy J, Liu Z: Structural characterisation and expression analysis of Toll-like receptor 2 gene from catfish. Fish Shellfish Immun. 2007, 22 (4): 418-426. 10.1016/j.fsi.2006.04.005.
Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim S, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B: Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003, 424: 743-748. 10.1038/nature01889.
Jiang Z, Georgel P, Du X, Shamel L, Sovath L, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B: CD14 is required for MyD88-independent LPS signaling. Nature Immunol. 2005, 6 (6): 565-573. 10.1038/ni1207.
Akira S: Mammalian Toll-like receptors. Curr opin. 2003, 15: 5-11.
Locksley RM, Killeen N, Lenardo MJ: The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001, 104 (4): 487-501. 10.1016/S0092-8674(01)00237-9.
Cha GH, Cho KS, Lee JH, Kim M, Kim E, Park J, Lee SB, Chung J: Discrete Functions of TRAF1 and TRAF2 in Drosophila melanogaster Mediated by c-Jun N-Terminal Kinase and NF-B-Dependent Signaling Pathways. Mol Cell Biol. 2003, 23 (22): 7982-7991. 10.1128/MCB.23.22.7982-7991.2003.
Acknowledgements
This study was supported by the Consolider-Ingenio Programme 2010, project CSD2007-0002 funded by the Spanish Ministry of Science and Education, Spain to SM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
SM, SB and FG participated in the conception of the experimental design. SB and SM carried out the analysis. SB, FRL and DM performed microarray experiments. SB edited expression data, carried all statistical analysis and validated array data with qRT-PCR. SB and SM wrote the manuscript and FG corrected it. All listed authors edited the manuscript. All authors read and approved the manuscript.
Electronic supplementary material
12864_2010_3173_MOESM2_ESM.XLS
Additional file 2:Comparison of expression data for selected transcripts obtained from microarray analyses and qRT-PCR validation. The results are presented as fold change relative to 18S abundance and mean ± std deviation. (XLS 34 KB)
12864_2010_3173_MOESM3_ESM.XLS
Additional file 3:Description of PGN (O111;B4) regulated transcripts/genes over the control (all cDNAs on the array) at early stage (1 h)(XLS 33 KB)
12864_2010_3173_MOESM4_ESM.XLS
Additional file 4:Description of PGN (O111;B4) regulated transcripts/genes over the control (all cDNAs on the array) at median stage (6 h). xls (XLS 38 KB)
12864_2010_3173_MOESM5_ESM.XLS
Additional file 5:Description of PGN (O111;B4) regulated transcripts/genes over the control (all cDNAs on the array) at late stage (12 h). xls (XLS 34 KB)
12864_2010_3173_MOESM6_ESM.XLS
Additional file 6:Description of PGN (K12) regulated transcripts/genes over the control (all cDNAs on the array) at early stage (1 h). (XLS 46 KB)
12864_2010_3173_MOESM7_ESM.XLS
Additional file 7:Description of PGN (K12) regulated transcripts/genes over the control (all cDNAs on the array) at median stage (6 h). (XLS 30 KB)
12864_2010_3173_MOESM8_ESM.XLS
Additional file 8:Description of PGN (K12) regulated transcripts/genes over the control (all cDNAs on the array) at late stage (12 h). (XLS 50 KB)
12864_2010_3173_MOESM9_ESM.XLS
Additional file 9:Quantitative summary of transcripts/genes differentially expressed over the control in both treatment and stages. (XLS 28 KB)
12864_2010_3173_MOESM10_ESM.PDF
Additional file 10:Relationship between intensity and magnitude of transcriptomic response in up (a) and down (b) regulated genes at different time stages during the PGNs challenge. The horizontal abscises (magnitude) show the number of transcripts grouped in biological processes expressed in both treatments as: Antigen presentation, Cell adhesion and proliferation, Cytokines and Chemokines, Cellullar defense response, MAPK/ERK, Inflammatory response, Cell homeostasis, Transcription. The vertical abscises (intensity) show fold change mean (FC: intensity) of the transcripts grouped in each biological process. The black circle and the blue slope represented the fit generated by the intensity and magnitude of the transcriptomic response under PGN-O111:B4 treatment. The white circle and the red slope represented the fit generated by the intensity and magnitude of the transcriptomic response under PGN-K12 treatment. Transcriptomic profiles were highly ranked dependent upon PGN-type (two-way ANCOVA on transcriptomic magnitudes of respective intensities α = 0.05; n = 68). (PDF 1 MB)
12864_2010_3173_MOESM11_ESM.XLS
Additional file 11:Summary of ANCOVA analysis for common slope of regression and adjusted means examining differences in intensity and magnitude of the transcriptomic response at different times (1, 6 and 12 h; n = 68)(XLS 36 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Boltaña, S., Reyes-Lopez, F., Morera, D. et al. Divergent responses to peptidoglycans derived from different E. coli serotypes influence inflammatory outcome in trout, Oncorhynchus mykiss, macrophages. BMC Genomics 12, 34 (2011). https://doi.org/10.1186/1471-2164-12-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2164-12-34