Abstract
Background
Polarised gene expression is thought to lead to the graded distribution of signaling molecules providing a patterning mechanism across the embryonic eye. Bone morphogenetic protein 4 (Bmp4) is expressed in the dorsal optic vesicle as it transforms into the optic cup. Bmp4 deletions in human and mouse result in failure of eye development, but little attempt has been made to investigate mammalian targets of BMP4 signaling. In chick, retroviral gene overexpression studies indicate that Bmp4 activates the dorsally expressed Tbx5 gene, which represses ventrally expressed cVax. It is not known whether the Tbx5 related genes, Tbx2 and Tbx3, are BMP4 targets in the mammalian retina and whether BMP4 acts at a distance from its site of expression. Although it is established that Drosophila Dpp (homologue of vertebrate Bmp4) acts as a morphogen, there is little evidence that BMP4 gradients are interpreted to create domains of BMP4 target gene expression in the mouse.
Results
Our data show that the level of BMP4 signaling is critical for the regulation of distinct Tbx2, Tbx3, Tbx5 and Vax2 gene expression domains along the dorso-ventral axis of the mouse optic cup. BMP4 signaling gradients were manipulated in whole mouse embryo cultures during optic cup development, by implantation of beads soaked in BMP4, or the BMP antagonist Noggin, to provide a local signaling source. Tbx2, Tbx3 and Tbx5, showed a differential response to alterations in the level of BMP4 along the entire dorso-ventral axis of the optic cup, suggesting that BMP4 acts across a distance. Increased levels of BMP4 caused expansion of Tbx2 and Tbx3, but not Tbx5, into the ventral retina and repression of the ventral marker Vax2. Conversely, Noggin abolished Tbx5 expression but only shifted Tbx2 expression dorsally. Increased levels of BMP4 signaling caused decreased proliferation, reduced retinal volume and altered the shape of the optic cup.
Conclusion
Our findings suggest the existence of a dorsal-high, ventral-low BMP4 signaling gradient across which distinct domains of Tbx2, Tbx3, Tbx5 and Vax2 transcription factor gene expression are set up. Furthermore we show that the correct level of BMP4 signaling is critical for normal growth of the mammalian embryonic eye.
Similar content being viewed by others
Background
Eye development begins in the fourth week of life in a human embryo, and at embryonic day (E) 8.5 in the mouse embryo. Bilateral protrusions of the developing forebrain neuroepithelium extend towards the surface ectoderm to form the optic vesicles. Invagination of the optic vesicle together with the overlying surface ectoderm forms the bi-layered optic cup and lens vesicle respectively. The distal region of the optic vesicle gives rise to the inner layer of the optic cup, the presumptive neural retina (NR). The proximal region of the optic vesicle forms the optic stalk and the outer layer of the optic cup forms the retinal pigmented epithelium (RPE). The optic cup is initially incomplete along the ventral surface and closure of the optic fissure marks the completion of the eye globe [1]. By around 6 weeks of human development (E13.5 in mouse) these critical stages of early eye morphogenesis are accomplished [2]. If disrupted, congenital malformations of the eye, such as anophthalmia, microphthalmia, and coloboma occur. These clinical conditions are heterogeneous and complex, probably involving genetic and environmental factors, however, a number of causative single gene mutations have been identified in humans or in murine models [3]. Elucidation of the interactions between key genes may lead to the identification of common pathways that are critical for the formation and growth of the embryonic optic cup.
During the morphological transition of the optic vesicle into the optic cup, formation of the dorsal (superior) part of the optic cup precedes formation of the ventral (inferior) part and closure of the optic fissure. As the optic cup forms in the chick and the mouse embryos, the ventral retinal region has a higher rate of proliferation compared with the dorsal retinal region [4, 5]. Recent cell lineage tracing experiments carried out in chick embryos, using replication defective retroviruses suggested that the dorso-ventral (D-V) axis of the neural retina is divided into multiple domains of restricted gene expression which exhibit features of lineage compartments, with clones of cells originating within one compartment rarely crossing to neighbouring compartments [6]. Thus, differential regulation of cell proliferation within domains of gene expression across the D-V axis may be a mechanism driving the formation of the globe-like structure of the optic cup. The molecular mechanisms that pattern and shape this early growth phase of eye development are not well understood.
Signaling centres are believed to define 'patterning axes' within developing tissues through the diffusion of signaling molecules/morphogens and/or propagation of secondary relay mechanisms [7–10]. Asymmetric distribution of several secreted signaling molecules including bone morphogenetic protein (BMP), sonic hedgehog (SHH) and retinoic acid (RA) are implicated in regulating the development of the vertebrate eye [11, 12]. RA signaling is needed for normal eye morphogenesis in mouse [13, 14] and alteration of levels of RA signaling causes deletion or duplication of the ventral zebrafish retina [15, 16]. SHH overexpression in zebrafish causes optic stalk hypertrophy and mutation of the human SHH gene is associated with holoprosencephaly and coloboma [17–19].
While important regulators of ventral retinal development have been identified [11, 20–23], there is less understanding of the requirements for dorsal regulators in patterning and development of the dorsal retina or how they may affect formation of the ventral eye. The studies reported here focus on the role of Bmp4, which is specifically expressed in the dorsal retina of several vertebrate species including the mouse [24–26].
BMPs direct D-V patterning in both invertebrate and vertebrate embryos and are thought of as morphogens acting throughout a field of cells in a graded fashion [27–29]. Decapentaplegic (Dpp), the BMP4 homologue in Drosophila melanogaster has been at the forefront of morphogen studies exploring how endogenous morphogen gradients are shaped and interpreted to create boundaries for BMP target gene expression [30–32]. In vertebrates, a common role for BMP4 in tissue morphogenesis is emerging, notably in the limb, the mandible, and the beak [33–37]. Variation in the pattern of Bmp4 expression was recently shown to affect the shape and size of closely related finch beaks originally described by Darwin; Bmp4 misexpression in chick embryos, causes morphological transformations paralleling the beak morphology of the large ground finch [36, 38].
BMP action in the nervous system includes effects on neural induction, cell fate determination, apoptosis and proliferation [39]. In the chick retina, Bmp4 has been implicated in regulating programmed cell death in the dorsal optic cup [40] and in regulating topographic mapping of retinal ganglion cells [41]. Overexpression of the BMP antagonist, Noggin, in chick, induces microphthalmia and coloboma, together with other ventral abnormalities, suggesting that endogenous BMPs have significant effects on the development of ventral optic cup structures [42]. Experiments in which optic vesicle explant cultures from Bmp4 null mice were treated with BMP4-soaked beads have shown that BMP4 signaling is essential, but not sufficient, for lens formation [26]. Bmp4 null embryos die at midgestation and optic vesicle development is arrested, hampering direct analysis of the role of Bmp4 in optic cup development. In Bmp4+/- mice, the optic cup forms, but mature eyes show anterior segment abnormalities as well as an increased incidence of microphthalmia [43, 44]. Deletions of human chromosome 14q, including the BMP4 gene have been identified in patients with anophthalmia [45–48].
Candidate downstream targets of BMP4 signaling in the mouse retina include members of the evolutionarily conserved T-box gene family of transcription factors. In Drosophila, Dpp regulates omb, a T-box gene critical for development of the fly visual system [31, 49]. We have previously characterised the expression of three omb-related genes of the Tbx2 subfamily, Tbx2, Tbx3, and Tbx5 to be dorsally restricted in the human and mouse optic cup [50]. These genes are closely related and likely evolved from an ancestral Tbx2/omb gene via an unequal recombination event, followed by gene duplication, prior to the divergence of the invertebrate and vertebrate lineages [51]. Several studies in other species also support the idea that Tbx2 subfamily genes are regulated by BMP4. Bmp4 overexpression by electroporation in chick and Xenopus embryos induces ectopic Tbx5 expression in the retina [41, 52]. In the chick, Tbx3 expression in the limb is also dependent on BMP signaling [53, 54]. There is not yet functional evidence for the importance of the Tbx2 subfamily genes in mammalian eye development, however misexpression of Tbx5 in chick embryos, perturbs retinotectal projections [41]. Recent work has also shown that Tbx2b (tbx-c) in zebrafish may be important specifically for differentiation of retinal neurons in the dorsal retina [55].
Here we have developed the use of whole mouse embryo culture to test the hypothesis that BMP4 signaling regulates T-box gene expression in the developing mouse eye. This is the first time that implantation of growth factor soaked beads have been used to examine patterning of the developing mouse eye in vivo. We examined whether perturbation of BMP4 levels affect optic cup growth and shape. We report that Tbx2, Tbx3, and Tbx5 are all responsive to manipulations of BMP4 signaling, but that each gene responds differently. We found that increasing BMP4 signaling by addition of exogenous BMP4 alters the molecular patterns of gene expression across the D-V axis in a ventral shift, while addition of the BMP antagonist, Noggin induces a dorsal shift. These changes in gene expression are associated with a reduced retinal volume, primarily due to reduced cell proliferation and alterations in the axial lengths of the optic cup, indicating that correct levels of BMP signaling are important for the growth and the shape of the embryonic eye.
Results
Nested expression of Bmp4, Tbx2, Tbx3 and Tbx5in the mouse optic cup
The expression pattern of Bmp4 and members of the Tbx2 subfamily were compared during development of the optic cup in mouse embryos. Bmp4 expression was detected in the dorso-distal optic vesicle from E9.5 (Fig. 1A, somite stage 15) in the region of neuroepithelium contacting the surface ectoderm. The distal optic vesicle is the presumptive neural retina. Tbx5 and Tbx2 were expressed in a similar region of the presumptive neural retina as Bmp4 at this stage (Fig. 1B,C). Bmp4 and Tbx2, but not Tbx5 were expressed in the mesenchyme ventral to the optic vesicle (white arrowheads in Fig. 1A and 1C). Bmp4 was also expressed in the surface ectoderm (Fig. 1A, black arrowhead), as previously reported [26].
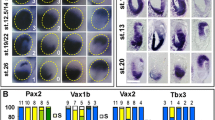
Expression of Bmp4 , Tbx2, Tbx3 , Tbx5 , and Vax2 during optic cup formation. (A-C) Bmp4 co-expressed with Tbx5 and Tbx2 in the optic vesicle at E9.5 (arrows; 15 somite stage). Black arrowhead in A indicates Bmp4 expression in the surface ectoderm. White arrowheads indicate expression in mesenchyme ventral to the developing eye. (D-F) Bmp4, Tbx5 and Tbx2 expression in the invaginating optic vesicle at E10.5 (24–29 somite stage). (G, H) Bmp4 and Tbx5 expression at E11.5 in the optic cup in dorsal domain 1, shown schematically in I. (J) Tbx5 expression in dorsal domain 1 in an E11.5 dissected optic cup. (K) Tbx3 expression in dorsal domain 1 and its extension into dorsal domain 2 in the temporal neural retina. (L) Tbx2 expression in domains 1 and 2, shown schematically in M. (N) Tbx3 expression in an E11.5 dissected optic cup. (O) Vax2 expression at E11.5 in the ventral optic cup in domain 4. (P) Double labeling of Tbx2 (red) and Vax2 (blue) shows a domain in between domain 2 and domain 4, which does not express either marker, and is designated domain 3, shown schematically in Q. (R) Tbx2 (red) and Vax2 (blue) expression in an E11.5 dissected optic cup, revealing domain 3 in white. Arrows in G-R indicate the boundaries of gene expression domains. Dashed circles in J, N, R demarcate the lens vesicle. A-F are coronal sections. Scale bars: A-F, 0.05 mm; G, H, K, L, O, P, 0.5 mm; J, N, R, 0.1 mm. Abbreviations: L, lens placode; MN, mandibular process of the first branchial arch; NR, neural retina; P-NR, presumptive neural retina; D-V indicates the dorso-ventral axis, N-T indicates the naso-temporal axis.
During optic cup invagination at E10.5, the Bmp4 expression domain in the dorsal neural retina remained similar to that of Tbx5 and Tbx2 (Fig. 1D,E,F). By E11.5, Tbx5 and Bmp4 were co-expressed in the dorsal-most region of the optic cup, designated domain 1 (Fig. 1G–J), whereas Tbx2 expression in the dorsal retina was broader and extended ventrally beyond domain 1 into a second domain designated domain 2 (Fig. 1L,M). A third Tbx2 subfamily gene, Tbx3, was also expressed in domain 1 and extended into domain 2 in the temporal, but not nasal, neural retina (Fig. 1K,N). Tbx2, Tbx3 and Bmp4 were also expressed in the mandibular and maxillary processes, ventral to the optic cup (Fig. 1G,K,L). Expression domains 1 and 2, were compared with the expression domain of a ventral marker, the Vax2 homeobox gene. Vax2 is expressed in the ventral-most region, designated domain 4 (Fig. 1O). The region lying between domains 2 and 4, which expresses neither Tbx2, nor Vax2, was designated domain 3 (Fig. 1P–R).
Bmp4 is thus highly expressed within the dorso-distal eye primordia, providing a dorsal source of BMP4 signaling throughout formation of the optic cup. The spatio-temporal overlap in the expression patterns of Bmp4, Tbx2, Tbx3 and Tbx5 in the dorsal eye supports the idea that BMP4 is an upstream regulator of these Tbx2 subfamily genes during mouse eye development. That Tbx2 and Tbx3 are expressed in a broader domain than Bmp4, suggests that these genes may respond to BMP4 signaling which is acting some distance from the source of Bmp4 expression. To test the hypothesis that Bmp4 expressed in domain 1, regulates T-box gene expression in domains 1 and 2, we aimed to increase the level of BMP4 signaling by implanting growth factor soaked agarose beads into the mouse eye primordia and then observed the effect on gene expression domains.
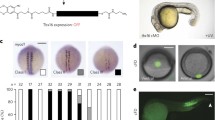
We developed a system using whole mouse embryo culture, analogous to the method of bead implantation used in the developing chick embryo in ovo [56, 57]. In contrast to widespread retroviral or plasmid overexpression approaches, growth factor soaked beads allow the establishment of a local signaling source to test the effect of diffusion of signaling factors across a field of cells. Mouse embryos were cultured using established conditions [58]. Control experiments were performed to ensure, first, that normal optic cup morphogenesis was achieved, and second, that implantation of beads soaked in the biologically inactive protein, bovine serum albumin (BSA), did not disrupt normal morphogenesis or gene expression. We found that normal eye development occurred ex utero during whole mouse embryo culture; embryos were cultured from three time points: E9.5 (optic vesicle); E10.5 (optic vesicle to optic cup transition); and E11.5 (early optic cup). In CBA/Ca embryos, optic vesicle invagination initiates at 24–26 somites and the optic cup forms by late E10.5, at 33–35 somites. This process occurred faithfully in culture (Fig. 2A–C, pre-culture optic vesicle, Fig. 2D–H post-culture optic cup compare with Fig. 2D'–H' stage matched non-cultured optic cup). Normal growth and pigmentation of the optic cup also occurred in culture (Fig. 2N–S post-culture optic cup compare to Fig. 2N'–S' stage matched non-cultured optic cup) and normal patterns of T-box gene expression were observed in post-culture embryos (Fig. 2 and data not shown). Implantation of BSA-soaked agarose beads around the optic vesicle and within the lens of the optic cup did not disrupt morphogenesis or gene expression (Fig. 2I–M and data not shown). Hence, embryo culture, combined with bead implantation provides a useful ex utero model for investigation of how alteration of BMP signaling affects patterning of the mammalian optic cup.
Optic cup development in whole mouse embryo culture. A-S' are serial transverse sections through the developing optic vesicle and optic cup, ordered with dorsal (D) sections uppermost. A-H, I-M, and N-S show Tbx5 expression (in blue). (A-C) E10.5 optic vesicle (not cultured). (D-H) Post-culture early optic cup. Compare with A-C for pre-culture stage of development and to D'-H' for a stage matched non-cultured optic cup. Invagination to form an optic cup with a thick neural retina (NR) surrounded by a thin layer of presumptive retinal pigmented epithelium (RPE), and formation of a lens vesicle (L) occur normally during culture. (I-M) Post-culture optic cup implanted with a control bead, showing normal eye morphology and gene expression. (N-S) Post-culture late optic cup. Compare with D'-H' for the approximate pre-culture stage of development and with N'-S' for the non-cultured stage matched equivalent. Growth of the NR, pigmentation of the RPE, and apposition of the optic fissure walls (indicated by arrows in S and S') proceed normally during culture. Scale bars: 0.1 mm. Abbreviations: D, dorsal; L, lens vesicle; NR, neural retina; P-NR; presumptive neural retina; OF, optic fissure; OS, optic stalk; RPE, retinal pigmented epithelium; P-RPE, presumptive RPE; ss, somite stage; V, ventral.
Exogenous BMP4 induces T-box gene expression in the developing optic cup
BMP4-soaked beads were implanted in the dorso-temporal or dorso-nasal peri-ocular mesenchyme adjacent to the optic vesicle of E9.5 and E10.5 embryos, which were cultured overnight. Beads soaked in recombinant BMP4 protein have previously been shown to be functionally active in the mouse embryo [26]. The bead implantation site was selected to provide an ectopic source of BMP4 signaling, and the effect of growth factor perturbation in the operated eye was compared to the contralateral non-treated eye in each embryo.
Exogenous BMP4 induced ectopic Tbx2 and Tbx5 expression in the proximal optic vesicle, the presumptive RPE (Table 1; Fig. 3A–F). The extension of the normal expression domain is visible in dorsal views of the embryos (Fig. 3A,B). Transverse sections showed Tbx2 and Tbx5 up-regulation in the presumptive RPE that normally does not express the T-box genes, with Tbx2 showing the most marked response (Fig. 3C,D). On the non-treated side, as well as in control embryos implanted with BSA-soaked beads, T-box gene expression was restricted to the dorso-distal optic vesicle (Fig. 3A,B,E,F and data not shown).
Induction of Tbx2 and Tbx5 expression by exogenous BMP4. (A, B) Dorsal views of post-culture embryos showing ectopic expression of Tbx2 (A) and Tbx5 (B) in the proximal optic vesicle (arrowheads) after implantation of a BMP4-soaked bead in the peri-ocular mesenchyme (asterisks). Expression is extended into the presumptive RPE on the treated side but restricted to the presumptive neural retina on the contralateral non-treated side shown by transverse sections through the optic vesicle (arrowheads,C-F). (G, H) Lateral views of a post-culture embryo showing normal Tbx5 expression in the non-treated eye (G) and increased Tbx5 expression after implantation of a BMP4-soaked bead in the periocular mesenchyme (H). Arrows demarcate the Tbx5 expression domain. (I-J) Transverse sections of the non-treated and BMP4-treated contralateral eyes showing the extended Tbx5 expression into the presumptive RPE next to the BMP4-soaked bead (arrow). (K-N) Serial transverse sections showing normal Tbx2 expression in a control eye (K, M) and the extension of Tbx2 expression ventrally after implantation of a BMP4-soaked bead in the treated eye (L, N). Sections M and N are immediately ventral to sections K and L. Scale bars: A, B, 0.1 mm; C-F, 0.05 mm; G, H, 0.5 mm; I-N, 0.1 mm. Abbreviations: L, lens vesicle; MN, mandibular region of the first branchial arch; MX, maxillary region of the first branchial arch; N, nasal; NR, neural retina; P-NR, presumptive neural retina; OS, optic stalk; P-RPE, presumptive retinal pigmented epithelium; T, temporal.
To assess the effect of changing the level of BMP4 signaling during optic cup formation, BMP4-soaked beads were implanted adjacent to the optic vesicle of early E10.5 (22–26 somites) embryos, prior to optic vesicle invagination, and embryos were cultured through the invagination process. Tbx5 expression was never extended to the ventral retina and remained dorsally restricted (Fig. 3G,H). Sections through the dorsal eye showed that Tbx5 was induced in the presumptive RPE adjacent to the bead, consistent with observations at E9.5 (Fig. 3I,J). Rarely, exogenous BMP4 extended Tbx2 expression into domain 3 in the ventral retina (Table 1; Fig. 3K–N). No change in expression of Vax2 or Tbx3 was observed after BMP4 bead implantation (Table 1). BMP4-soaked beads placed temporal to the eye gave similar results to nasal implantation (nasal data are shown in Table 1).
Tbx2 and Tbx5 could not be induced in the ventral-most region, domain 4, even when BMP4-soaked beads were placed ventral to the optic vesicle in E9.5 and E10.5 culture (data not shown). Exogenous BMP4 did not disrupt the process of optic vesicle invagination; normal optic cup formation occurred during the culture period. In summary, exogenous BMP4 protein induced ectopic expression of both Tbx2 and Tbx5 during optic cup formation.
Noggin abolishes Tbx5 and reduces Tbx2expression
To provide further evidence supporting the role of BMP4 signaling in regulating T-box gene expression, we used the BMP antagonist, Noggin, to block BMP4 signaling in the eye (Fig. 4). Noggin has high affinity for BMP4 and prevents BMP receptor-ligand association, hence disrupting the signaling pathway [59].
Repression of Tbx2 and Tbx5 expression by exogenous Noggin. (A) Post-culture embryo showing Tbx5 expression in the non-treated dorsal optic cup. (B) Contralateral Noggin-treated eye, showing absence of Tbx5 expression in the optic cup. (C) Post-culture embryo showing Tbx2 expression in the non-treated dorsal optic cup. (D) Contralateral Noggin-treated eye showing a reduced Tbx2 expression domain. Tbx5 expression (E, F) and Tbx2 expression (G, H) are not altered in non-operated and BSA-treated eyes respectively of post-culture embryos. A'-H' show higher magnifications of eyes in A-H. Arrows indicate boundaries of gene expression domains. Implanted beads are marked by asterisks. (I-P) Serial transverse vibratome sections through the optic cup showing altered morphology after Noggin treatment, with the dorsal-most sections in upper panels. (I-L) Post-culture non-treated eye showing invagination of the presumptive neural retina (P-NR) in the ventral optic cup. (M-P) Contralateral Noggin-treated eye showing dorsal extension of the optic stalk region as compared to the non-treated eye. (Q) Schematic representation of normal Tbx2 and Tbx5 expression domains in a lateral view of the optic cup, and the dorsal shift of the expression domains induced by exogenous Noggin. Scale bars: A-H, 0.5 mm; I-P, 0.1 mm. Abbreviations; MN, mandibular process of the first branchial arch; MX, maxillary process of the first branchial arch; N, nasal process; P-NR, presumptive neural retina; OS, optic stalk.
Noggin-soaked beads were implanted close to the site of endogenous Bmp4 expression, dorsal to the optic vesicle of E10.5 embryos. Noggin abolished Tbx5 expression (Fig. 4A,B,A',B'), but only reduced the Tbx2 expression domain to the dorsal-most region of the eye, domain 1, and did not markedly affect Tbx3 expression (Fig. 4C,D,C',D' and [see Additional File 1A, B]; Table 1). Implantation of BSA-soaked beads in the same position as the Noggin-soaked beads had no effect on either Tbx5 or Tbx2 expression (Fig. 4E–H,E'–H'). Analysis of Vax2 after Noggin treatment showed that Vax2 expression was maintained ([see Additional File 1C, D]; Table 1).
Analysis of the morphology of manipulated eyes revealed that dorsal optic vesicle invagination initiated in the presence of Noggin. However, the ventral region of the operated eye, showed a marked delay in the invagination process (Fig. 4M–P) when compared to the contralateral control eye (Fig. 4I–L; n = 6/8). In operated eyes, invagination to form the optic cup had not occurred in ventral sections, and only the single layered neuroepithelium of the optic vesicle was observed (Fig. 4N–P), By contrast, formation of the bi-layered optic cup and increased growth of the presumptive neural retina was advanced in the ventral region of control eyes (Fig. 4J–L).
In summary, at E10.5, Noggin-soaked beads induced a dorsal shift in expression domains by repressing expression of Tbx5 in domain 1 and Tbx2 in domain 2. Thus, the dorsal-most region of the retina acquired the expression profile of domain 2, in that it was Tbx2-positive, Tbx5-negative (Fig. 4Q). Although Tbx2 and Tbx5 expression was altered by increased or decreased levels of BMP4 signaling , Tbx3 and Vax2 expression appeared tolerant of the same changes in BMP signaling levels.
Effect of disruption of BMP4 signaling on dorso-ventral patterning
To explore whether the concentration of BMP4 is important for defining distinct domains of gene expression in the mouse optic cup, and whether genes show different responses to alterations in the level of signaling, BMP4-soaked beads were placed centrally in the eye, within the lens vesicle of optic cup stage E11.5 embryos. Exogenous BMP4 added to the centre of the optic cup is at an equivalent distance from both the dorsal and the ventral retina, and by raising BMP4 levels throughout the eye, may disrupt the normal putative BMP4 (dorsal-high, ventral-low) signaling gradient.
Exogenous BMP4 resulted in the extension of Tbx5 expression from domain 1 across the dorsal half of the retina, covering the normal site of Tbx2 expression in domain 2 (Table 1; Fig. 5A,B). Ectopic Tbx5 expression never crossed to the ventral half of the retina. Tbx2 was expanded into the ventral retina to cover domain 3 (Fig. 5C,D), and Tbx3 was expanded across domains 2, 3 and 4 (Fig. 5E,F). Strikingly,Vax2 expression in domain 4, was completely abolished in BMP4-treated eyes (Fig. 5G,H). Tbx2, but not Tbx3 or Tbx5, was also induced in the lens (which does not normally express Bmp4; Fig. 5C,D). Double in situ hybridisation for Tbx2 and Vax2 confirmed the expansion of Tbx2 to entirely cover domain 3, but not 4, and the loss of Vax2 in domain 4 (Fig. 5I–N). Control experiments, carried out by implantation of BSA-soaked beads in the same location as BMP4-soaked beads either in the contralateral eye of the same embryo, or in parallel on somite-matched embryos never showed alterations in patterns of gene expression (Fig. 5C,E,G,I; Table 1, and data not shown).
Ventral shift of gene expression along the D-V axis of the optic cup after BMP4 treatment. Beads are indicated with asterisks and domains of gene expression are demarcated with arrows. (A) Post-culture embryo showing normal dorsal Tbx5 expression in the control optic cup. (B) Contralateral BMP4-treated eye, showing extension of the Tbx5 expression domain. (C) Post-culture embryo showing normal dorsal Tbx2 expression in the control BSA-treated optic cup. (D) Contralateral BMP4-treated eye, showing extension of the Tbx2 expression domain into the ventral optic cup and induction of expression in the lens. (E) Post-culture embryo showing normal dorsal Tbx3 expression in the control BSA-treated optic cup. (F) Contralateral BMP4-treated eye, showing induction of Tbx3 expression throughout the ventral optic cup. (G) Post-culture embryo showing normal ventral expression of Vax2 in the control BSA-treated optic cup. (H) Contralateral BMP4-treated eye, showing complete absence of Vax2 expression. (I, J) Double in situ hybridisation of Tbx2 expression (red) and Vax2 expression (blue) in control BSA-treated (I) and contralateral BMP4-treated eyes (J). (K-N) Transverse sections through the planes indicated in I and J, showing induction of ectopic Tbx2 expression (K, L) and repression of Vax2 expression (M, N) upon BMP4 treatment. (O) Schematic representation of gene expression domains 1–4 along the D-V axis of the whole mount optic cup and the ventral shift of domains upon BMP4 treatment. Domain 3* is Tbx2-negative, Tbx3-positive, Vax2-negative. Scale bars: A-J, 0.5 mm; K-N, 0.1 mm. Abbreviations: L, lens vesicle; MN, mandibular process of the first branchial arch; MX, maxillary process of the first branchial arch; NR, neural retina.
Our findings show that exogenous BMP4 provided to the central region of the eye has shifted the normal gene expression boundaries ventrally by one or more domains (Fig. 5O), suggesting that the molecular division of the retina into gene expression domains is dependent on precise levels of BMP4 signaling. Although all genes showed a ventral shift, their response was non-uniform suggesting that additional regulatory mechanisms exist. Domain 1 (Tbx5-positive, Tbx2-positive), was expanded ventrally to cover the former site of the Tbx5-negative, Tbx2-positive domain 2; whereas the latter domain is now shifted to the more ventral former site of domain 3. Tbx3 showed a different response and shifted ventrally by two domains into the entire ventral retina. Domain 3*, which shows the expression profile Tbx5-negative, Tbx2-negative, Tbx3-positive is in the ventral-most region of BMP4-treated eyes and the Vax2-positive domain 4 is lost in this ventral shifting of expression domains.
To assess BMP signaling in the optic cup we performed immunohistochemistry for the Phospho-Smad 1/5/8 proteins which transduce the extracellular BMP signal to the nucleus [60]. Phospho-Smad 1/5/8 labeling was highest in the dorsal retina (consistent with a previous report [61]), and in the lens in non-treated control eyes, while labeling was detected across a wider region in the retina of BMP4-treated eyes [see Additional File 1F–I]. These data suggest that BMP signaling in the retina extends beyond the Bmp4 expression domain upon addition of exogenous BMP4 [see Additional File 1E–I]. BMP4 has previously been shown to upregulate the Msx2 homeobox gene in other systems [62–64]. Since Msx2 is expressed in the mouse optic cup [65] we tested the effect of BMP4 bead implantation and compared the Msx2 response to that of the Tbx2 subfamily genes. Exogenous BMP4 induced Msx2 gene expression in a different pattern to any of the other marker genes tested; high level ectopic Msx2 expression was detected in the lens, optic cup and surface ectoderm, as compared with control eyes [see Additional File 1J, K].
Change in eye size and shape after BMP4 treatment
BMP4-treated eyes of E11.5 embryos, displaying a ventral shift in Tbx2 subfamily gene expression domains, were examined for morphological abnormalities. These eyes appeared smaller in size and this difference was analysed by estimating retinal volumes. Retina from BMP4-treated eyes were consistently smaller than those of contralateral eyes treated with BSA control beads (n = 4 embryos; mean volume ± 1 S.D., BMP4-treated retina = 7.35 × 10-3 mm3 ± 2.3 × 10-3; mean volume ± 1 S.D., BSA-treated retina = 10.90 × 10-3 mm3 ± 1.95 × 10-3; p = 0.033; Fig. 6A).
Effect of BMP4 treatment on eye size and shape. (A) Retinal volume in BMP4-treated and contralateral BSA-treated eyes estimated from four post-culture embryos and represented by bar charts as mean ± 1 S.D. p = 0.033 by the paired t-test indicates a significant reduction in retinal volume in BMP4-treated eyes. (B, C) Coronal sections of control (B) and contralateral BMP4-treated (C) eyes stained with H&E and showing a reduction in eye size and retinal thickness upon BMP4-treatment. Scale bars: 0.05 mm.
We next investigated how the eye had changed in shape, and whether there was a change in the axial lengths of the optic cup associated with the changed gene expression patterns. For each embryo, the D-V, naso-temporal (N-T) and proximo-distal (P-Di) axes of the BMP4-treated eye were measured and compared to the control eye of the same embryo. The percentage change in axial length between treated and contralateral control eyes were calculated (Table 2). In the BMP4-treated group, the D-V axis of the BMP4-treated eye was smaller than the non-treated contralateral eye in every case (n = 25, P < 0.0001). By contrast there was no significant difference between BSA-treated eyes and the non-treated control (n = 8; p = 0.07). Neither was there a significant natural variance between the axial length of right and left eyes of non-treated cultured embryos (n = 10; p = 0.10).
Similarly, the N-T axis of BMP4-treated eyes was reduced, although the reduction along the N-T axis of BMP4-treated eyes was less pronounced than that along the D-V axis (Table 2, n = 25; p = 0.001). There was no significant difference in the BSA-treated group (n = 8, p = 0.15), nor in non-treated cultured embryos (n = 10, p = 1). By contrast, the P-Di axis (from the ventricular edge of the retina to the surface ectoderm) was unchanged by the exogenous BMP4 (n = 4, 0.58).
These data show that the addition of exogenous BMP4 reduced optic cup size and the D-V and N-T axial lengths, though not the P-Di axial length, thus inducing a change in the size and the shape of the optic cup. Histological sections also showed the small eye size and the abnormally thin neural retina of BMP4-treated optic cups (Fig. 6B,C).
Cell proliferation and cell death in BMP4-treated eyes
The differences in gene expression domains and optic cup size/shape observed after the addition of exogenous BMP4 to developing optic cups could be due to regional decreases in retinal cell proliferation or increases in apoptosis (or both). To establish whether the altered gene expression domains correlated with regionalised changes in cell proliferation, retinal sections were immuno-labelled using the pH3 antibody, which labels mitotic cells in the retina at the ventricular surface. BMP4-treated embryos (n = 4) were examined for differences in the number of pH3-positive cells either throughout the retina, or in the dorsal region (containing domains 1 to 3) and the ventral region (containing domain 4). There was a significant overall decrease in the mitotic index (number of pH3-labelled cells as a percentage of total cells) in BMP4-treated compared with contralateral non-treated eyes in cultured embryos (p = 0.005, 2-way ANCOVA, n = 30 sections; Fig. 7A–C). Comparisons of both dorsal and ventral cell counts between eyes showed that the number of pH3-labelled cells were significantly reduced after BMP4 treatment in both the dorsal (p = 0.007; 2-way ANOVA, n = 44 sections) and the ventral (p = 0.018; 2-way ANOVA, n = 44 sections) retina [see Additional File 2A, B]. Non-treated eyes showed a significantly higher level of mitosis (pH3 per mm2) in the ventral retina, compared to the dorsal region (p = 0.013; 2-way ANOVA, n = 19 sections, data not shown), but this difference was abolished in BMP4-treated eyes (p = 0.4, data not shown).
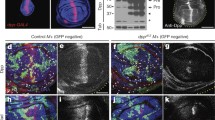
Effect of BMP4 treatment on proliferation and cell death. (A, B) pH3-positive cells (green) in retinal sections of control and BMP4-treated contralateral eyes of a post-culture embryo. Sections are counterstained with propidium iodide (red). (C) Graph showing the mitotic index per section per eye in BMP4-treated optic cups, compared with contralateral control optic cups (n = 30 sections from 4 embryos; p = 0.005 by ANCOVA). Data was Ln transformed for normalisation. Between embryo variability likely reflects the differences in growth rate of individual embryos. (D, E) Increased dorsal apoptosis detected by the TUNEL assay (green) in BMP4-treated eyes (arrow in E) compared to contralateral control eyes (D) of post-culture embryos. (F, G) Apoptosis detected in eyes of post-culture embryos after high dose BMP4 treatment. Widespread apoptosis detected throughout the neural retina. Scale bars: 0.1 mm. Abbreviations: D, dorsal; L, lens vesicle; NR, neural retina; pH3, phospho-histone H3; TUNEL, Terminal deoxynucleotidyl transferase-mediated dUTP Nick End Labeling; V, ventral.
Finally we used TUNEL labeling to assess whether the altered gene expression domains observed after BMP4 treatment were associated with increased or altered patterns of apoptosis. Regionalised apoptosis could cause tissue ablation and loss of gene expression domains. For example ablation of ventral tissue could cause loss of the Vax4 expression domain and reduction of eye size. BMP4 has been shown to promote apoptosis in other systems [57, 62, 66, 67].
In control eyes, TUNEL labeling was detected in the region of the optic disc and the optic stalk. Occasionally, a few apoptotic cells were detected in the dorsal aspect of the retina (not shown). This low level pattern of cell death correlates well with previous reports of cell death in E11.5 mouse embryos [40]. Levels of TUNEL-labelled cells were compared in BMP4-treated and contralateral control eyes. No significant differences in apoptosis were detected in the ventral (domain 4-containing) or the central retina between BMP4-treated and control eyes, suggesting that loss of Vax2 expression was not associated with apoptosis (Fig. 7D,E). Instead, more TUNEL-positive cells were found at the vitreal edge in the dorsal retina (domain 1; Fig. 7D,E), close to the source of endogenous BMP4 expression and where BMP4 levels appear highest after exogenous BMP4 addition. In total, 79 TUNEL-positive cells were detected in the dorsal region of BMP4-treated retina compared with 26 in contralateral non-treated eyes (n = 26 sections from 5 embryos; TUNEL-positive cells: p = 0.036 by 2-way ANOVA; TUNEL index: p = 0.04 by ANCOVA; [see Additional File 2C]). We also carried out BMP4-bead implantations using higher doses of BMP4 to test whether this increased the level of apoptosis. Widespread apoptosis and tissue ablation were detected throughout the neural retina (Fig. 7G). Therefore, while excess BMP4 can induce widespread apoptosis in the retina, the level of BMP4 that caused ventral shifts of gene expression domains, was only associated with a modest increase in apoptosis in the dorsal eye.
Taken together these data support the view that BMP4 signaling from the dorsal eye primordia is important for defining gene expression domains across the D-V axis of the retina. An increase in the level of BMP4 in the optic cup causes a shift in gene expression domains and expands the dorsal retinal territory at the expense of the ventral territories. These changes in gene expression domains fundamentally alter the molecular character of the optic cup. Furthermore, these data suggest that alterations in the BMP4 signaling gradient disrupt differential regulation of cell proliferation across the D-V axis, thus affecting growth and shape of the optic cup.
Discussion
Using whole embryo culture and bead implantation, our analyses have provided new evidence for the critical importance of the level of BMP4 signaling for D-V patterning and growth of the developing mouse eye. Our data suggest that BMP4 patterns the optic cup by dosage dependent regulation of target genes, Tbx2, Tbx3 and Tbx5.
A conserved genetic pathway: BMP4 regulation of Tbx2 subfamily genes
In whole embryo culture experiments, exogenous BMP4 induced Tbx2, Tbx3 and Tbx5 expression, while Noggin repressed/reduced Tbx2 and Tbx5 expression in the developing retina, providing strong support for the idea that BMP4 signaling regulates T-box gene expression during mouse eye development. This is the first demonstration that all three members of the Tbx2 subfamily, Tbx2, Tbx3 and Tbx5 genes are downstream targets of BMP signaling in the mouse eye. A recent paper reported loss of Tbx5 expression in the optic vesicles of Bmp4-/- mice [61]. Previous reports have demonstrated BMP2/4/Dpp regulation of Tbx2 subfamily genes in the heart, limb and retina in other vertebrates and of omb in Drosophila [41, 49, 52–54, 68]. Considered together, these data indicate that BMP2/4 regulation of Tbx2 subfamily genes is an ancient and evolutionarily conserved pathway, which has been recruited to regulate development and patterning of different tissues in vertebrates and invertebrates.
In the developing eye, Tbx2, Tbx3 and Tbx5show differential responses to BMP4 signaling
Our data suggest that in the eye, each T-box gene responds to BMP4 in a different manner. They are consistent with a model in which Tbx5 responds to BMP4 close to the dorsal signaling source, while Tbx2 and Tbx3 can be induced in a wider area by lower levels of BMP4 secreted from the source. Based on the expression pattern of Bmp4 in the dorsal region of the optic vesicle and the optic cup, it is likely that BMP4 protein concentrations will be high in the dorsal and low in the ventral region of the eye; indeed the dorsal retina shows high levels of BMP signaling activity as determined by immunohistochemistry for Phospho-Smad 1/5/8 proteins.
The different spatial response that each T-box gene showed and their close evolutionary relationship support a mechanism of direct regulation by BMP4 signaling, although we have not excluded the possibility that other intermediate factors regulate these genes. Significantly, a recent study showed that the Tbx3 promoter is directly regulated by BMP Smads, in chromatin immunoprecipitation experiments using extracts from embryonic hindlimbs, as well as cotransfection assays [69]. In Drosophila, molecular dissection of cis-regulatory sequences of omb, the homologue of vertebrate Tbx2/3, revealed a Dpp response element [70] suggesting that omb may also be a direct target of Dpp signaling. Interestingly, Tbx3 responded more vigorously than Tbx2 to exogenous BMP4 administered to the lens, extending all the way to the ventral retina, whereas Tbx2 did not extend as far and may need other factors or is being inhibited by factors present in the ventral retina. The fact that Tbx3 expression is initially more restricted than the Tbx2 expression domain, yet after exogenous BMP4 treatment it shows the greatest expansion suggests that other factors are regulating the expression of T-box genes along with BMP4.
It has been proposed that Tbx5 represses cVax and vice versa in the chick based on overexpression studies [21, 41]. The cascade in the mouse appears to differ, as with increased BMP4 levels, we observed repression of Vax2 without ventral expression of Tbx5. Also, in Vax2 null mice, the Tbx5 expression domain is unchanged [71]. Moreover the separation of Vax2 and Tbx5 expression in two non-overlapping domains (domains 1 and 4) indicate that these factors are unlikely to control each other's expression.
Tbx2 subfamily genes as regulators of apoptosis and proliferation
Downstream targets regulated by the Tbx2, Tbx3 and Tbx5 transcription factors have not been identified in the eye. Several in vitro studies implicate these genes in the regulation of cell proliferation and apoptosis. Ectopic expression of TBX5 in osteosarcoma cells inhibits cell proliferation and induces apoptosis [72, 73]. TBX2 gene amplification has been detected in primary human breast cancer tumours, pancreatic cancer cell lines, and its overexpression detected in melanoma cell lines [74–78]. Both TBX2 and TBX3 repress the expression of p14ARF, and there is evidence for the repression of other cyclin dependent kinase inhibitors p21, p16INK4a, and p15INK4b by TBX2 in vitro [74, 79–81]. Moreover, both TBX2 and TBX3 show anti-senescence properties [74, 82], while a dominant negative form of Tbx2 can induce senescence in a melanoma cell line [76].
During embryogenesis, a similar pro-proliferative role has not been identified for these genes. In fact, it has been shown that the expression of p21, p19ARF, p16INK4a, and p15INK4b is normal in mice with a targeted mutation of Tbx2, and there is no evidence for a genetic interaction between Tbx2 and p53 (Trp53) [83]. Furthermore, Tbx2 expression has been associated with a low rate of proliferation in the atrioventricular canal during heart development [84]. Tbx5 has also been associated with the regulation of cell proliferation during embryogenesis. Misexpression of TBX5 in the chick heart leads to inhibition of cell proliferation [72] and in the limb, ectopic expression of Tbx5 (or Tbx4) leads to a truncated limb phenotype due to cell proliferation arrest [68].
We observed a dosage sensitive increase in the amount of apoptosis in BMP4-treated eyes, which correlates with Tbx5 expression in the dorsal retina, but not with the ectopic Tbx2 or Tbx3 expression in the ventral retina. Alternatively, the homeobox gene Msx2, which is normally expressed in the dorsal neural retina [40, 65], could be mediating the apoptotic effect of the elevated BMP4 levels in the dorsal neural retina. Msx genes mediate BMP induced apoptosis during development of other organs such as the hindbrain and the limbs [57, 62]. The different response of Msx2 and the T-box genes to BMP4 treatment suggests that these genes do not regulate each other. If Tbx2 acts to reduce proliferation during optic cup formation, as recently found in the developing heart [84], then Tbx2 expansion into the ventral retina may contribute to the observed reduction in levels of proliferation after BMP4 treatment.
BMP4 dosage in the regulation of eye size and shape
Addition of exogenous BMP4 in the mouse optic cup in embryo cultures resulted in a reduction in retinal volume and alterations in eye shape. In chick and Xenopus, malformation of the ventral retina has previously been described after overexpression of BMP4 by electroporation [41, 52]. Increases in gene dosage, as well as loss of function are known to cause congenital eye malformations in humans and mice, for example in the case of the transcription factor genes Pax6 and Foxc1 [85, 86]. Our findings suggest that increased levels of BMP4 signaling may be detrimental; in the human population, such changes could occur, for instance, via genetic variation in regulatory sequences, gene duplication, or gain of function mutations. The recent report that anophthalmia occurs in embryos from crosses between Bmp4 heterozygous mutants and mice homozygous for a disrupted allele of Twisted gastrulation (Tsg), a BMP binding protein that is thought to enhance BMP4 signaling in the eye [87], also indicates that excess BMP4 signaling may be as harmful as loss of function.
The adverse consequence of reduced levels of BMP4 signaling for eye development has been explored in different systems. That optic vesicle development is arrested in Bmp4 null mice [26] first indicated the importance of Bmp4 for normal growth. We observed an expansion of optic stalk and a reduction in the neural retina after addition of the BMP antagonist, Noggin, close to the dorsal BMP4 signaling source in the mouse optic cup. In the chick embryo, misexpression of Noggin induces primarily ventral abnormalities that include optic stalk hyperplasia [42]. Conditional gene inactivation has also been used to analyse the effect of loss of the serine/threonine kinase membrane bound BMP receptors; BmprIa, BmprIb, and BmprII are expressed in the developing mouse eye [26, 88, 89]. Whereas targeted deletion of Bmpr1b alone disrupts the ability of many ventrally located ganglion cells to enter the optic nerve head [88], conditional Bmpr1a-/fx/Bmpr1b-/-;cre double mutant embryos exhibit more profound structural abnormalities due to excess apoptosis and reduced proliferation at E11.5, and show anophthalmia at birth [61]. In these mutants, the loss of Bmpr1a and Bmpr1b likely interfered with BMP4 signaling, as well as signaling by BMP7, BMP2 and BMP3, from non-neural retinal sources [90].
The findings of altered eye size and shape with loss and gain of BMP4 function, suggest that a BMP4 signaling gradient mediates control of proliferation across the D-V axis during optic cup morphogenesis. With exogenous BMP4, we observed a significantly decreased level of retinal cell proliferation in both the dorsal and the ventral retina. Regional differences in proliferation have recently been found during optic cup growth [4, 5], and we found that the exogenous BMP4 acted to equalise the differences in levels of proliferation across the D-V axis. Other studies have linked excess BMP4 to reduced proliferation and increased apoptosis. For example, the BMP expressing region of the mouse telencephalon has a lower proliferative activity and higher level of apoptosis than the rest of the forebrain, and BMP4-soaked beads can induce apoptosis in telencephalic explants [66]. In the chick, exogenous BMP4 applied to the dorsal optic cup, or at early stages to the telencephalon also increases apoptosis [40, 91]. However, in the same studies, addition of BMP4 to chick retinal cultures at later stages induced proliferation [40], while reduced levels of BMP signaling (via Noggin misexpression) in the telencephalon was associated only with a decrease in proliferating cells [91], highlighting that the cellular response to BMP4 signaling appears stage and context dependent. In our study, only at higher doses of BMP4 was widespread apoptosis induced.
Threshold-specific requirements for BMP4 in optic cup development
Our data show that a major role for dorsal BMP4 signaling in the developing mouse optic cup is to regulate gene expression across the D-V axis. It suggests that BMP4 may be critically important for providing a signaling gradient, which is used to demarcate gene expression domains across the D-V axis, with retinal cells expressing different transcription factor genes in response to different threshold levels of BMP4 activity. Variations in Smad binding site distribution between target genes or levels of other active cofactors are likely to contribute to the differential gene responses.
Exogenous BMP4 treatment prior to optic cup formation affected Tbx2 and Tbx5, but not Vax2 or Tbx3, and T-box gene expression changes were not induced in the ventral retina in contrast to the effect of exogenous BMP4 addition to the lens. These differences may be due to the different developmental stage, and/or the different range of BMP signaling from bead implantation in the lens compared with the extraocular mesenchyme. In the latter case the different intervening tissue between the bead and the retina that is exposed to the BMP signal may be significant; when a bead is implanted in the lens the intervening tissue is the lens, whereas from a bead located in the mesenchyme, the BMP signal has to pass through the presumptive RPE. We were only able to detect increased levels of Phospho-Smad 1/5/8 immuno-labeling after BMP4 bead implantation in the lens, which correlates with our findings that lens bead implantation showed the most dramatic effect on gene expression domains. In this study it was not possible to distinguish if the BMP gradient regulates the expression of the Tbx2 subfamily genes directly or indirectly. Rather than the BMP gradient being directly responsible for the nested expression domains it is possible that there are other secondary molecules induced by BMP that carry the signal. However, our data considered together with recent data showing that the Tbx3 promoter is directly regulated by Smads [69] strongly support the idea that these genes respond directly to a BMP4 signaling gradient.
Although there are some differences in the specific gene expression patterns between chick and mouse, the broadly conserved pattern of gene expression domains across the D-V axis of the optic cup suggests that compartment-like units of clonally related cells defined by restricted gene expression domains, as previously described in the chick [6], may also exist in the mouse. If this is the case, then our data suggests that altering the BMP4 concentration in the eye re-specifies the compartments of the retina along the D-V axis; increased BMP causes compartments to express markers normally expressed by their more dorsal neighbours, although the response to BMP4 is not uniform in the different genes examined. Our analysis of proliferation and apoptosis support the conclusion that these changes in gene expression domains reflect changes in the identity of retinal territories rather than tissue loss. Instead of being made up of domains 1, 2, 3 and 4, the post BMP-treatment eye consists only of domains 1, 2 and a modified domain 3 (domain 3* that lacks Tbx2 and Vax2 expression but expresses Tbx3). The importance of correct spatial restriction of gene expression for topographic mapping of retinal ganglion cells to the brain is well established [21, 41, 92]; our data suggests it is also important in regulating regional growth of the developing optic cup.
Our finding that exogenous BMP4 not only extends expression of the dorsally expressed T-box genes in the optic cup, but also reduces the ventral marker Vax2 support the idea that BMP4 signaling has a long-range action across the D-V axis, and that BMP4 may repress Vax2 expression. BMP inhibitors, in particular Ventroptin and Dan, are known to be expressed in a ventral-high gradient in the chick eye [93, 94], although these expression patterns have not been reported in the mouse. The role of such inhibitors may normally be to block long range BMP4 signaling from acting in the ventral-most region (domain 4), allowing Vax2 expression, and preventing induction of dorsally expressed genes. In our study, increased levels of BMP4 at optic cup stage are apparently sufficient to overcome endogenous inhibitors, leading to Vax2 repression and induction of T-box gene expression in the ventral retina.
Also consistent with the proposal that BMP regulates Vax2, the recent informative study by Murali et al [61], showed that with reduced levels of BMP signaling, Tbx5 expression was lost whereas Vax2 expression was expanded into the dorsal mouse retina in Bmpr1a-/fx/Bmpr1b+/-;Cre double mutants. In chick, retroviral overexpression of Bmp4 was previously shown to repress expression of Ventroptin [94]. Alternative to direct regulation of Vax2 by BMP4, interaction with other ventral signaling pathways could be important, as in zebrafish and chick, hedgehog signaling from the ventral midline contributes to regulation of the Vax gene [95, 96]. Mouse embryos cannot be cultured ex utero for long enough to analyse the effect of the shifted gene expression domains on late stages of eye development. Nevertheless, the effect of loss of Vax2 expression in the ventral eye has already been carefully studied in Vax2 null mice and shown to cause profound defects in ventral eye formation and coloboma [20, 22, 97].
Conclusion
Our data suggest that BMP4 signaling from a dorsal site of expression, patterns the mammalian optic cup across the entire D-V axis, by dosage dependent regulation of target genes, Tbx2, Tbx3, Tbx5 and Vax2. Our findings fit with the idea that a BMP4 signaling gradient across the developing optic cup is interpreted to create domains of BMP4 target gene expression. A conserved genetic pathway is emerging whereby BMP4 regulates members of the Tbx2 subfamily in different tissues and organisms during organogenesis. Increasing evidence suggests that Tbx2 and Tbx3 may regulate proliferation in cancer and in development and our data is consistent with a role for these T-box genes downstream of BMP4 in regulating proliferation of the optic cup. Upon increasing the level of BMP4 signaling, growth of the embryonic eye was perturbed resulting in a reduction in retinal volume as well as the D-V and N-T axial lengths of the optic cup. That precise levels of BMP4 signaling are critical for normal mammalian eye development is becoming progressively more clear; it is likely that altered dosage of BMP4 is a mechanism underlying human congenital eye defects.
Methods
Whole mouse embryo culture
Mouse embryos at E9.5, E10.5, and E11.5 were obtained from timed matings of CBA/Ca mice. Days on which plugs were found after overnight matings were considered to be E0.5. Embryos were dissected and cultured based on the method of D.L Cockroft [58, 98]. E9.5 embryos were cultured with intact yolk sacs, whereas E10.5 and E11.5 embryos were cultured outside the yolk sac. For E10.5 and E11.5 embryos, a small incision was made in the yolk sac adjacent to the placenta, avoiding large blood vessels. The yolk sac and the amnion were then drawn over the head of the embryo. Embryos were incubated at 37°C in a rolling incubator (B.T.C. Engineering, Cambridge, UK) in 3 ml culture medium consisting of 100% rat serum (prepared as described in [98]) for E9.5 embryos or 25% rat serum 75% culture saline (0.12 M NaCl, 4 M KCl, 0.4 mM MgSO4, 0.25 mM MgCl2, 0.60 mM NaH2PO4, 1.8 mM CaCl2, 11.1 mM glucose, 24 mM NaHCO3) for E10.5–11.5 embryos. The medium was manually gassed prior to culture for one minute with a 20% O2/5% CO2 source for E9.5, and 95% O2/5% CO2 (Crysoservice Ltd, UK) for E10.5–E11.5. Embryos were cultured for 1 hour before bead implantation.
Bead implantation
10 μl of Affi-Gel Blue beads, diameter 80–150 μm (Bio-Rad Laboratories, CA, USA), were washed three times in embryo water (Sigma, UK), then incubated in 5 μl of either 100 μgml-1 human recombinant BMP4 (R&D Systems, UK), or 1 mgml-1 Noggin (R&D Systems, UK), or 1 mgml-1 bovine serum albumen (BSA; Promega, USA), for 1 hour at 37°C prior to implantation. High concentration BMP4 beads were prepared as previously described [99]. E9.5 mouse embryos with intact yolk sacs were transferred to a dish of explanting saline (same as culture saline except 0.83 mM glucose, 0.6 mM NaHCO3). To enable bead implantation, a small tear was made using watchmaker's forceps (Raymond Lamb, UK) in the yolk sac adjacent to the ectoplacental cone, then an arc was cut using iridectomy scissors around one third of the cone (adjacent to the embryonic head). Watchmaker's forceps were used to tear the amniotic sac and expose the eye region. E10.5 and E11.5 embryos with their yolk sac removed, were rested on their side in explanting saline to expose the eye. The site of bead implantation was pierced using a tungsten needle and then using a mouth-operated glass capillary pipette, a bead was inserted into the extra-ocular mesenchyme (at E9.5 and E10.5) or in the lens vesicle (at E11.5). Following bead implantation, embryos were gassed again for 1 minute and cultured for 18 hours at 37°C. Embryos were scored after culture for blood circulation, number of somites and head length [98]. Embryos were only analysed when circulation was maintained and somite number had increased by at least 6 pairs during culture. Overnight ex utero growth of optic vesicle stage embryos (somite stage 24–26) to somite stages 33–35 resulted in optic vesicle invagination. Overnight ex utero growth of optic cup stage embryos (somite stage 33–35) to somite stages ≥ 44 resulted in normal growth and pigmentation of the optic cup. For each treatment, viable post culture embryos from at least two litters were analysed in independent experiments. After culture, embryos were fixed overnight in 4% para-formaldehyde (PFA) for analysis.
Wholemount in situhybridisation
RNA in situ hybridisation was performed on 4% PFA fixed cultured, operated and control embryos using standard protocols [100]. For detection of single genes, digoxigenin (DIG)-labelled RNA probes were prepared for Tbx2, Tbx3, Tbx5, Msx2, and Vax2 using a DIG RNA labeling kit (Roche, Germany) according to the manufacturer's description. For detection of Tbx2 and Vax2 by double in situ hybridisation, Tbx2 DIG-labelled and Vax2 fluorescein-labelled probes were prepared and hybridised simultaneously. Plasmids used were a 2.2 kb Tbx2 cDNA in pBK-CMV, 3 kb Tbx5 cDNA in pBK-CMV [50], 1 kb Tbx3 cDNA in pB(II)KS (kindly provided by V. Papaioannou, Columbia University), a 700 bp Vax2 cDNA in pBS SK (kindly provided by C. Cepko, Harvard Medical School), and a 1000 bp Msx2 cDNA in pCR2.1 (kindly provided by P. Sharpe, King's College London). Hybridisation of labelled probe was visualised using alkaline phosphatase conjugated anti-DIG antibody and the colour reaction was developed with NBT/BCIP (Roche, Germany); treated and control eyes of the same embryo were compared ensuring identical reaction conditions. For double in situ hybridisation, the Vax2 probe was detected with alkaline phosphatase conjugated anti-fluorescein antibody and the colour reaction developed with NBT/BCIP. Embryos were then incubated at 65°C for 30 minutes in TBST (137 mM NaCl, 2.7 mM KCl, 0.25 M Tris.HCl pH 7.5, 1% Tween-20, 2 mM Levamisol), blocked in TBST containing 10% heat-inactivated sheep serum for 1 hour at room temperature and incubated with anti-DIG antibody for detection of the Tbx2 probe with INT/BCIP (Roche, Germany).
Microscopy
Images of whole mount embryos were captured with a Leica MZ FLIII microscope fitted with a Leica DC500 camera using the Leica IM1000 Image manager V1.20 software (Leica, Germany), and imported into Adobe Photoshop (6.0). Vibratome sections (50 μm) prepared after whole mount in situ hybridisation were imaged using an Axiophot 2 (Zeiss, Germany) with differential interference contrast (DIC) optics, a Leica digital MZIII camera and Openlab 4.0.4 software (Improvision Ltd., UK). Fluorescent images of sections after immunohistochemistry were digitally captured using an Axiophot (Zeiss, Germany) microscope, ProgRes Cl4 camera (Jenoptik Jena, Germany) and Openlab 4.0.4 software.
Morphometric analysis
To estimate retinal volume, post-culture embryos were embedded in 0.45% w/v gelatine, 28% w/v egg albumin, 18 % w/v sucrose, 0.2% glutaraldehyde (all from Sigma, UK) in phosphate buffered saline (PBS). Serial transverse vibratome sections (50 μm) of each eye were mounted on slides with 50% glycerol/PBS and glass coverslips. Retinal surface area per section was estimated using Openlab 4.0.4 software. Retinal volume per section was calculated as retinal surface area multiplied by section thickness, and total retinal volume was calculated by addition of retinal volumes of all sections per eye. BMP4-treated eyes were compared to contralateral BSA-treated eyes using the paired t-test. To determine the proximo-distal (P-Di) axis, the distance from the ventricular edge of the retina to the surface ectoderm was measured using Openlab 4.0.4 software on serial sections and the longest distance for each optic cup was compared with the paired t-test. To determine the dorso-ventral (D-V) and naso-temporal (N-T) axial lengths of optic cups after culture, digital images of optic cups captured using identical conditions, were measured using the Photoshop (6.0) measure tool. Operated eyes were compared to contralateral eyes in each embryo using the paired t-test.
Immunohistochemistry, analysis of cell proliferation, apoptosis, and histology
Phospho-Smad 1/5/8 immunohistochemistry was performed on coronal wax sections of 4% PFA fixed post-culture embryos. Sections (7 μm) were de-parafinised and un-masked by steaming in Declear (Cellmarque, USA) for 40 minutes and then incubated in 3% H2O2 in TBS (137 mM NaCl, 2.7 mM KCl, 0.25 M Tris pH7.5) containing 0.1% Triton-X100 (BDH, UK) for 10 minutes. Sections were incubated in blocking solution (5% v/v goat serum, 0.15% w/v glycine, 2 mg/ml BSA in TBS-0.1% Triton-X100) for 30 minutes and then in blocking solution containing a 1:100 dilution of the Phospho-Smad1/5/8 antibody (Cell Signaling technology, USA) for 1 hour at room temperature. This was followed by incubation in a 1:250 dilution of Biotinylated goat anti-rabbit (Dako, Denmark) secondary antibody in blocking solution for 1 hour at room temperature. The secondary antibody was detected with the Vectastain ABC kit (Vector Laboratories, USA) and the diaminobenzidine tetrahydrochloride (DAB; Sigma, UK) chromogen according to the manufacturers' descriptions. Sections were counterstained with 0.5% w/v methyl green (BDH, UK). Histology was examined on 7 μm thick coronal wax sections stained with Haematoxylin and Eosin (BDH, UK). Frozen coronal sections of 4% PFA fixed, OCT (BDH, UK) embedded, post-culture embryos were prepared for analysis of cell proliferation and apoptosis. Anti-phospho-histone H3 (pH3) immunohistochemistry was used to detect mitotic cells in the neural retina. Sections were incubated in blocking solution (10% foetal calf serum, 1% bovine serum albumin, 0.1% Tween-20 in PBS) for 1 hour, followed by incubation with primary antibody, rabbit anti-pH3 (1:100 dilution in blocking solution, Upstate, USA), overnight at 4°C. Sections were incubated with FITC-conjugated goat anti-rabbit secondary antibody (1:100 in blocking solution, Jackson Immunoresearch laboratories, USA) and propidium iodide (1 μgml-1, Sigma, UK), for 1 hour at room temperature. pH3-positive cells were counted in 3–5 midline sections per eye per embryo (n = 4 embryos). A 2-way Analysis of Variance (ANOVA) was used to test whether the number of pH3 cells per section differed between paired BMP4-treated and contralateral non-treated eyes. The Shapiro-Wilk's test of normality was used to confirm that residual values (observed value – value predicted by the model) were normally distributed. Total cell number per section was counted on digital images in Photoshop (6.0). The effect of total cell number on the number of pH3-positive cells was tested by incorporating total cell number per section as a covariate in the Analysis of Co-Variance (ANCOVA) test. To investigate whether the reduction in the number of mitotic cells is regional along the D-V axis of the neural retina, retinal sections were divided into a dorsal region above the optic stalk (including domains 1, 2 and 3) and a ventral region below the optic stalk (containing the Vax2 expression domain 4) and pH3 labelled cells were counted in each region.
TUNEL (Terminal deoxynucleotidyl transferase-mediated dUTP Nick End Labeling, Roche, UK) was used to detect apoptotic cells according to the manufacturer's description. Briefly, frozen sections were permeabilised with 0.1% Triton X-100 in PBS for 2 minutes and incubated at 37 C° for 1 hour with TUNEL mix. Afterwards, sections were counterstained with propidium iodide (1 μgml-1, Sigma, UK) in PBS and mounted with CITIFLUOR. The number of TUNEL-positive cells was counted in dorsal, central, and ventral retinal regions in 2–3 midline sections of eyes in five embryos and compared between the BMP4-treated and non-treated contralateral eyes using a 2-way ANOVA; retinal area was incorporated into an ANCOVA to test for the effect of retinal size on the number of TUNEL positive nuclei (TUNEL index).
References
Chow RL, Lang RA: Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001, 17: 255-296.
Larsen WJ: Human embryology. 2001
Graw J: The genetic and molecular basis of congenital eye defects. Nat Rev Genet. 2003, 4: 876-888.
Calvente R, Carmona R, Abadia-Molina F, Abadia-Fenoll F: Stereological study on the mode of optic cup expansion and the accumulation of mitoses in the early stages of chick embryo development. Anat Rec. 1988, 222: 401-407.
Morcillo J, Martínez-Morales JR, Trousse F, Fermin Y, Sowden JC, Bovolenta P: Proper patterning of the optic fissure requires the sequential activity of BMP7 and SHH. Development. 2006, 133: 3179-3190.
Peters MA, Cepko CL: The dorsal-ventral axis of the neural retina is divided into multiple domains of restricted gene expression which exhibit features of lineage compartments. Dev Biol. 2002, 251: 59-73.
Reilly KM, Melton DA: Short-range signaling by candidate morphogens of the TGF beta family and evidence for a relay mechanism of induction. Cell. 1996, 86: 743-754.
Sanson B: Generating patterns from fields of cells. Examples from Drosophila segmentation. EMBO Rep. 2001, 2: 1083-1088.
Kerszberg M: Morphogen propagation and action: towards molecular models. Semin Cell Dev Biol. 1999, 10: 297-302.
Wolpert L, Kerszberg M: Patterning and positional information. In Patterning in vertebrate development Edited by: Tickle C. Oxford, Oxford University Press; 2003:1-9.
Peters MA: Patterning the neural retina. Curr Opin Neurobiol. 2002, 12: 43-48.
Lupo G, Harris WA, Lewis KE: Mechanisms of ventral patterning in the vertebrate nervous system. Nat Rev Neurosci. 2006, 7: 103-114.
Molotkov A, Molotkova N, Duester G: Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006, 133: 1901-1910.
Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB: Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005, 132: 4789-4800.
Hyatt GA, Schmitt EA, Marsh-Armstrong N, McCaffery P, Drager UC, Dowling JE: Retinoic acid establishes ventral retinal characteristics. Development. 1996, 122: 195-204.
Marsh-Armstrong N, McCaffery P, Gilbert W, Dowling JE, Drager UC: Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc Natl Acad Sci U S A. 1994, 91: 7286-7290.
Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW: Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995, 121: 3267-3278.
Schimmenti LA, de la Cruz J, Lewis RA, Karkera JD, Manligas GS, Roessler E, Muenke M: Novel mutation in sonic hedgehog in non-syndromic colobomatous microphthalmia. Am J Med Genet. 2003, 116A: 215-221.
Roessler E, Belloni E, Gaudenz K, Vargas F, Scherer SW, Tsui LC, Muenke M: Mutations in the C-terminal domain of Sonic Hedgehog cause holoprosencephaly. Hum Mol Genet. 1997, 6: 1847-1853.
Mui SH, Kim JW, Lemke G, Bertuzzi S: Vax genes ventralize the embryonic eye. Genes Dev. 2005, 19: 1249-1259.
Schulte D, Furukawa T, Peters MA, Kozak CA, Cepko CL: Misexpression of the Emx-related homeobox genes cVax and mVax2 ventralizes the retina and perturbs the retinotectal map. Neuron. 1999, 24: 541-553.
Barbieri AM, Broccoli V, Bovolenta P, Alfano G, Marchitiello A, Mocchetti C, Crippa L, Bulfone A, Marigo V, Ballabio A, Banfi S: Vax2 inactivation in mouse determines alteration of the eye dorsal-ventral axis, misrouting of the optic fibres and eye coloboma. Development. 2002, 129: 805-813.
Torres M, Gomez-Pardo E, Gruss P: Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996, 122: 3381-3391.
Hemmati-Brivanlou A, Thomsen GH: Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4. Dev Genet. 1995, 17: 78-89.
Francis PH, Richardson MK, Brickell PM, Tickle C: Bone morphogenetic proteins and a signalling pathway that controls patterning in the developing chick limb. Development. 1994, 120: 209-218.
Furuta Y, Hogan BL: BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998, 12: 3764-3775.
Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N: Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature. 2002, 419: 304-308.
Dosch R, Gawantka V, Delius H, Blumenstock C, Niehrs C: Bmp-4 acts as a morphogen in dorsoventral mesoderm patterning in Xenopus. Development. 1997, 124: 2325-2334.
Jones CM, Smith JC: Establishment of a BMP-4 morphogen gradient by long-range inhibition. Dev Biol. 1998, 194: 12-17.
Raftery LA, Sutherland DJ: Gradients and thresholds: BMP response gradients unveiled in Drosophila embryos. Trends Genet. 2003, 19: 701-708.
Nellen D, Burke R, Struhl G, Basler K: Direct and long-range action of a DPP morphogen gradient. Cell. 1996, 85: 357-368.
Lecuit T, Brook WJ, Ng M, Calleja M, Sun H, Cohen SM: Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature. 1996, 381: 387-393.
Zhao Z, Rivkees SA: Programmed cell death in the developing heart: regulation by BMP4 and FGF2. Dev Dyn. 2000, 217: 388-400.
Duprez D, Bell EJ, Richardson MK, Archer CW, Wolpert L, Brickell PM, Francis-West PH: Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev. 1996, 57: 145-157.
Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, Martin JF: Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol. 2005, 283: 282-293.
Sinervo B: Darwin's finch beaks, Bmp4, and the developmental origins of novelty. Heredity. 2005, 94: 141-142.
Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM: Molecular shaping of the beak. Science. 2004, 305: 1465-1466.
Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ: Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004, 305: 1462-1465.
Mehler MF, Mabie PC, Zhang D, Kessler JA: Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997, 20: 309-317.
Trousse F, Esteve P, Bovolenta P: Bmp4 mediates apoptotic cell death in the developing chick eye. J Neurosci. 2001, 21: 1292-1301.
Koshiba-Takeuchi K, Takeuchi JK, Matsumoto K, Momose T, Uno K, Hoepker V, Ogura K, Takahashi N, Nakamura H, Yasuda K, Ogura T: Tbx5 and the retinotectum projection. Science. 2000, 287: 134-137.
Adler R, Belecky-Adams TL: The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development. 2002, 129: 3161-3171.
Chang B, Smith RS, Peters M, Savinova OV, Hawes NL, Zabaleta A, Nusinowitz S, Martin JE, Davisson ML, Cepko CL, Hogan BL, John SW: Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet. 2001, 2: 18-
Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL: Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997, 188: 235-247.
Lemyre E, Lemieux N, Decarie JC, Lambert M: Del(14)(q22.1q23.2) in a patient with anophthalmia and pituitary hypoplasia. Am J Med Genet. 1998, 77: 162-165.
Ahmad ME, Dada R, Dada T, Kucheria K: 14q(22) deletion in a familial case of anophthalmia with polydactyly. Am J Med Genet A. 2003, 120: 117-122.
Bennett CP, Betts DR, Seller MJ: Deletion 14q (q22q23) associated with anophthalmia, absent pituitary, and other abnormalities. J Med Genet. 1991, 28: 280-281.
Nolen LD, Amor D, Haywood A, St Heaps L, Willcock C, Mihelec M, Tam P, Billson F, Grigg J, Peters G, Jamieson RV: Deletion at 14q22-23 indicates a contiguous gene syndrome comprising anophthalmia, pituitary hypoplasia, and ear anomalies. Am J Med Genet A. 2006
Grimm S, Pflugfelder GO: Control of the gene optomotor-blind in Drosophila wing development by decapentaplegic and wingless. Science. 1996, 271: 1601-1604.
Sowden JC, Holt JK, Meins M, Smith HK, Bhattacharya SS: Expression of Drosophila omb-related T-box genes in the developing human and mouse neural retina. Invest Ophthalmol Vis Sci. 2001, 42: 3095-3102.
Agulnik SI, Garvey N, Hancock S, Ruvinsky I, Chapman DL, Agulnik I, Bollag R, Papaioannou V, Silver LM: Evolution of mouse T-box genes by tandem duplication and cluster dispersion. Genetics. 1996, 144: 249-254.
Sasagawa S, Takabatake T, Takabatake Y, Muramatsu T, Takeshima K: Axes establishment during eye morphogenesis in Xenopus by coordinate and antagonistic actions of BMP4, Shh, and RA. Genesis. 2002, 33: 86-96.
Yamada M, Revelli JP, Eichele G, Barron M, Schwartz RJ: Expression of chick Tbx-2, Tbx-3, and Tbx-5 genes during early heart development: evidence for BMP2 induction of Tbx2. Dev Biol. 2000, 228: 95-105.
Tumpel S, Sanz-Ezquerro JJ, Isaac A, Eblaghie MC, Dobson J, Tickle C: Regulation of Tbx3 expression by anteroposterior signalling in vertebrate limb development. Dev Biol. 2002, 250: 251-262.
Gross JM, Dowling JE: Tbx2b is essential for neuronal differentiation along the dorsal/ventral axis of the zebrafish retina. Proc Natl Acad Sci U S A. 2005, 102: 4371-4376.
Eichele G, Tickle C, Alberts BM: Microcontrolled release of biologically active compounds in chick embryos: beads of 200-microns diameter for the local release of retinoids. Anal Biochem. 1984, 142: 542-555.
Ganan Y, Macias D, Duterque-Coquillaud M, Ros MA, Hurle JM: Role of TGF beta s and BMPs as signals controlling the position of the digits and the areas of interdigital cell death in the developing chick limb autopod. Development. 1996, 122: 2349-2357.
Copp A, Cogram P, Fleming A, Gerrelli D, Henderson D, Hynes A, Kolatsi-Joannou M, Murdoch J, Ybot-Gonzalez P: Neurulation and neural tube closure defects. In Developmental Biology Protocols Volume136. Edited by: Tuan RS and Lo CW. Totowa, NJ, Humana Press Inc.; 2000:135-160.
Zimmerman LB, De Jesus-Escobar JM, Harland RM: The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996, 86: 599-606.
Shi Y, Massague J: Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003, 113: 685-700.
Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y: Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Development. 2005, 132: 913-923.
Graham A, Francis-West P, Brickell P, Lumsden A: The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994, 372: 684-686.
Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A: Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999, 274: 19838-19845.
Barlow AJ, Francis-West PH: Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997, 124: 391-398.
Monaghan AP, Davidson DR, Sime C, Graham E, Baldock R, Bhattacharya SS, Hill RE: The Msh-like homeobox genes define domains in the developing vertebrate eye. Development. 1991, 112: 1053-1061.
Furuta Y, Piston DW, Hogan BL: Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997, 124: 2203-2212.
Zou H, Niswander L: Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996, 272: 738-741.
Rodriguez-Esteban C, Tsukui T, Yonei S, Magallon J, Tamura K, Izpisua Belmonte JC: The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature. 1999, 398: 814-818.
Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S: Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006, 133: 1575-1585.
Sivasankaran R, Vigano MA, Muller B, Affolter M, Basler K: Direct transcriptional control of the Dpp target omb by the DNA binding protein Brinker. Embo J. 2000, 19: 6162-6172.
Barbieri AM, Lupo G, Bulfone A, Andreazzoli M, Mariani M, Fougerousse F, Consalez GG, Borsani G, Beckmann JS, Barsacchi G, Ballabio A, Banfi S: A homeobox gene, vax2, controls the patterning of the eye dorsoventral axis. Proc Natl Acad Sci U S A. 1999, 96: 10729-10734.
Hatcher CJ, Kim MS, Mah CS, Goldstein MM, Wong B, Mikawa T, Basson CT: TBX5 transcription factor regulates cell proliferation during cardiogenesis. Dev Biol. 2001, 230: 177-188.
He ML, Chen Y, Peng Y, Jin D, Du D, Wu J, Lu P, Lin MC, Kung HF: Induction of apoptosis and inhibition of cell growth by developmental regulator hTBX5. Biochem Biophys Res Commun. 2002, 297: 185-192.
Jacobs JJ, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof PM, van Welsem T, van de Vijver MJ, Koh EY, Daley GQ, van Lohuizen M: Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified in a subset of human breast cancers. Nat Genet. 2000, 26: 291-299.
Sinclair CS, Adem C, Naderi A, Soderberg CL, Johnson M, Wu K, Wadum L, Couch VL, Sellers TA, Schaid D, Slezak J, Fredericksen Z, Ingle JN, Hartmann L, Jenkins RB, Couch FJ: TBX2 is preferentially amplified in BRCA1- and BRCA2-related breast tumors. Cancer Res. 2002, 62: 3587-3591.
Vance KW, Carreira S, Brosch G, Goding CR: Tbx2 is overexpressed and plays an important role in maintaining proliferation and suppression of senescence in melanomas. Cancer Res. 2005, 65: 2260-2268.
Barlund M, Monni O, Kononen J, Cornelison R, Torhorst J, Sauter G, Kallioniemi OP, Kallioniemi A: Multiple genes at 17q23 undergo amplification and overexpression in breast cancer. Cancer Res. 2000, 60: 5340-5344.
Mahlamaki EH, Barlund M, Tanner M, Gorunova L, Hoglund M, Karhu R, Kallioniemi A: Frequent amplification of 8q24, 11q, 17q, and 20q-specific genes in pancreatic cancer. Genes Chromosomes Cancer. 2002, 35: 353-358.
Lingbeek ME, Jacobs JJ, van Lohuizen M: The T-box repressors TBX2 and TBX3 specifically regulate the tumor suppressor gene p14ARF via a variant T-site in the initiator. J Biol Chem. 2002, 277: 26120-26127.
Prince S, Carreira S, Vance KW, Abrahams A, Goding CR: Tbx2 directly represses the expression of the p21(WAF1) cyclin-dependent kinase inhibitor. Cancer Res. 2004, 64: 1669-1674.
Brummelkamp TR, Kortlever RM, Lingbeek M, Trettel F, MacDonald ME, van Lohuizen M, Bernards R: TBX-3, the gene mutated in Ulnar-Mammary Syndrome, is a negative regulator of p19ARF and inhibits senescence. J Biol Chem. 2002, 277: 6567-6572.
Carlson H, Ota S, Campbell CE, Hurlin PJ: A dominant repression domain in Tbx3 mediates transcriptional repression and cell immortalization: relevance to mutations in Tbx3 that cause ulnar-mammary syndrome. Hum Mol Genet. 2001, 10: 2403-2413.
Harrelson Z, Kelly RG, Goldin SN, Gibson-Brown JJ, Bollag RJ, Silver LM, Papaioannou VE: Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development. 2004, 131: 5041-5052.
Cai CL, Zhou W, Yang L, Bu L, Qyang Y, Zhang X, Li X, Rosenfeld MG, Chen J, Evans S: T-box genes coordinate regional rates of proliferation and regional specification during cardiogenesis. Development. 2005, 132: 2475-2487.
Schedl A, Ross A, Lee M, Engelkamp D, Rashbass P, van Heyningen V, Hastie ND: Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell. 1996, 86: 71-82.
Lehmann OJ, Ebenezer ND, Jordan T, Fox M, Ocaka L, Payne A, Leroy BP, Clark BJ, Hitchings RA, Povey S, Khaw PT, Bhattacharya SS: Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am J Hum Genet. 2000, 67: 1129-1135.
Zakin L, De Robertis EM: Inactivation of mouse Twisted gastrulation reveals its role in promoting Bmp4 activity during forebrain development. Development. 2004, 131: 413-424.
Liu J, Wilson S, Reh T: BMP receptor 1b is required for axon guidance and cell survival in the developing retina. Dev Biol. 2003, 256: 34-48.
Faber SC, Robinson ML, Makarenkova HP, Lang RA: Bmp signaling is required for development of primary lens fiber cells. Development. 2002, 129: 3727-3737.
Dudley AT, Robertson EJ: Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997, 208: 349-362.
Ohkubo Y, Chiang C, Rubenstein JL: Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002, 111: 1-17.
McLaughlin T, Hindges R, O'Leary DD: Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol. 2003, 13: 57-69.
Ogita J, Isogai E, Sudo H, Sakiyama S, Nakagawara A, Koseki H: Expression of the Dan gene during chicken embryonic development. Mech Dev. 2001, 109: 363-365.
Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura S, Yamamoto TS, Ueno N, Noda M: Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science. 2001, 293: 111-115.
Take-uchi M, Clarke JD, Wilson SW: Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003, 130: 955-968.
Zhang XM, Yang XJ: Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001, 233: 271-290.
Mui SH, Hindges R, O'Leary DD, Lemke G, Bertuzzi S: The homeodomain protein Vax2 patterns the dorsoventral and nasotemporal axes of the eye. Development. 2002, 129: 797-804.
Cockroft DL: Dissection and culture of postimplantation embryos. Postimplantation Mammalian Embryos: A Practical Approach. Edited by: Copp AJ and Cockroft DL. 1990, Oxford, UK., IRL Press at Oxford Univeristy Press, 15–40.-
Lee SH, Fu KK, Hui JN, Richman JM: Noggin and retinoic acid transform the identity of avian facial prominences. Nature. 2001, 414: 909-912.
Wilkinson DG: In situ hybridisation. 1992, Oxford, Oxford University Press
Acknowledgements
We are very grateful to Professor Andrew Copp for help in establishing the mouse embryo culture system and to Dr Deborah Henderson and Dr Patrizia Ferretti for critical comments and discussions. We would like to thank Professor Tim Cole for guidance with the statistical analysis and Ms Katy Gardner for provision of material. The study was supported by grants from the Child Health Research Appeal Trust and the Medical Research Council UK (G120/191). Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from Research and Development funding received from the NHS Executive.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
HB and JKLH carried out the bead implantation and whole embryo culture experiments. HB carried out the proliferation and apoptosis study, the morphometric and statistical analyses, prepared the figures, and assisted with the preparation of the manuscript. JCS conceived of the study and wrote the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12861_2006_141_MOESM1_ESM.pdf
Additional File 1: Tbx3 , Vax2 , Msx2 expression and Phospho-Smad 1/5/8 localisation after alteration of BMP4 signaling in embryo culture by addition of exogenous Noggin or BMP4. (A, B) Post-culture embryo showing normal dorsal Tbx3 expression in the control non-treated and Noggin-treated optic cups respectively. (C, D) Post-culture embryo showing normal ventral Vax2 expression in the control non-treated and Noggin-treated optic cups respectively. (E) The boundary of Bmp4 expression (arrow) in the dorsal neural retina of an E11.5 embryo. (F) Post-culture embryo showing high levels of Phospho-Smad 1/5/8 labeling in the dorsal neural retina and in the lens. The extent of the highest level of BMP signaling in the retina is demarcated by arrow. (G) BMP4-treated contralateral eye showing a wider region of high level Phospho-Smad 1/5/8 labeling (arrow). (H, I) Another example of the extension of BMP signaling in the BMP4-treated optic cup (I) as compared with the control eye (H) in a post-culture embryo. Sections H and I are counterstained with methyl green. (J) Post-culture embryo showing normal Msx2 expression restricted to the dorsal neural retina and lens. (K) BMP4-treated contralateral eye showing widespread induction of Msx2 expression in the lens and the optic cup. Noggin or BMP4-soaked beads are indicated with asterisks (in A-K) and domains of gene expression are demarcated with arrows (in A-D). E-I show coronal sections of eyes. Abbreviations: L, lens vesicle; NR, neural retina. (PDF 3 MB)
12861_2006_141_MOESM2_ESM.pdf
Additional File 2: Analysis of pH3 and TUNEL in the dorsal and ventral retina after BMP4 treatment. (A, B) Graphs show the number of mitotic cells per section per eye in the dorsal (A) and ventral (B) regions of BMP4-treated optic cups compared with contralateral control optic cups (n = 44 sections from 4 embryos; p = 0.007 in dorsal, p = 0.018 in ventral by ANOVA). (C) Graph shows the programmed cell death index (per section per eye) in the dorsal neural retina of BMP4-treated and contralateral control eyes (n = 26 sections in 5 embryos; p = 0.044 by ANCOVA). Data was Ln transformed for normalisation. (PDF 294 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Behesti, H., Holt, J.K. & Sowden, J.C. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev Biol 6, 62 (2006). https://doi.org/10.1186/1471-213X-6-62
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-213X-6-62