Abstract
Background
The adaptive response of bone cells to mechanical strain is a primary determinant of skeletal architecture and bone mass. In vivo mechanical loading induces new bone formation and increases bone mineral density whereas disuse, immobilisation and weightlessness induce bone loss. The potency of mechanical strain is such that a single brief period of loading at physiological strain magnitude is able to induce a long-lasting osteogenic response that lasts for days. Although the process of mechanotransduction in bone is incompletely understood, observations that responses to mechanical strain outlast the duration of stimulation necessitate the existence of a form of cellular memory through which transient strain episodes are recorded, interpreted and remembered by bone cells. Recent evidence supports the existence of a complex multicellular glutamate-signalling network in bone that shares functional similarities to glutamatergic neurotransmission in the central nervous system. In neurones, these signalling molecules coordinate synaptic communication required to support learning and memory formation, through a complex process of long-term potentiation.
Presentation of the hypothesis
We hypothesise that osteoblasts use a cellular mechanism similar or identical to neuronal long-term potentiation in the central nervous system to mediate long-lasting changes in osteogenesis following brief periods of mechanical strain.
Testing the hypothesis
N-methyl-D-aspartate (NMDA) receptor antagonism should inhibit the saturating response of mechanical strain and reduce the enhanced osteogenicity of segregated loading to that of an equivalent period of uninterrupted loading. Changes in α-amino-3-hydroxy-5-methyl-isoxazole propionate (AMPA) receptor expression, localisation and electrophysiological responses should be induced by mechanical strain and inhibited by modulators of neuronal long-term potentiation.
Implications of the hypothesis
If true, this hypothesis would provide a mechanism through which the skeleton could be pharmacologically primed to enhance or retrieve the normal osteogenic response to exercise. This would form a basis through which novel therapies could be developed to target osteoporosis and other prevalent bone disorders associated with low bone mass.
Similar content being viewed by others
Background
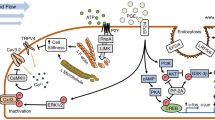
Numerous animal and human studies have demonstrated that mechanical strain is an important determinant of skeletal architecture and bone mass, although the precise mechanotransductory events that direct this biological phenomenon are unclear. Like neurones, bone cells express complex glutamate signalling machinery including functional ionotropic and metabotropic receptors that share comparable basic pharmacological and electrophysiological properties to their neuronal counterparts [1–7]. Osteoblasts also spontaneously release glutamate and express functional glutamate transporters, providing evidence for endogenous receptor activation and agonist recycling in the bone microenvironment [8–10]. Together these data support the existence of a complex, highly orchestrated and functionally significant multicellular glutamate-signalling network in bone. But what role does glutamate play in musculoskeletal physiology and bone cell function? One possibility is the regulation of cell differentiation. Glutamate receptor antagonism has been reported to inhibit differentiation of osteoblasts and osteoclasts in vitro, although the in vivo significance of these findings remains unclear [4, 11].
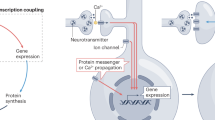
Over recent years the excitatory amino-acid glutamate has emerged as an important molecular determinant of plasticity and synaptic enhancement in the central nervous system responsible for the processes of learning and memory – the best studied of which is NMDA receptor-dependent long-term potentiation (LTP). LTP can be crudely defined as a long-lasting activity dependent change in synaptic efficacy in which synaptic strength is persistently increased in response to brief periods of repetitive electrical stimulation lasting only seconds. At a molecular level, LTP is initiated by NMDA receptor activation and calcium influx, which mediates increased delivery to, and/or conductance of existing AMPA-type glutamate receptors at the postsynaptic membrane. Further episodes of signalling are subsequently enhanced as a result of increased probability of receptor activation and glutamate mediated synaptic conductivity. NMDA receptor mediated calcium influx and activation of CaMKII plays a key role in the expression of LTP [12, 13]. Inhibition or knockout of CaMKII has been reported to prevent LTP induction [14–16] while constitutively activated CaMKII increases synaptic strength [17, 18]. Studies have demonstrated that CaMKII activation occurs immediately after induction of LTP, increasing single channel conductance of AMPA receptors through serine phosphorylation of GluR1 and mediating delivery and clustering of new AMPA receptors at synapses through the regulation of PDZ interactions between GluR1, Stargazin protein and PSD-95 [19–22].
Presentation of the hypothesis
Long-term potentiation in osteoblasts, a cellular basis of memory in bone?
Neuronal LTP has clear analogies to osteogenesis resulting from brief periods of mechanical loading particularly in as much as they are both long-lasting forms of use-dependent potentiation. This prompted the hypothesis that memory formation in bone and brain share a common cellular pathway. Mechanical loading is arguably the most potent environmental influence on the skeleton and promotes new bone formation through modelling and remodelling. In contrast, disuse, immobilisation and weightlessness are associated with bone loss [23–26]. Mechanical influences on the skeleton and the adaptive responses of bone cells to recent loading history have been described as the primary functional determinant of skeletal architecture [27]. In simple terms, dynamic alteration in bone structure induced by loading serves to improve its structural suitability to subsequent loading events. Bone cells learn from changes in their loading environment so that the activity of cells are precisely orchestrated to control modelling and remodelling and preserve optimum skeletal structure. This protective mechanism of cause and effect acts to prevent skeletal damage that would otherwise result from habitual physical activity. The osteogenic potency of mechanical strain on bone cells is such that single brief periods of loading are able to exert a long-lasting osteogenic response in vivo [28]. The temporal nature of this response, transducing mechanical stimuli into an appropriate level of consolidatory bone formation that outlasts the stimulus, necessitates the existence of a form of cellular memory.
Evidence exists for conventional memory formation in bone in which responses to mechanical loading are affected by preceding episodes of loading, at least fulfilling the conceptual requirement for LTP. For instance, it has been demonstrated that mechanical loading is more osteogenic when separated into discrete episodes, compared to an equivalent period of uninterrupted loading. Using a well-characterised model of loading induced bone formation in vivo, several studies have demonstrated that the osteogenic potential of mechanical loading is enhanced in terms of bone formation and biomechanical and structural properties, by separation of loading cycles into discrete episodes with varying degrees of rest between periods of stimulation [29–31]. In these studies, the order of osteogenic potency was such that 6 periods of 60 cycles > 4 periods of 90 cycles > 360 cycles at once. These data were interpreted as bone cells becoming increasingly less responsive or 'deaf' to prolonged periods of mechanical stimulation, a form of depression or desensitisation to the stimulus. Based on a hypothesis of LTP in bone described here, in contrast to losing responsiveness, bone cells could be considered primed by earlier periods of loading, so that further episodes are increasingly efficacious and subsequent responses potentiated. This would account for the extrapolatory deduction that less total loading cycles should be required to produce the equivalent level of osteogenic response if loading is separated into discrete bouts compared to a single sustained, and less effective period of loading. Moreover, other studies have reported that the osteogenic response of mechanical strain increases with loading frequency [32], which has clear similarities to LTP in central neurones, which is more effectively induced by high frequency stimulation. Lower frequency stimulation on the other hand is associated with short-term potentiation (STP) or long-term depression (LTD). Perhaps most significantly, LTP in bone would also account for the observation that levels of osteogenic stimulus saturate rapidly so that only a few loading cycles are required to achieve the maximum osteogenic effect [33]. In support of LTP-like mechanisms mediating the osteogenic response of bone cells, key events involved in LTP are observed in osteoblasts following mechanical stimulation in vitro, including specific and rapid initiation of calcium transients and activation of neuronal CaMKII. Furthermore, CaMKII activity appears to be required for normal osteoblast differentiation, which temporally precedes new bone formation post-loading [34, 35]. In vivo evidence also exists for the involvement of an LTP-like mechanism in loading induced osteogenesis; potent inhibition of AMPA/Kainate receptor activation with DNQX causes a significant decrease in loading induced bone formation in the 4-point bending rat ulnae model [36]. This could be interpreted as functional antagonism of potentiated AMPA receptor responses induced by LTP-like mechanotransduction in bone.
Testing the hypothesis
Our hypothesis could be tested using a combined in vitro electrophysiological, and in vivo approach. One would expect electrophysiological responses of bone cells to glutamate receptor agonists to be potentiated following mechanical strain in vitro coincident with an increase in AMPA receptor number and/or conductance. The signalling pathways responsible for mediating these effects could be investigated using known agonists and antagonists of neuronal LTP. Similarly pharmacological intervention could be used to investigate the in vivo significance of LTP in which antagonists of NMDA receptors and CaMKII should diminish the potentiated osteogenic responses of segregated loading.
Implications of the hypothesis
Naturally this hypothesis raises the question as to why bone cells would require such an exquisitely sensitive and rapid signalling system as fast excitatory neurotransmission. The millisecond time-scale through which these events are mediated may be required to detect and respond to the very rapid strain changes experienced during forms of exercise with high osteogenic potential. Perhaps the most significant implication of this would be the ability to pharmacologically prime the skeleton to retrieve or potentiate the osteogenic capacity of mechanical strain, an effect desirable for the treatment of bone disorders associated with low bone mass and architectural abnormalities such as osteoporosis.
Abbreviations
- AMPA:
-
α-amino-3-hydroxy-5-methyl-isoxazole propionate
- CaMKII:
-
Calcium/calmodulin-dependent protein kinase II
- LTP:
-
Long-term potentiation
- NMDA:
-
N-methyl-D-aspartate.
References
Chenu C, Serre CM, Raynal C, Burt-Pichat B, Delmas PD: Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998, 22: 295-299. 10.1016/S8756-3282(97)00295-0.
Patton AJ, Genever PG, Birch MA, Suva LJ, Skerry TM: Expression of an N-methyl-D-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone. 1998, 22: 645-649. 10.1016/S8756-3282(98)00061-1.
Laketic-Ljubojevic I, Suva LJ, Maathuis FJ, Sanders D, Skerry TM: Functional characterization of N-methyl-D-aspartic acid-gated channels in bone cells. Bone. 1999, 25: 631-637. 10.1016/S8756-3282(99)00224-0.
Peet NM, Grabowski PS, Laketic-Ljubojevic I, Skerry TM: The glutamate receptor antagonist MK801 modulates bone resorption in vitro by a mechanism predominantly involving osteoclast differentiation. FASEB J. 1999, 13: 2179-2185.
Itzstein C, Espinosa L, Delmas PD, Chenu C: Specific antagonists of NMDA receptors prevent osteoclast sealing zone formation required for bone resorption. Biochem Biophys Res Commun. 2000, 268: 201-209. 10.1006/bbrc.2000.2097.
Gu Y, Publicover SJ: Expression of functional metabotropic glutamate receptors in primary cultured rat osteoblasts. Cross-talk with N-methyl-D-aspartate receptors. J Biol Chem. 2000, 275: 34252-34259. 10.1074/jbc.M004520200.
Hinoi E, Fujimori S, Nakamura Y, Yoneda Y: Group III metabotropic glutamate receptors in rat cultured calvarial osteoblasts. Biochem Biophys Res Commun. 2001, 281: 341-346. 10.1006/bbrc.2001.4355.
Bhangu PS, Genever PG, Spencer GJ, Grewal TS, Skerry TM: Evidence for targeted vesicular glutamate exocytosis in osteoblasts. Bone. 2001, 29: 16-23. 10.1016/S8756-3282(01)00482-3.
Genever PG, Skerry TM: Regulation of spontaneous glutamate release activity in osteoblastic cells and its role in differentiation and survival: evidence for intrinsic glutamatergic signaling in bone. FASEB J. 2001, 15: 1586-1588.
Spencer GJ, Bhangu PS, Genever PG, Grewal TS, Skerry TM: Functional GLAST; An essential molecular link in glutamatergic signalling during osteoblast differentiation. J Bone Miner Res. 1999, 14 (6): O12-
Birch MA, Genever PG, Laketic-Ljubojevic I, Patton AJ, Peet NM, Skerry TM: Glutamate receptor activation is necessary for bone formation in vitro. J Bone Miner Res. 1997, 12 (9): O10-
Fukunaga K, Stoppini L, Miyamoto E, Muller D: Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993, 268: 7863-7867.
Fukunaga K, Miyamoto E: A working model of CaM kinase II activity in hippocampal long-term potentiation and memory. Neurosci Res. 2000, 38: 3-17. 10.1016/S0168-0102(00)00139-5.
Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA: An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989, 340: 554-557. 10.1038/340554a0.
Malinow R, Schulman H, Tsien RW: Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989, 245: 862-866.
Silva AJ, Stevens CF, Tonegawa S, Wang Y: Deficient hippocampal long-term potentiation in alpha-calcium- calmodulin kinase II mutant mice. Science. 1992, 257: 201-206.
Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA: Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci U S A. 1995, 92: 11175-11179.
Pettit DL, Perlman S, Malinow R: Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science. 1994, 266: 1881-1885.
Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R: Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000, 287: 2262-2267. 10.1126/science.287.5461.2262.
Derkach V, Barria A, Soderling TR: Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999, 96: 3269-3274. 10.1073/pnas.96.6.3269.
Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ: Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000, 408: 936-943. 10.1038/35046031.
Nakagawa T, Sheng M: Neurobiology. A stargazer foretells the way to the synapse. Science. 2000, 290: 2270-2271.
Rodan GA: Bone mass homeostasis and bisphosphonate action. Bone. 1997, 20: 1-4. 10.1016/S8756-3282(96)00318-3.
Hillam RA, Skerry TM: Inhibition of bone resorption and stimulation of formation by mechanical loading of the modeling rat ulna in vivo. J Bone Miner Res. 1995, 10: 683-689.
Bourrin S, Palle S, Pupier R, Vico L, Alexandre C: Effect of physical training on bone adaptation in three zones of the rat tibia. J Bone Miner Res. 1995, 10: 1745-1752.
Heinonen A, Oja P, Kannus P, Sievanen H, Haapasalo H, Manttari A: Bone mineral density in female athletes representing sports with different loading characteristics of the skeleton. Bone. 1995, 17: 197-203. 10.1016/8756-3282(95)00151-3.
Lanyon L, Skerry TM: Postmenopausal osteoporosis as a failure of bone's adaption to functional loading: A Hypothesis. J Bone Miner Res. 2001, 16: 1937-1947.
Pead MJ, Skerry TM, Lanyon LE: Direct transformation from quiescence to bone formation in the adult periosteum following a single brief period of bone loading. J Bone Miner Res. 1988, 3: 647-656.
Robling AG, Burr DB, Turner CH: Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Miner Res. 2000, 15: 1596-1602.
Robling AG, Hinant FM, Burr DB, Turner CH: Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002, 17: 1545-1554.
Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS: Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002, 17: 1613-1620.
Hsieh YF, Turner CH: Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res. 2001, 16: 918-924.
Rubin CT, Lanyon LE: Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984, 66: 397-402.
Spencer GJ, Grewal TS, Genever PG, Skerry TM: Long-term potentiation in bone: A cellular basis of memory in osteoblasts. Bone. 2001, 28 (5S): S65-OR72.
Spencer GJ, Grewal TS, Genever TM, Skerry : Calcium dependent protein kinase II activity is obligate for bone formation in vitro. J Bone Miner Res. 2001, 16 (6): OC25-
Taylor AF, Brabbs AC, Peet NM, Laketic-Ljubojevic I, Dobson KR, Skerry TM: Bone formation/resorption and osteoblast/adipocyte plasticity mediated by AMPA/kainate glutamate receptors in vitro and in vivo. J Bone Miner Res. 2000, 15 (S1): SA222-
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
GJS and PGG discussed this hypothesis, GJS drafted and PGG critically reviewed the paper. Both authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Spencer, G.J., Genever, P.G. Long-term potentiation in bone – a role for glutamate in strain-induced cellular memory?. BMC Cell Biol 4, 9 (2003). https://doi.org/10.1186/1471-2121-4-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2121-4-9