Abstract
Background
Models of the maintenance of sex predict that one reproductive strategy, sexual or parthenogenetic, should outcompete the other. Distribution patterns may reflect the outcome of this competition as well as the effect of chance and historical events. We review the distribution data of sexual and parthenogenetic biotypes of the planarian Schmidtea polychroa.
Results
S. polychroa lives in allopatry or sympatry across Europe except for Central and North-Western Europe, where sexual individuals have never been reported. A phylogenetic relationship between 36 populations based on a 385 bp fragment of the mitochondrial cytochrome oxidase I gene revealed that haplotypes were often similar over large geographic distances. In North Italian lakes, however, diversity was extreme, with sequence differences of up to 5% within the same lake in both sexuals and parthenogens. Mixed populations showed "endemic" parthenogenetic lineages that presumably originated from coexisting sexuals, and distantly related ones that probably result from colonization by parthenogens independent from sexuals.
Conclusions
Parthenogens originated repeatedly from sexuals, mainly in Italy, but the same may apply to other Mediterranean regions (Spain, Greece). The degree of divergence between populations suggests that S. polychroa survived the ice ages in separate ice-free areas in Central, Eastern and Southern Europe and re-colonised Europe after the retreat of the major glaciers. Combining these results with those based on nuclear markers, the data suggest that repeated hybridisation between sexuals and parthenogenetic lineages in mixed populations maintains high levels of genetic diversity in parthenogens. This can explain why parthenogens persist in populations that were originally sexual. Exclusive parthenogenesis in central and western populations suggests better colonisation capacity, possibly because of inbreeding costs as well as hybridisation of sexuals with parthenogens.
Similar content being viewed by others
Background
Theory predicts that stable coexistence of sexual and parthenogenetic conspecifics must be rare [1, 2]. Mixed associations are therefore seen as a transition towards the extinction of one of the two forms [3]. A non-overlapping spatial distribution pattern of sexuals and parthenogens is frequently found and has been termed geographic parthenogenesis [4]. Typically, the (ancestral) sexual population is located in the distribution centre of the species, while parthenogens are present at the margin of the distribution [5]. An ecological explanation for this pattern does not exist [6]. Lynch [7] proposed that parthenogens might fail to establish themselves in the presence of sexuals as backcrosses with the ancestral sexual forms may hamper the independence of purely parthenogenetic forms. But parthenogens may have better colonizing capacities: They have a higher intrinsic growth rate and do not pay the deleterious effects of population bottlenecks (e.g. inbreeding) that may act on sexual populations [7]. As a result, they may colonize areas where sexuals have difficulties establishing a population [6].
Non-adaptive explanations for geographic parthenogenesis take historical events into account, e.g. when colonisation was recent. In Europe, as elsewhere, the climate changed dramatically during the past 20,000 years from the last glacial height to the present interglacial period [8, 9]. As a result, previously glaciated areas have become available for species that could not withstand the earlier low temperatures [10]. Recolonization, however, also requires migration routes that allow dispersal from 'refugia'. Hence, distributions will be affected by dispersal barriers such as mountain ranges like the Pyrenees, the Alps and the Balkans [11, 10]. Using mitochondrial DNA sequences it has become possible to reconstruct the phylogenetic relationships between populations of the same species, thereby identifying the source populations of the lineages that (re)colonized Central and Northern Europe [12].
Intraspecific phylogenies may also reveal age and origin of parthenogenetic lineages [13–15]. Since mtDNA is maternally inherited, it is also a suitable tool to identify the maternal ancestors of hybrid parthenogens [16–20]. Both allow identification of the rate at which new parthenogenetic lineages arise, and to what extent the presence of sexual conspecifics is required.
Schmidtea polychroa is a freshwater planarian with a Europe-wide distribution. It is a simultaneous hermaphrodite with sexual and parthenogenetic biotypes and has been used for various studies on the costs and benefits of sex [21–24]. In much of Western and Central Europe sexuals are absent [25], whereas mixed populations of sexuals and parthenogens occur in Italy [25, 26] and Sweden [27]. It is not known whether coexistence is in equilibrium or whether invasion and displacement are still in progress. Allozyme markers indicated a polyphyletic origin of parthenogens from sexuals [26]. Parthenogens were more closely related to sympatric sexuals from the same population than to parthenogens elsewhere.
Here, we studied the phylogenetic relationships of sexual and parthenogenetic S. polychroa collected at 38 sites in Europe to explain their distribution. In a first step we review all available distribution data from the literature. Using an mtDNA marker, we then investigate (1) whether parthenogens arose repeatedly or whether they are monophyletic, and (2) whether there are indications for recent or ancient parthenogenetic strains. Special attention was paid to mixed populations and to evidence for hybridisation between sexuals and parthenogens. We discuss what these data imply for the recolonisation of Central and Northern Europe by sexual and parthenogenetic S. polychroa.
Schmidtea polychroa: Diploid sexuals and polyploid parthenogens
Schmidtea (formerly Dugesia) polychroa consists of four karyologic forms, or biotypes A to D [28]. B, C and D represent polyploid, parthenogenetic forms derived from the diploid sexual biotype A (2x = 8). Benazzi [28] differentiated between synaptic (B) and asynaptic (C, D) oogenesis. In the synaptic biotypes, production of triploid or tetraploid eggs is achieved by endoduplication of the polyploid chromosome set, followed by meiosis during which the identical, duplicated sister chromatids pair. This means that normal meiotic processes, including segregation and recombination, occur, but eggs still have the same genetic information as their mother. The other biotypes produce triploid (C) and tetraploid (D) eggs mitotically. In all parthenogens (B–D) egg development requires fusion with allosperm. The sperm nucleus, however, does not fuse with the egg nucleus, but degrades and is expelled [29].
Schmidtea polychroa is a generalist and can be found in meso- to eutrophic freshwater habitats like lowland rivers, streams, ditches, and lakes. Sexuals and parthenogens are widespread and live in sympatry in several localities in Italy. Sexuals have never been reported from Central and Western Europe, but are present in Sweden (see results). It is therefore unlikely that differences in climatic preferences or variance in colonization ability alone can explain the distribution pattern.
Results
Distribution of sexual and parthenogenetic Schmidtea polychroa
Sampling coverage is good for much of Europe, except for Eastern Europe and the Iberian Peninsula (Table 1). Large numbers of collection sites in close proximity of a single study area [e.g. locality 30] were pooled as a single locality. Due to a large number of data points in some areas, not every single location was listed when no additional biotype was found. This applies to the British Isles and some Mediterranean islands.
The majority of studies reported either sexuals or parthenogens for a given locality (Fig. 1). Sexual populations are abundant in Italy, including Sardinia and Elba. Reports from sexual forms elsewhere are rare, but span a wide geographical range: Hungary (2 sites), Austria (Graz), Spain (3 sites) and Sweden (3 sites). Sexual populations have not been found North and West of the Alps, including France, Germany, Switzerland, large parts of Austria, the Netherlands, Belgium, Denmark, Great Britain and Ireland. In all those countries, only polyploid (mainly triploid) populations have been reported. No data are available for much of Eastern Europe, except for the more general statement that Dugesia [=Schmidtea] polychroa reaches its eastern distribution limit at the Volga [31, 32].
Overview of the geographic distribution of diploid sexual and polyploid parthenogenetic Schmidtea polychroa in Europe. Numbers indicate sample ID for COI phylogeny (see Table 2).
Parthenogenetic S. polychroa are found all over Europe. Biotype B appears to be most widely distributed, but one needs to consider that most studies do not distinguish between biotypes B, C and D. Mixed populations of sexuals and parthenogens were only reported from Pisa, Italy (Monti Pisani: [25, 30]), North Italian lakes (Lago Maggiore: [33]; Lago di Caldonazzo: [22]; Lago d'Iseo: this study), and one locality in Spain [34].
Phylogenetic relationships among COI haplotype sequences
From 176 individuals we identified 31 different sequences (haplotypes hp01-31; Fig. 2 and 3). Within haplotypes, letters were used to differentiate between individuals with the same haplotype but different ploidy (2x, 3x or 4x) or geographic origin. This resulted in 82 S. polychroa sequences for phylogenetic analysis and allowed the comparison of single or multiple origins of parthenogens in the analysis (see below). Note that hlE03 (S. lugubris, biotype E) and hlF01 (S. nova, biotype F) were used as outgroups.
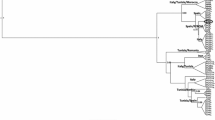
Bayesian phylogenetic analysis of haplotype sequences hp01-hp31 of S. polychroa. hlE03 (S. lugubris, biotype E) and hlF01 (S. nova, biotype F) were used as outgroups. Numbers adjacent to the nodes indicate the posterior probability for the Bayesian analysis. Locality names are followed from left to right by ploidy number (between brackets), locality name (between square brackets) and haplotype code. Haplotypes with equal number but followed by a different letter (e.g. hp03a, hp03b, hp03c, etc.), represent identical haplotypes (identical COI sequences) found in animals with different ploidy and/or from a different geographical locality. Grey boxes highlight all sexual (diploid) S. polychroa included in this study. Outgroup branch with dashed line has been shortened for aesthetics of the figure.
Minimum spanning network of all identified haplotypes. Haplotype codes, and the number of sexual (S) and partenogens (P) that have a particular haplotype are indicated inside the circles. Small filled circles separating haplotypes represent a single nucleotide substitution difference. Squares represent the ancestral haplotype of every particular network. Dashed lines indicate a possible joining place for the most divergent haplotypes, whose connection to other haplotypes could not be justified by the parsimony criterion.
Within S. polychroa, polymorphisms were found at 59 positions, 28 of which were parsimony informative. Forty-nine out of a total of 63 substitutions were synonymous. Many of the rare haplotypes differed by a single substitution from a common haplotype (hp03, Fig. 2 and 3). Within S. polychroa, the largest genetic distance was 24 nucleotides, or 6.2% (Table 3). Between Schmidtea species, the differences ranged between 61 and 87 substitutions. S. polychroa sequences, sampling site and biotype information are accessible in GenBank (AF287052 – AF287133).
Both phylogenetic methods (Bayesian and maximum-likelihood ML) gave very similar results recovering most monophyletic groups with posterior probability ≥90% (Fig. 2). In both analyses parthenogens (3x and 4x) were scattered on several branches indicating repeated origin from sexuals. To test this possibility, three constraint analyses were performed using the Shimodaira-Hasegawa test (one-tailed). The first assumed a single origin for all parthenogens (both 3x and 4x). The next two assumed a single origin for either 3x or 4x parthenogens (Table 4). All three are significantly worse than the Bayesian phylogeny and therefore we reject the possibility of a single origin for parthenogenesis. Figs. 2 and 3 show that a single haplotype (hp03) is widespread among Central European parthenogens. The four sexual S. polychroa with hp03 are restricted to 4 lakes in Northern Italy (Fig. 2).
The network analysis shows hp03 as a central, presumably ancestral haplotype in the TCS analysis. Some haplotypes could not be joined without exceeding the maximum number of mutational steps as specified by the parsimony criterion. One of these was the very divergent hp31 from the only Spanish sexual S. polychroa. Another four (hp06, hp14, hp25 and hp26) clustered together in a small separate network. All haplotypes within this cluster are from Northern Italy (Fig. 3). Two other divergent groups were represented by parthenogens from France (hp12, hp13 and hp21) and sexuals from Central Italy (hp02, hp18 and hp23). Three more highly divergent, single haplotypes (hp05, hp17 and hp19) were only found in parthenogens.
From all sampled areas, Northern Italy shows most diversity. Although part of this must be attributed to the high sampling effort in this area, it is also the region where two widespread, but distinct clusters overlap. These two differ by about 5% and consistently appeared on different branches of the tree, irrespective of the method used.
Parthenogenesis: recent or ancient?
The degree to which parthenogenetic lineages diverge from their closest sexual progenitors should correlate with their ability to persist for a time long. Differences between parthenogenetic strains in the degree of divergence would then be a relative measure of the age of such lineages, and allow to distinguish relatively "young" from relatively "old" lineages. In all main clusters both sexuals and parthenogens were found (Fig. 2, 3). Some parthenogenetic lineages had haplotypes identical or similar to sympatric or geographically close sexuals, indicating recent (sexual) ancestors (Table 2, Fig. 2). Other parthenogens, however, showed large differences to nearby sexual haplotypes. The largest observed difference was 13 nucleotide substitutions and was found between parthenogenetic S. polychroa from Lac d'Annecy (France) and sexual individuals from Northern Italy, which represents a divergence of approximately 3%. Hp19, present in parthenogens from Central Italy, differs by six substitutions from sympatric sexuals (hp18, hp23), a 1.8% divergence. Parthenogens in Lago di Como (hp05) and Ammersee (hp17) show a similar pattern when compared to the nearest sexual populations. Divergent lineages like these may represent ancient parthenogenetic lineages. However, it cannot be excluded that some more closely related sexual haplotypes are present in the field, but were not sampled.
Discussion
Geographic distribution
Although the distribution of sexual and parthenogenetic S. polychroa suggests 'geographic parthenogenesis' [4], the picture is complicated. On a large scale, the pattern is clear: sexuals are absent from areas North of the Alps, including France, Germany, parts of Austria, Belgium, Luxembourg, the Netherlands, Denmark, and Great Britain. Yet, mixed populations occur in Italy, Spain and Sweden. Data for Eastern Europe are poor, but sexuals occur in Hungary. Since sexuals have never been observed in any other place North of the Alps, their occurrence in Sweden raises questions about long-distance dispersal aided by waterfowl [35, 36] or humans. Anthropogenic dispersal is invoked for the success of the North-American Girardia (Dugesia) tigrina in Europe. Planaria torva may have invaded Great Britain by such a process [37]. Similarly, human activity is the likely cause of the introduction of S. polychroa to North America [38]. But there are also alternative explanations.
As Scandinavia was ice-covered during the last glaciation, present-day populations in Southern Sweden must be descendants of lineages that persisted in refugia. The Baltic Sea may have aided dispersal into Scandinavia, since its salinity dropped to freshwater levels during certain periods ('Ancylus Sea') [39]. At present, several freshwater planarians, including Schmidtea polychroa, can be found in brackish regions along the Swedish East coast [27, 40]. Colonization of Scandinavia after the last glaciation through the Baltic has been shown for the European perch using genetic data [41, 42]. The fact that sexual S. polychroa are absent from Denmark and Germany suggests that Sweden was colonized by Eastern populations. Extensive karyological and genetic data from Eastern Europe and Scandinavia are needed to confirm this possibility.
Our (limited) genetic data for parthenogens from Denmark suggests that they are descendants of Central European populations. It suggests that the Kattegat Strait between Denmark and Sweden is a dispersal barrier for S. polychroa. Support for this possibility comes from another planarian, Polycelis nigra, which occurs throughout Europe up to Northern Germany and Denmark, but is missing from Sweden [43]. Yet, P. nigra is one of the commonest European planarians and often lives under conditions similar to those prevalent in southern Sweden [35].
Phylogeography of European species, and geographic distribution of haplotypes
Hewitt [44] analysed the effects of recurrent ice ages and concluded that species ranges have contracted and expanded repeatedly. The consequences are loss of variation, divergence among populations due to isolation, genome reorganization and hybridisation of slightly divergent genomes [44, 45]. In principle, geographic and genetic variability allow identification of recolonisation routes and hybrid zones after the last ice ages [10, 11]. However, in our data the intraspecific divergence among mtDNA lineages is so extreme, that it appears as if major lineages split long before the quarternary ice ages. This means that the latter did not cause, but possibly maintained or reinforced existing divergence [11].
During the ice ages not all species went extinct in Central Europe. Cold-adapted species survived in the ice-free corridor between the Scandinavian and the Alpine ice sheets. Some planarians, including S. polychroa, may well have belonged to this community [46]. Planarians, including S. polychroa, also survived the last ice age in Britain's ice-free Southwest [35]. This implies that recolonization of previously glaciated habitats may well have started from persisting nearby populations rather than from remote refugia. Although low temperatures in ice-covered lakes does not seem to affect adult survival, S. polychroa requires temperatures to rise above 7.5°C for cocoon production [35]. This coincides with Central European summer temperatures during glaciations, which were around 9°C [47].
With few exceptions, clusters of related haplotypes belong to certain geographical regions (Fig. 2). Spanish samples differed clearly from all others. Similarly, no haplotypes were shared between Central Italy and any other region. Two very different clusters were exclusively found in Northern Italy, coexisting with even more types. North of the Alps, however, haplotype variation is low. This does not necessarily indicate recolonization from a single source population after the last glaciation. The COI gene may be too conserved to allow resolution of divergence caused by the most recent glaciation. In contrast to other phylogenetic studies, the Alps do not seem to represent an absolute genetic barrier for some haplotypes. Hp03, dominant in Central Europe parthenogens, also occurs in Northern Italy. However, the Alps do seem to represent a dispersal barrier for sexual, diploid S. polychroa, which are absent from countries on their western and northern edge (France to Austria).
Origin and divergence time of parthenogenetic lineages
Our results support the multiple, repeated origin of parthenogenetic lineages [26] from sexuals. A single origin is rejected by the constraint analyses (Table 4).
Inferring the age of a parthenogenetic lineage from genetic data is based on nucleotide divergence with its closest sexual relative (the hypothetical ancestor). If the true ancestor has gone extinct or has not been sampled, this procedure overestimates the age of the lineage. It also requires a reliable molecular clock for the focal sequence. Nevertheless, if the sampling pattern is dense enough, at least a qualitative identification of "ancient" parthenogens should be possible. Estimates for the mutation rate of the mitochondrial COI gene of arthropods are in the range of about 2% per million years [48–50]. Although mutation rates differ between taxa, we apply this rate to S. polychroa for a preliminary estimate. It suggests that most parthenogens are not older than 500,000 years. The parthenogenetic lineages from France (hp13, hp12 and hp21), however, differ by about 1.5 to 3% from their closest sexuals. This suggests divergence times of 750,000 to 1.5 million years. If this is true, parthenogens from the same or adjacent areas should form a monophyletic clade of exclusively and rather ancient parthenogenetic lineages. Better phylogeographic coverage for Southern France and Switzerland is required to prove this.
Finally, tetraploid parthenogens of localities 14 and 15 (Fig 1) differ strikingly from coexisting sexuals (Fig 2 and 3). Further sampling from more populations from adjacent areas is required to confirm their relationship. As the Italian distribution of sexuals and parthenogens is a true mosaic, these tetraploids may be descendants from extant but as yet unsampled diploid populations.
Hybridisation between sexuals and parthenogens in mixed populations
Mixed populations encourage ecological and genetic interactions between sexuals and parthenogens, allowing the study of the evolutionary advantage of sex and the origin of parthenogenesis. Hermaphroditic parthenogens that have a functional male gender may spread genes for parthenogenesis in the sexual gene pool, thus diluting and ultimately displacing sexuality. Jaenike & Selander [51] explored the ecological conditions under which such processes work and applied them to explain the distribution of parthenogenetic oligochaetes in North America.
Being a hermaphrodite, S. polychroa always has both sex functions in the same individual, also in parthenogens. Unusual is that parthenogens are sperm-dependent and produce haploid sperm, an unusual trait for a polyploid [53]. They require sperm from a partner (sexual or parthenogenetic) to activate parthenogenetic development of their eggs [52]. However, neither sexuals nor parthenogens are able to self-fertilize. Hybridisation between sexuals and parthenogens has been studied extensively in the population of Lago di Caldonazzo (Trento, N-Italy). The frequency distribution of sexuals and parthenogens varied strongly between sites but was not explained by ecological parameters [22]. Genetic and karyological data indicate that new triploid, parthenogenetic lineages arise regularly as a result of hybridisation between sexuals and parthenogens [21, 26, 54]. Higher heterozygosity and diversity among parthenogens may be attributed to triploidy and fixed heterozygosity. However, it is also evidence for secondary contact of sexuals and parthenogens. If parthenogens had exclusively originated from local sexuals, their genetic variability should be within that of sexuals, which is not the case. Our results show that although some haplotypes are shared (Hp01, Hp07), others are exclusive to parthenogens (Hp06, Hp14). This pattern is supported by a detailed study with large samples from several sites within Caldonazzo [54]. Interestingly, parthenogenetic lineages differ not only with regard to their mitochondrial COI sequence, but also with regard to sex allocation and female fecundity. This indicates that they may represent different stages of adaptation to clonal reproduction [54].
Conclusions
Sexual S. polychroa are absent from Central and Western Europe, where populations are exclusively parthenogenetic. The pattern South of the Alps reveals a complicated overlapping mosaic between parthenogens and sexuals. Coexistence also exists in Spain and Southern Sweden. Clustering of COI haplotypes identified groups of geographically close lineages. In northern Italy, two haplotype clusters are present that differ by about 5%, but overlap geographically. Both types were found among sexuals as well as parthenogens. The data suggest that postglacial colonisation in Europe was not from a single refugium, but from several sources that already had diverged to some extent. It is likely that Scandinavia was colonised from the East, whereas Central and Western Europe were colonised from the South or Centre. The data further indicate that parthenogenetic lineages are present on several branches in a phylogeographic tree, indicating repeated origin. The latter may be enhanced by hybridisation between sexuals and parthenogens, as shown in studies using microsatellites [54]. This can explain the coexistence of identical sexual and parthenogenetic haplotypes. Yet other parthenogenetic haplotypes differed clearly from coexisting sexuals, indicating dispersal and secondary contacts. Coexistence of sexual and parthenogenetic forms must therefore be seen as a dynamic process of genetic exchange, arisal (and extinction) of new lineages, and dispersal. Exclusive parthenogenesis in Central and Western populations suggests better colonisation capacity of parthenogens. It is unclear why this is, but since sexuals are obligate outcrossers, they may suffer from inbreeding costs in small founder populations, slowing down their colonisation rate relative to parthenogens. Sexuals may also be poor competitors when invading already established parthenogenetic populations. Sexuals cannot obtain paternity in parthenogenetic partners, and even "help" to generate new parthenogenetic lineages through hybridisation.
Methods
Review of distribution data
Distribution records were extracted from publications and personal communications. All studies with karyological data and a description of sample origins were considered, but with an emphasis on continental Europe. Because field samples were collected differently in different studies, only presence or absence of a certain ploidy level was scored, not abundance. Differentiation of biotypes B, C and D requires cytological analysis of the final stages of oogenesis in fresh cocoons. Most studies, however, were limited to simple chromosome counts and only allow identification of (diploid) sexual and (polyploid) parthenogenetic lineages (Table 1).
Own data
Additional data were obtained from field trips to 38 localities in Europe between 1996 and 1999. Collection, transportation, and preparation of metaphase chromosome spreads are described in Pongratz [26]. For two samples (Sarca and Palancia), ploidy was not determined by karyotyping but inferred from allele numbers at up to four, highly polymorphic microsatellite loci (data not shown)[55].
mtDNA sequencing
Genomic DNA was extracted following the protocol in Pongratz [55]. In total, we analysed 176 individual S. polychroa from 36 different localities (Table 2). 81 samples were from diploids, 75 from triploids, and 20 from tetraploids. For use as an outgroup in phylogenetic analyses, several specimens of Schmidtea biotype E and F [28, 56] were sequenced. These were collected in populations 37 and 38 (Table 2).
Sequencing of COI
We amplified a fragment of the mitochondrial cytochrome-oxidase I (COI) gene using primers pr-a2 (5'-AGCTGCAGTTTTGGTTTTTTGGACATCCTGAGGT-3') and pr-b2 (5'-ATGAGCAACAACATAATAAGTATCATG-3') [57]. Although these primers were developed for Dugesia japonica, they amplify the same region in S. polychroa. For details see Pongratz [58]. PCR products were purified using Geneclean® Kit (Bio 101), yielding 15 μl solution of clean template in appropriate concentration for sequencing reactions with the ABI PRISM™ Dye Terminator Cycle Sequencing Kit (PE Applied Biosystems). Fragments were analysed on an ABIPRISM310. All samples were sequenced in both directions.
Phylogenetic analyses
All sequences were identical in length (385 bp) and were aligned manually. Polymorphisms were analysed with DNASP3.0 [59] and DAMBE3.7 [60]. The latter was also used for translation into amino acid sequences using the flatworm mitochondrial code in order to differentiate between synonymous and non-synonymous substitutions.
A total of 82 S. polychroa sequences, plus one sequence from each of the two outgroup taxa, S. lugubris (biotype E; GenBank accession No. AF290021) and S. nova (biotype F; AF290023) were used in the phylogenetic analyses. Phylogenetic relationships were estimated using Bayesian analysis and maximum-likelihood (ML). Modeltest v. 306 [61] was used to select the most appropriate model of sequence evolution under the Akaike Information Criterion. This was the General Time Reversible model (GTR) taking into account the shape of the gamma distribution (G). ML analyses were performed in PAUP*4.0b10 [62]. These consisted of heuristic searches involving tree bisection and reconnection (TBR) branch swapping. Bayesian phylogenetic analyses [63, 64] were performed with MRBAYES v. 2.01 [65] using the GTR+G model with parameters estimated as part of the analysis and four incrementally heated Markov chains with the default heating values. The analysis ran for 1. 5 × 106 generations, with sampling at intervals of 100 generations that produced 15000 sampled trees. After the run, the log-likelihood values of sample points were plotted against the generation time and all trees prior to reaching stationarity were discarded. A 50% majority rule consensus tree was generated combining only the last 5000 sampled trees. The frequency of any particular clade of the consensus tree represent the posterior probability of that clade [65]; only values above 95% were considered to indicate that nodes were significantly supported. Intra-specific mtDNA haplotype networks were reconstructed using the program TCS v1.13 [66]. Where appropriate, topological constraints were generated using MacClade v.4.0 [67] and compared to our optimal topology using the Shimodaira-Hasegawa (SH) test implemented in PAUP*4.0b10.
References
Case TJ, Taper ML: On the coexistence and evolution of asexual and sexual competitors. Evolution. 1986, 40: 366-387.
Peck JR, Yearsley J, Barreau G: The maintenance of sexual reproduction in a structured population. Proc R Soc Lond B Biol Sci. 1999, 266: 1857-1863. 10.1098/rspb.1999.0857.
Chaplin JA: The local displacement of a sexually reproducing ostracod by a conspecific parthenogen. Heredity. 1993, 71: 259-268.
Vandel A: La parthénogenèse géographique: Contribution à l'étude biologique et cytologique de la parthénogenèse naturelle. Bull Biol France Belg. 1928, 62: 164-281.
Cuellar O: Animal parthenogenesis. Science. 1977, 197: 837-843.
Cuellar O: Biogeography of parthenogenetic animals. Compte Rendu des Séances de la Société de Biogeographie. 1994, 70: 1-13.
Lynch M: Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Q Rev Biol. 1984, 59: 257-290.
COHMAP Members: Climatic changes of the last 18, 000 years: observations and model simulations. Science. 1988, 241: 1043-1052.
Bartlein PJ, Prentice IC: Orbital variations, climate and palaeoecology. Trends Ecol Evol. 1989, 4: 195-199. 10.1016/0169-5347(89)90072-4.
Hewitt GM: Post-glacial re-colonization of European biota. Biol J Linn Soc. 1999, 68: 87-112. 10.1006/bijl.1999.0332.
Taberlet P, Fumagalli L, Wust-Saucy AG, Cossons JF: Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol. 1998, 7: 453-464. 10.1046/j.1365-294x.1998.00289.x.
Bermingham E, Moritz C: Comparative phylogeography: concepts and applications. Mol Ecol. 1998, 7: 367-369. 10.1046/j.1365-294x.1998.00424.x.
Normark BB: Phylogeny and evolution of parthenogenetic weevils of the Aramigus tesselatus species complex (Coleoptera: Curculionidae: Naupactini): Evidence from mitochondrial DNA sequences. Evolution. 1996, 50: 734-745.
Sandoval C, Carmean DA, Crespi BJ: Molecular phylogenetics of sexual and parthenogenetic Timema walking-sticks. Proc R Soc Lond B Biol Sci. 1998, 265: 589-595. 10.1098/rspb.1998.0335.
Johnson SG, Bragg E: Age and polyphyletic origins of hybrid and spontaneous parthenogenetic Campeloma (Gastropoda: Viviparidae) from the Southeastern United States. Evolution. 1999, 53: 1769-1781.
Brown WM, Wright JW: Mitochondrial DNA analyses and the origin and relative age of parthenogenetic lizards (genus Cnemidophorus). Science. 1979, 203: 1247-1249.
Spolsky C, Uzzell T: Evolutionary history of the hybridogenetic frog Rana esculenta as deduced from mtDNA analyses. Mol Biol Evol. 1986, 3: 44-56.
Densmore LD, Wright JW, Brown WM: Mitochondrial-DNA analyses and the origin and relative age of parthenogenetic lizards (genus Cnemidophorus). Evolution. 1989, 43: 943-957.
Quattro JM, Avise JC, Vrijenhoek RC: Molecular evidence for multiple origins of hybridogenetic fish clones (Poeciliidae: Poeciliopsis). Genetics. 1991, 127: 391-398.
Moritz C, Heideman A: The origin and evolution of parthenogenesis in Heteronotia bionoei Gekkonidae. Reciprocal origins and diverse mitochondrial DNA in western populations. Syst Biol. 1993, 42: 293-306.
Weinzierl RP, Schmidt P, Michiels NK: High fecundity and low fertility in parthenogenetic planarians. Invert Biol. 1999, 118: 87-94.
Weinzierl RP, Beukeboom LW, Gerace L, Michiels NK: Spatial and ecological overlap between coexisting sexual and parthenogenetic Schmidtea polychroa (Tricladida; Platyhelminthes). Hydrobiologia. 1999, 392: 179-185. 10.1023/A:1003519418925.
Storhas M, Weinzierl RP, Michiels NK: Paternal sex in parthenogenetic planarians: a tool to investigate the accumulation of deleterious mutations. J Evol Biol. 2000, 13: 1-8. 10.1046/j.1420-9101.2000.00140.x.
Michiels NK, Beukeboom LW, Pongratz N, Zeitlinger J: Parthenogenetic flatworms have more symbionts than their coexisting, sexual conspecifics, but does this support the Red Queen?. J Evol Biol. 2001, 14: 110-119. 10.1046/j.1420-9101.2001.00249.x.
Beukeboom LW, Weinzierl RP, Reed KM, Michiels NK: Distribution and origin of chromosomal races in the freshwater planarian Dugesia polychroa (Turbellaria: Tricladida). Hereditas. 1996, 124: 7-15.
Pongratz N, Sharbel TF, Beukeboom LW, Michiels NK: Allozyme variability in sexual and parthenogenetic freshwater planarians – evidence for polyphyletic origin of parthenogenetic lineages through hybridization with coexisting sexuals. Heredity. 1996, 81: 38-47. 10.1038/sj.hdy.6883590.
Melander Y: Cytogenetic aspects of embryogenesis in Paludicola Tricladida. Hereditas. 1963, 49: 119-166.
Benazzi M: Cariologia di Dugesia lugubris (Schmidt) (Tricladida Paludicola). Caryologia. 1957, 10: 276-303.
Benazzi Lentati G: Gametogenesis and egg fertilization in planarians. Int Rev Cytol. 1970, 27: 101-179.
Canovai R: Distribuzione dei Tricladi dulcacquicoli dei Monti Pisani con riferimento ai biotipi cariologici delle diverse specie. Atti Soc Tosc Sci Nat, Mem, (Serie B). 1989, 96: 107-120.
Hoffmann JA: Faune des Triclades Paludicoles du Grand-Duché de Luxembourg. Arch Inst Grand-Ducal de Luxembourg, Sect Sci nat phys et math. 1964, 30: 181-261.
Dyganova R: Species structures and zoogeographical relations of Triclads of the European part of the USSR. In Free-living and symbiotic Plathelminthes. Fortschritte der Zoologie. Edited by: Ax P, Ehlers U and Sopott-Ehlers B. 1988, 36: 517-522. Gustav Fischer Verlag Stuttgart 1988
Mirolli M: La distribuzione dei tricladi sulla costa del Lago Maggiore e del Lago di Mergozzo. Verh Internat Ver Limnol. 1961, 14: 972-977.
Ribas M: Cariologia, sistemàtica i biogeografía de les Planaries d'aigües dolces al Paisos Catalans. Barcelona: PhD Thesis, University of Barcelona. 1990
Reynoldson TB: The distribution and abundance of lake-dwelling triclads – towards a hypothesis. In Advances in Ecological Research. Edited by: Cragg JB. 1966, New York: Academic Press New York, 3: 1-71.
Ball IR: A contribution to the phylogeny and biogeography of the freshwater triclads (Platyhelminthes: Turbellaria). Biology of the Turbellaria. Edited by: Riser NW and Morse MP. 1974, New York: McGraw-Hill New York, 339-401.
Young JO, Reynoldson TB: Continuing dispersal of freshwater triclads (Platyhelminthes; Turbellaria) in Britain with particular reference to lakes. Freshw Biol. 1999, 42: 247-262. 10.1046/j.1365-2427.1999.444488.x.
Ball IR: Dugesia lugubris (Tricladida: Paludicola), a European immigrant into North American fresh waters. J Fish Res B Canada. 1969, 26: 221-228.
Heinsalu A, Kohonen T, Winterhalter B: Early post-glacial environmental changes in the western Gulf of Finland based on diatom and lithostratigraphy of sediment core B-51. Baltica. 2000, 13: 51-60.
Ball IR, Reynoldson TB:British Planarians. Cambridge: Cambridge University Press. 1981, 5:
Refseth UH, Nesbø CL, Stacy JE, Vollestad LA, Fjeld E, Jakobsen KS: Genetic evidence for different migration routes of freshwater fish into Norway revealed by analysis of current perch (Perca fluviatilis) populations in Scandinavia. Mol Ecol. 1998, 7: 1015-1027. 10.1046/j.1365-294x.1998.00423.x.
Nesbø CL, Fossheim T, Vollestad LA, Jakobsen KS: Genetic divergence and phylogeographic relationships among European perch (Perca fluviatilis) populations reflect glacial refugia and postglacial colonization. Mol Ecol. 1999, 8: 1387-1404. 10.1046/j.1365-294x.1999.00699.x.
Dahm AG, Gourbault N: Tricladida et Temnocephalida (Turbellaria). In: Limnofauna Europaea 1. Edited by: Illies J. 1978, Stuttgart, Gustav Fischer Verlag Stuttgart 1978, 16-20.
Hewitt GM: Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc. 1996, 58: 247-276. 10.1006/bijl.1996.0035.
Hewitt GM: Hybrid zones – natural laboratories for evolutionary studies. Trends Ecol Evol. 1988, 3: 158-167. 10.1016/0169-5347(88)90033-X.
Thienemann A: Verbreitungsgeschichte der Süβwassertierwelt Europas. Stuttgart: E. Schweizerbart'sche Verlagsbuchhandlung Stuttgart. 1950
Ullyott P: A note on the zoogeographical history of north western Europe. Proc Prehist Soc. 1936, 2: 169-177.
Brower AVZ: Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proc Natl Acad Sci USA. 1994, 91: 6491-6495.
Juan C, Oromi P, Hewitt GM: Mitochondrial DNA phylogeny and sequential colonization of Canary Islands by darkling beetles of the genus Pimelia (Tenebrionidae). Proc R Soc Lond B Biol Sci. 1995, 261: 173-180.
Juan C, Oromi P, Hewitt GM: Phylogeny of the genus Hegeter (Tenebrionidae, Coleoptera) and its colonization of the Canary Islands deduced from Cytochrome Oxidase I mitochondrial DNA sequence. Heredity. 1996, 76: 392-403.
Jaenike J, Selander RK: Evolution and Ecology of Parthenogenesis in Earthworms. Am Zool. 1979, 19: 729-737.
Beukeboom LW, Vrijenhoek RC: Evolutionary genetics and ecology of sperm-dependent parthenogenesis. J Evol Biol. 1998, 11: 755-782. 10.1007/s000360050117.
Benazzi M, Benazzi Lentati G: Pseudogamy (gynogenesis) in planarians: annotations some forty years on. In: Selected Symposia and Monographs U. Z. I. Edited by: Dallai R. 1992, Mucchi, Modena, 6: 87-102.
Storhas M: Sex versus Asex in a Hermaphrodite Flatworm – Evolution and Coexistence of Reproductive Types in the Freshwater Planarian Schmidtea polychroa. Münster: Ph. D. Thesis, University Muenster. 2000
Pongratz N, Gerace L, Martin Alganza A, Beukeboom LW, Michiels NK: Microsatellite development and inheritance in the planarian flatworm Schmidtea polychroa. Belg J Zool. 2000, 131 (Supplement): 3-7.
Benazzi M: Speciation events evidenced in Turbellaria. In: Mechanisms of Speciation. Edited by: Barigozzi C. 1982, Alan R. Liss, New York, 307-344.
Bessho Y, Ohama T, Osawa S: Planarian mitochondria I. Heterogeneity of cytochrome c oxidase subunit I gene sequences in the freshwater planarian, Dugesia japonica. J Mol Evol. 1992, 34: 324-330.
Pongratz N: Genetic analysis of the mating system and population differentiation in a simultaneous hermaphrodite. Münster: Inaugural-Dissertation, Westfälische Wilhelms-Universität Münster. 2000
Rozas J, Rozas R: DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics. 1999, 15: 174-175. 10.1093/bioinformatics/15.2.174.
Xia X: Data Analysis in Molecular Biology and Evolution. Boston: Kluwer Academic Publishers, Boston/Dordrecht/London. 2000
Posada D, Crandall KA: MODELTEST: Testing the model of DNA substitution. Bioinformatics. 1998, 14: 817-818. 10.1093/bioinformatics/14.9.817.
Swofford DL: PAUP*: phylogenetic analysis using parsimony (and other methods), v 4.0. Sunderland, MA. Sinauer Associates. 1998
Rannala B, Yang ZH: Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J Mol Evol. 1996, 43: 304-311.
Mau B, Newton MA, Larget B: Bayesian phylogenetic inference via Markov chain Monte Carlo methods. Biometrics. 1999, 55: 1-12.
Huelsenbeck JP, Ronquist F: MRBAYES: Bayesian inference of phylogeny. Bioinformatics. 2001, 17: 754-755. 10.1093/bioinformatics/17.8.754.
Clement M, Posada D, Crandall KA: TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000, 9: 1657-1659. 10.1046/j.1365-294x.2000.01020.x.
Maddison WP, Maddison DR: MacClade, version 3.06. Massachusetts: Sinauer Associates. 1992
Vacca RA, Casu S, Pala M: Popolamento planariologico dei fiumi del Nord Sardegna. II. I cariotipi dei tricladi d'acqua dolce rinvenuti nel bacino idrografico del fiume Coghinas. Boll Soc Sarda Sc Naturali. 1993, 29: 59-73.
Casu S, Pala M, Vacca RA: Distribuzione geografica in Sardegna di planarie d'acqua dolce appartenenti alla specie "Dugesia (S.) polychroa" e "Dugesia (S.) mediterranea". Boll Soc Sarda Sc Naturali. 1982, 21: 177-184.
Canovai R, Galleni L: I Tricladi dulcacquicoli dei Monti Pisani e della pianura circostante. Lunigiana: Boll Mus St Nat Lunigiana. 1988, 6–7: 145-149.
Benazzi M: Sulle basi genetiche del polycromatismo nella planaria Dugesia lugubris. Atti Ass Genet It Pavia. 1965, 10: 257-258.
Gremigni V, Puccinelli I: A contribution to the problem of the origin of blastema cells in planarians: a caryological and ultrastructural investigation. J Exp Zool. 1977, 199: 57-71.
Benazzi M: Genetics of reproductive mechanisms and chromosome behaviour in some freshwater-triclads (Platyhelminthes: Turbellaria). The Lower Metazoa. 1963, California: Univ. California Press, Berkeley and Los Angeles, 405-422.
Gourbault N: The karyotypes of Dugesia species from Spain. In: The Biology of the Turbellaria. Edited by: Schockaert ER and Ball IR. 1981, Junk, The Hague, 45-52.
Keller JM, Stéphan-Dubois F: Seasonal variations and regeneration of copulatory apparatus of Dugesia lugubris (Tricladida Paludicola). Bull Soc Zool France. 1984, 109: 199-210.
Dutrillaux B, Lenicque P: Analyse du caryotype de cinq espèces de planaires par la méthode du choc hypotonique. Acta Zool. 1971, 52: 241-248.
Reynoldson TB, Bellamy LS: The status of Dugesia lugubris and D. polychroa (Turbellaria, Tricladida) in Britain. J Zool Lond. 1970, 162: 157-177.
Magagnini G: Cytological researches on populations of the planarian Dugesia lugubris s.l. from North Ireland. Processi verb Soc tosc Sci nat Pisa. 1961, 68: 181-187.
Ball IR: Planarians, plurality and biogeographical explanations. In Evolution, Time and Space: The Emergence of the Biosphere. Edited by: Simms RW, Price JH and Whalley PES. 1983, London: Academic Press London, 409-430.
Acknowledgements
We thank the following people for sending samples, or for help with collecting planarians: Letizia Gerace, Jaco Greeff, Andrea Hohner, Aline Kuehl, John McKay, Marta Riutort, Tim Sharbel, Kim Teltscher and Sandrine Trouvé. Thanks also to two unknown referees for constructive criticism of a previous draft. We also thank Jesus Gómez-Zurita for providing advice during the network analysis. This study was supported by a grant from the German Science Foundation (DFG) no. Mi 482/1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
NP, MS and NKM planned the study. NP carried out most field samples and did the molecular genetic analyses, a first analysis of the genetic data and drafted the manuscript for his Ph.D. MS assisted in field sampling and analysis. SC assisted in establishment of COI mtDNA markers in our system, redid the phylogenetic analyses and made the graphs. NKM supervised the work and edited the manuscript for publication. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pongratz, N., Storhas, M., Carranza, S. et al. Phylogeography of competing sexual and parthenogenetic forms of a freshwater flatworm: patterns and explanations. BMC Evol Biol 3, 23 (2003). https://doi.org/10.1186/1471-2148-3-23
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2148-3-23