Abstract
Background
In this study, we analyzed maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) values in a stable COPD population compared with normal subjects. We evaluated the possible correlation between functional maximal respiratory static pressures and functional and anthropometric parameters at different stages of COPD. Furthermore, we considered the possible correlation between airway obstruction and MIP and MEP values.
Subject and methods
110 patients with stable COPD and 21 age-matched healthy subjects were enrolled in this study. Patients were subdivided according to GOLD guidelines: 31 mild, 39 moderate and 28 severe.
Results
Both MIP and MEP were lower in patients with severe airway impairment than in normal subjects. Moreover, we found a correlation between respiratory muscle function and some functional and anthropometric parameters: FEV1 (forced expiratory volume in one second), FVC (forced vital capacity), PEF (peak expiratory flow), TLC (total lung capacity) and height. MIP and MEP values were lower in patients with severe impairment than in patients with a slight reduction of FEV1.
Conclusion
The measurement of MIP and MEP indicates the state of respiratory muscles, thus providing clinicians with a further and helpful tool in monitoring the evolution of COPD.
Similar content being viewed by others
Background
In several diseases, the evaluation of respiratory muscle strength can prove to be very useful. The measurement of the maximum static mouth pressures made against an occluded airway (maximal expiratory pressure and maximal inspiratory pressure) is the most widely used and is a simple way to gauge respiratory muscle strength and to quantify its severity [1–3].
When we analyze maximal respiratory pressure, we should consider both the difficulty that some subjects have in performing a maximal effort and the normal biological variability of respiratory muscle strength [4].
Maximal inspiratory pressure (MIP) is the maximum negative pressure that can be generated from one inspiratory effort starting from functional residual capacity (FRC) or residual volume (RV) [5, 6]. Maximal expiratory pressure (MEP) measures the maximum positive pressure that can be generated from one expiratory effort starting from total lung capacity (TLC) or FRC. Unlike inspiratory muscles, expiratory muscles (abdominal and thoracic muscles) reach their optimal force-length relationship at elevate pulmonary volumes [7].
During normal breathing, most of the respiratory work depends on the diaphragm function and the accessory respiratory muscles become necessary only during deep inspiration [8].
The mouth pressures recorded during these maneuvers are assumed to reflect respiratory muscle strength [9].
It is known that a reduction of MIP and MEP has been associated with several neuromuscular diseases, but it is also possible to point up lower values in patients with chronic obstructive pulmonary disease (COPD) [10–12].
The factors contributing to respiratory muscle weakness in many patients with COPD are: a) malnutrition related to biochemical, anatomical and physiological changes; b) muscular atrophy; c) steroid-induced myopathy; d) pulmonary hyperinflation with increased residual volume; e) reduced blood flow to the respiratory muscles [13–19].
The measurement of MIP and MEP is indicated in any of these situations or when dyspnea or hypercapnia are not proportional to FEV1 reduction [6].
Age and sex could influence MIP and MEP values; these are lower in females than in males and quite constant until seventy years of age when they start to decrease [6].
The objectives of this study were: (1) to describe MIP and MEP values in a stable COPD population, (2) to explore the effect of varying degrees of obstructive ventilatory impairment on MIP and MEP measurement, (3) to evaluate the possible correlation between functional maximal respiratory pressures and functional parameters at different stages of COPD.
Methods
During 1 year 110 patients suffering from COPD were enrolled in this study. All patients were in a clinically stable condition and the mean age was 70 ± 8 years (Table 1).
At the first examination, we excluded those patients whose FEV1 improvement, after a bronchodilatory test, was ≥ 12% and 200 mL of the baseline value and with a history of asthma. Other patients, with clinically significant diseases such as fibrothorax, bronchiectasis, tuberculosis or neuromuscular diseases were also excluded. Some patients were excluded because of lack of compliance during the forced expiratory test or during the MIP and MEP maneuvers.
All patients gave their written informed consent before the study and the Ethics Committee approved the protocol.
At the enrolment, all the subjects were examined. Anthropometric measurements (age, height and weight) were taken and pulmonary function tests, including flow/volume spirometry and N2 Washout, were conducted. Maximal static inspiratory and expiratory mouth pressures were measured using a portable mouth pressure meter (Spirovis – COSMED – Pavona – Italy); this had a disposable mouthpiece, and a small leak to prevent glottic closure. MIP was obtained at the level of RV and MEP was measured at the level of TLC. The measurements were made in standing position. The subjects were verbally encouraged to achieve maximal strength. The measurements were repeated until five values varying by less than 5% and sustained for at least 1 s were obtained; the best value achieved was considered in the data analysis.
Forced expiratory tests were recorded with a Cosmed Quark spirometer (PFT4 SUITE – COSMED – Pavona – Italy) which was calibrated each morning using the same 3 L precision syringe.
We divided patients into three groups on the basis of airway obstruction: mild (FEV1 > 80%), moderate (FEV1 between 80 and 50%), and severe (FEV1 < 50%) with FEV1/FVC ratio <70% in all the groups.
The control group included 21 age-matched normal subjects who were non-smokers, free of respiratory symptoms and disease and with normal functional parameters (Table 1).
Statistical analysis was performed for all measured functional parameters using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego California USA). Standards descriptive statistics based on mean and standard deviation (SD) for quantitative variables were used. The mean difference between MIP/MEP in healthy subjects and MIP/MEP in patients with COPD was determined by the unpaired Student's t test. Statistical differences for respiratory muscle strength and different stage of COPD were analyzed by analysis of variance (ANOVA). The Pearson's test was performed to assess possible correlation between MIP and MEP values and anthropometric and functional parameters.
All p values were two sided and values below 0.05 were considered statistically significant.
Results
During this study, 12 COPD patients were withdrawn: 5 because of a contemporary restrictive deficit and 7 because of their insufficient compliance during the forced expiratory test or during the MIP and MEP maneuvers. Therefore, 98 patients took part in this study. Table 1 shows the anthropometric parameters and lung function of the patients studied.
The MIP and MEP values of healthy subjects were used as a control group for the comparison with patients with different stages of COPD.
MEP was significantly lower (p = 0.0014) in patients with severe airway obstruction than in the control group; no differences were observed in mild and moderate patients (p > 0.05).
At the same time, MIP was significantly lower at all the stages of COPD than in the control group: mild p = 0.010; moderate p = 0.018; severe p < 0.0001.
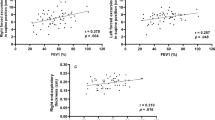
Statistical analysis performed on the total of COPD patients to assess the possible correlation between MIP and MEP values and anthropometric and functional parameters showed significant (p < 0.05) positive correlations among maximal static inspiratory pressure and FEV1 (L, r2 = 0.13, Figure 1), FVC (L, r2 = 0.20), PEF (L/sec, r2 = 0.19), TLC (L, r2 = 0.11) and height (r2 = 0.09).
Similar results were showed between MEP and the same functional and anthropometric parameters (FEV1 r2 = 0.13, figure 2; FVC r2 = 0.19; PEF r2 = 0.22; TLC r2 = 0.12; height r2 = 0.15).
Respiratory muscle strength, however, had no significant correlation with RV and RV/TLC (Figures 3, 4, 5, 6), weight and age.
Moreover, we evaluated whether there was a possible correlation between COPD stage and respiratory muscular strength. The analysis of variance (ANOVA) showed no statistically significant difference between mild and moderate patients (p > 0.005), while the difference between mild and severe was significant as well as that between moderate and severe patients.
Discussion
To our knowledge, this is the first study that analyzes MIP and MEP variation in the different stages of COPD severity to understand when MIP and MEP start to decrease.
The main finding of this work is that airway obstruction may be closely associated with decreased respiratory pressures in patients with COPD. In fact, both MIP and MEP values were lower in patients with severe obstruction compared with healthy subjects. MIP decreased also in patient with mild and moderate functional impairment: this could suggest an earlier deterioration of the inspiratory muscles in this sort of patients.
Similar results have been showed in a recent work that limited the evaluations only to the inspiratory muscle strength [20].
Dynamic functional parameters are a measure of the respiratory muscular strength as well as maximal respiratory pressures. In fact with the reduction of FEV1, PEF and FVC, as in severe COPD, we have observed a similar decrease in MIP and MEP. Interestingly, our study highlights the importance of the predictivity of functional parameters (FEV1, PEF, FVC, and TLC) on MIP and MEP reduction in COPD patients.
Nishimura and colleagues showed a similar relation between respiratory muscle force and FEV1 [18].
Our findings could be explained by several factors. Patients with severe disease probably have a decrease in tension produced by inspiratory muscle shortening. However, our study did not show a correlation between maximal respiratory pressure and residual volume at any of the different stages even though air-trapping was different among patients.
As reported by Rochester, chronic airflow limitation permanently shortens the diaphragm, in this way muscles lose sarcomeres, but the length of individual sarcomeres is restored to normal and the force-length relationship of the shortened diaphragm is reset to a new and shorter muscle length so, the relation of diaphragm length to lung volume is the same as in normal subjects [12].
Further support for the concept that COPD does not permanently alter diaphragm muscle length in human COPD comes from another study by the same author who reported that the diaphragm undergoes an adaptation to compensate for the mechanical stresses that pulmonary hyperinflation places on it [21].
Similarly, Nishimura and colleagues showed no significant correlation between respiratory muscular strength and RV [18].
Similowsky et al. demonstrated that the diaphragm of COPD patients undergoes some structural adaptation which preserves or even increases its capacity to generate pressure even if the muscular function is impaired because of an alteration in chest wall geometry [22, 23].
Walsh and coworkers found that the size of the rib cage and the arrangement of the ribs where not different between severely hyperinflated patients with COPD and healthy subjects [24].
McKenzie et al observed that at resting functional residual capacity the curvature of the diaphragm is only 3.5% smaller in patients with severe COPD than in healthy subjects [25].
Additional mechanisms of muscular impairment in COPD may include malnutrition, which predisposes the diaphragm to a greater loss in muscle mass in proportion to a patient's body-weight reduction [26–30]. Prolonged malnutrition can lead to skeletal and respiratory muscle wasting with severe effects on the contractile and fatigue properties of the diaphragm [26–30]. This suggests that a nutritional supplementation should be a primary intervention in patients with lean body mass [31].
Corticosteroids, routinely used to manage chronic inflammation, have negative consequences, including steroid myopathy of respiratory and skeletal muscles, even at low doses [26–28].
Further, electrolyte imbalance and hypoxemia alter muscle function and should be corrected, when possible [26, 27].
Oxidative stress, disuse, and systemic inflammation may contribute to the observed muscle abnormalities and each factor has its own potential for innovative treatment approaches [26, 27].
These processes contribute to the reduced capacity of the respiratory muscles in COPD and translate to measurable decreases in maximal pressure generation, exhibited as lower values for maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), sniff testing, maximal voluntary ventilation (MVV), and exercise tolerance.
There is also a strength correlation between thoracic morphology dimension and anthropometric variables even if some studies have obtained conflicting results with age, weight and height [32].
Wilson and co-workers reported that MIP and MEP in men were significantly correlated with age and weight, whereas in women they were correlated with height and weight, while Leech et al found that age had no consistent effect on respiratory muscular strength [33, 34].
In contrast with the study of Enright et al [35], our study did not find any correlation with weight and age, probably because our patients had reached approximately 60 years of age with the same normal body weight.
For these reasons our study shows a significant linear relationship between respiratory muscles pressure and height. This is likely to reflect an association between stature, diaphragm position and inspiratory muscle strength.
Nishimura showed that only lean body mass and abnormal body weight were associated with decreased respiratory strength [18].
To conclude, when we treat a patient with severe airflow obstruction we should consider the possible respiratory muscle deterioration that could affect this sort of patients. The periodical evaluation of the respiratory muscle strength could represent a further and helpful tool in monitoring the disease severity.
Our study is only a "snapshot" of the maximal respiratory pressures and their correlation with functional parameters at different stages of COPD severity in a hundred patients living in Rome.
Further studies on a larger population sample are needed to confirm our result.
Abbreviations
- COPD:
-
Chronic Obstructive Pulmonary Disease
- FEV1 :
-
Forced Expiratory Volume in one second
- FRC:
-
Functional Residual Capacity
- FVC:
-
Forced Vital Capacity
- MIP:
-
Maximal Inspiratory Pressure
- MEP:
-
Maximal Expiratory Pressure
- PEF:
-
Peak Expiratory Flow
- RV:
-
Residual Volume
- TLC:
-
Total Lung Capacity
- VC:
-
Vital Capacity
References
Black LF, Hyatt RE: Maximal respiratory pressure: normal values and relationship to age and sex. Am Rev Respis Dis 1969, 99:696–702.
Karvonen J, Saarelainen S, Nieminen MM: Measurements of respiratory muscle forces based on maximal inspiratory and expiratory pressures. Respiration 1994, 61:28–31.
Syabbalo N: Assessment of respiratory muscle function and strength. Postgrad Med J 1998, 74:208–215.
Neder JA, Andreoni S, Lerario MC, Nery LE: Reference values for lung function test, II. Maximal respiratory pression and voluntary ventilation. Brazilian Journal of Medical and Biological Research 1999, 32:719–727.
ATS/ERS statement on respiratory muscle testingAm J Respir Crit Care Med 2002, 166:518–624.
Terzano C: Il Polmone nelle malattie neuromuscolari. In Malattie dell'apparato respiratorio. Springer-Verlag; 2006:666–667.
Rochester DF: The diaphragm: contractile properties and fatigue. J Clin Invest 1985, 75:1397–1402.
Polla B, D'Antona G, Bottinelli R, Reggiani C: Respiratory muscle fibres: specialisation and plasticity. Thorax 2004, 59:808–817.
Epstein SK: An overview on respiratory muscle function. Clin Chest Med 1994, 15:619–39.
Iandell I, Gorini M, Misuri G, Gigliotti F, Rosi E, Duranti R, Scano G: Assessing inspiratory muscle strenght in patients with neurologic and neuromuscolar disease. Comparative evaluation of two noninvasive techiques. Chest 2001, 119:1108–1113.
Cheng BC, Chang WN, Chang CS, Tsai NW, Chang CJ, Hung PL, Wang KW, Chen JB, Tsai CY, Hsu KT, Chang HW, Lu CH: Predictive factors and long term outcome of respiratory failure after Guillain-Barre syndrome. Am J Med Sci 2004, 327:336–340.
Rochester DF: The respiratory muscle in COPD. Chest 1984, 85:47S-50S.
American Thoracic Society/European Respiratory Society: Dysfunction of skeletal muscle in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999,159(Suppl):1–40.
Rochester DF: Malnutrition and the respiratory muscles. Clin Chest Med 1986, 7:91–99.
Openbrier DR, Irwin MM, Rogers RM, Gottlieb GP, Dauber JH, Van Thiel DH, Pennock BE: Nutritional status and lung function in patients with emphysema and chronic bronchitis. Chest 1983, 83:17–22.
Decramer M, Stas KJ: Corticosteroids induced myophaty involving respiratory muscles in patient with chronic obsctuctive pulmonary disease and asthma. A Rev Respir Dis 1992, 146:800–802.
Van Balkom RHH, Zhan WZ: Corticosteroids effects on isotonic contractile properties of rat diaphragm muscle. J Appl Physiol 1997, 83:1062–1067.
Nishimura Y, Tsutsumi M, Nakata H, Tsunenari T, Maeda H, Yokoyama M: Relationship between respiratory muscle strenght and lean body mass in men with COPD. Chest 1995, 107:1232–1236.
Heijdra YF, Dekhuijzen PN, Van Herwaarden CL, Folgering HT: Effects of body position, hyperinflation, and blood gas tensions on maximal respiratory pressures in patients with chronic obstructive pulmonary disease. Thorax 1994, 49:453–458.
Kabitz HJ, Walterspacher S, Walker D, Windisch W: Inspiratory muscle strength in chronic obstructive pulmonary disease depending on disease severity. Clin Sci (Lond) 2007, 113:243–249.
Rochester DF, Braun NMT, Arora NS: Respiratory muscle strength in chronic obstructive pulmonary disease. Am Rev Respir Dis 1979, 119:151–154.
Orozco-Levi M: Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation? Eur Respir J 2003, 22:41S-51S.
Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F: Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med 1991, 325:917–923.
Walsh JM, Webber CL Jr, Fahey PJ, Sharp JT: Structural change of the thorax in chronic obstructive pulmonary disease. J Appl Physiol 1992, 72:1270–1278.
McKenzie DK, Gorman RB, Tolman J, Pride NB, Gandevia SC: Estimation of diaphragm length in patients with severe chronic obstructive pulmonary disease. Respir Physiol 2000, 123:225–234.
Gosker HR, Wouters EF, van der Vusse GJ, Schols AM: Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr 2000, 71:1033–47.
Laghi F, Tobin MJ: Disorders of the Respiratory Muscles. Am J Respir Crit Care Med 2003, 168:10–48.
Marchand E, Decramer M: Respiratory muscle function and drive in chronic obstructive pulmonary disease. Clin Chest Med 2000, 1:679–692.
Lewis MI, Sieck GC, Fournier M, Belman MJ: Effects of nutritional deprivation on diaphragm contractility and muscle fiber size. J Appl Physiol 1986, 60:596–603.
Lewis MI, Belman MJ: Nutrition and respiratory muscles. Clin Chest Med 1988, 9:337–358.
Gosselink R, Trooster T, Decramer M: Distrbution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2000, 20:353–360.
Bellemare JF, Cordeau MP, Leblanc P, Bellemare F: Thoracic dimension at maximum lung inflation in normal subject and in patients with obstructive and restrictive lung disease. Chest 2001, 119:376–386.
Wilson SH, Cooke NT, Edwards RHT, Spiro SG: Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax 1984, 39:535–538.
Leech JA, Ghezzo H, Stevens D, Becklake : Respiratory pressures and function in young adults. Am Rev Respir Dis 1983, 128:17–23.
Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE: Respiratory muscle strength in the elderly: correlates and reference value. Cardiovascular Health Study Research Group. Am J Respir Crit Care Med 1994, 149:430–438.
Acknowledgements
Our study was sponsored by the First School of Specialization in Respiratory Diseases, University of Rome "La Sapienza".
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
CT conceived the trial, participated in its design, study procedures, interpretation of results, performed the statistical analysis and helped to draft the manuscript. DC participated in the study procedures, in its design, interpretation of results, performed the statistical analysis and helped to draft the manuscript. VC participated in the study procedures, interpretation of results and helped to draft the manuscript. EG participated in study design and helped to draft the manuscript. AR participated in the study procedures and helped to draft the manuscript. AP participated in study design, study procedures, interpretation of results and helped to draft the manuscript. All of the authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Terzano, C., Ceccarelli, D., Conti, V. et al. Maximal respiratory static pressures in patients with different stages of COPD severity. Respir Res 9, 8 (2008). https://doi.org/10.1186/1465-9921-9-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1465-9921-9-8