Abstract
Background
Active smoking increases asthma severity and is related to diminished treatment efficacy. Animal models in which inhalation of both allergen and mainstream cigarette smoke are combined can help us to understand the complex interaction between both agents. We have recently shown that, in allergic mice, the airway inflammation can be cleared by repeated allergen challenge, resulting in the establishment of a state of inhalational tolerance.
Methods
In this study, we assessed in vivo the impact of cigarette smoke on the efficacy and time course of this form of tolerance induction. We exposed sensitized mice to concurrent mainstream cigarette smoke and allergen (Ovalbumin- OVA) and measured the airway inflammation at different time points.
Results
We first confirmed that aerosolized OVA administered for a prolonged time period (4–8 weeks) resulted in the establishment of tolerance. Concurrent OVA and smoke exposure for 2 weeks showed that tobacco smoke enhanced the Th-2 driven airway inflammation in the acute phase. In addition, the induction of the tolerance by repeated inhalational OVA challenge was delayed significantly by the tobacco smoke, since 4 weeks of concurrent exposure resulted in a more persistent eosinophilic airway inflammation, paralleled by a more mature dendritic cell phenotype. However, smoke exposure could not prevent the establishment of tolerance after 8 weeks of antigen exposure as shown by both histopathology (disappearance of the Th-2 driven inflammation) and by in vivo functional experiments. In these tolerized mice, some of the inflammatory responses to the smoke were even attenuated.
Conclusion
Cigarette smoke enhances acute allergic inflammation and delays, but does not abrogate the development of tolerance due to prolonged challenge with inhaled antigen in experimental asthma.
Similar content being viewed by others
Background
Immune-mediated tolerance encompasses a number of mechanisms by which the immune system avoids unnecessary inflammatory responses, not only in face of auto-allergens but also to harmless environmental antigens [1]. It has been suggested that the beneficial and often long-lasting effects of allergen specific immunotherapy in patients with allergy are due to a stimulation of these immune-suppressive mechanisms [2]. We and others have found that prolonged exposure to aerosolized allergen in sensitized mice is accompanied with a disappearance of the eosinophilic airway inflammation [3–6] under certain conditions of antigenic stimulation, resulting in a state of inhalational tolerance [3, 6]. Although a more persistent inflammation in experimental models of asthma can be obtained [7–10], the observation that the inflammation disappears under particular conditions of antigenic stimulation remains of potential importance, as it indicates that the vertebrate immune system has an inherent capacity to avoid unnecessary immunity and to restore homeostasis after an episode of allergen-induced airway inflammation. Studying the mechanisms involved in this phenomenon can provide us valuable new insights in the mechanisms regulating immune responses in the respiratory tract.
As a state of inhalational tolerance can be re-established in vivo [3, 6], the question arises of its susceptibility and robustness in face of the numerous environmental factors that are known to aggravate the allergic condition [11, 12]. One of the most important of such factors is mainstream cigarette smoke. To date, little is known about the effects of smoking on the efficacy of immunotherapy treatments in patients. Nevertheless, from animal models, we know that mainstream cigarette smoke has the potential to break primary inhalational tolerance to allergens in naïve animals [13] and to increase the systemic sensitization to surrogate and environmental allergens [14]. Several other studies have documented similar properties of second hand smoke or environmental tobacco smoke [15, 16]. More conflicting data were reported on the effects of mainstream cigarette smoke in an ongoing acute Th-2 driven inflammatory response. While concurrent exposure to allergen and cigarette smoke was shown to aggravate allergic airway inflammation [17], tobacco smoke exposure following an initial period of allergen challenge, attenuated airway hyperresponsiveness and airway inflammation [14, 18].
We hypothesized that mainstream cigarette smoke exposure could interfere with the induction of inhalational tolerance, thereby inducing a more persistent eosinophil-rich airway inflammation. This basic hypothesis seemed plausible from a theoretical point of view, since active smoking is associated with asthma exacerbations and reduced efficacy of treatment in humans [19] and mainstream cigarette smoke was able to break primary inhalational tolerance in naïve mice [13]. Active smoking could thus serve as an environmental factor that helps to explain why in patients with allergic asthma, the failure of immune tolerance persists. Therefore, we here analysed the effects of cigarette smoke exposure on the efficacy of inhalational tolerance induction in allergic mice.
Methods
Animals
Male C57BL/6 mice, 6 to 8 weeks old, were purchased from Harlan (Zeist, the Netherlands). All experimental procedures were approved by the local ethical committee for animal experiments (Faculty of Medicine and Health Sciences, Ghent University).
Experimental Protocols
Allergen Protocol 1
OVA aerosol exposure
1.1. Allergen Protocol 1A
Allergic airway inflammation
Two groups of 8 mice were sensitized with 10 μg intraperitoneal (i. p.) OVA (Grade III; Sigma, St-Louis, MO) adsorbed to 1 mg Al(OH)3 on day 0 (d0) and d7. From d14 onward, the mice were exposed to aerosolized (Ultraschallvernebler Sirius Nova, Heyer Medizintechnologie, Bad Ems, Germany) OVA (1% wt/v) or PBS 30 min/d, 3 times a week for 2 weeks (Figure 1 – Allergen Protocol 1A).
Cigarette smoke and allergen exposure protocols. Allergen exposure protocols (Allergen Protocols): Allergen Protocol 1A: Exposure of sensitized C57BL/6 mice to PBS or OVA aerosols for 2 weeks. (n = 8 mice/group). Allergen Protocol 1B: Prolonged PBS or OVA aerosol exposure in sensitized mice (8 weeks). (n = 8 mice/group). Allergen Protocol 2: OVA sensitized mice were exposed to OVA for 8 weeks ("chronic"), followed by HEL/alum immunisation and short-term ("acute") HEL aerosol challenge (OVA/HEL group). Control mice were exposed to PBS for 8 weeks, immunized with HEL/alum and challenged with either PBS (PBS/PBS group) or with HEL (PBS/HEL group). (n = 8–12 mice/group). Smoke exposure protocol (Smoke Protocol): C57BL/6 mice are exposed to Smoke or Air for 8 weeks. (n = 6 mice/group). Combined protocols (Combination Protocols):Combination Protocol 1: similar to Allergen Protocol 1B, combined with Smoke exposures or Air. (n = 8 mice/group). Combination Protocol 2: similar to Allergen Protocol 2, combined with or without Smoke. (n = 8 mice/group). Combination Protocol 3A: similar to Allergen Protocol 1A, combined with Smoke exposures or Air. (n = 8 mice/group). Combination Protocol 3B: 4 weeks of OVA aerosol or PBS exposures in sensitized mice, combined with Smoke exposures or Air. (n = 8 mice/group). Vertical arrows = aerosol challenges (OVA, HEL or PBS). Stripped horizontal bars = period of Smoke or Air exposures.
1.2. Allergen Protocol 1B
Tolerance
Two groups of mice (n = 8/group) were sensitized with i. p. OVA/alum on day 0 (d0) and d7. From d14 onward, the mice were subjected to prolonged OVA or PBS aerosol exposures for 8 weeks (Figure 1 – Allergen Protocol 1B).
Smoke Protocol
Mainstream cigarette smoke exposure in vivo
To show that exposure to mainstream cigarette smoke for 8 weeks is biologically active in C57BL/6 mice, two groups of 6 mice were exposed to either Smoke or Air for 8 weeks. Tobacco smoke exposures were performed in a plexiglass chamber (volume: 7500 cm3) with an inlet for pressured air (2.5 l/min). The chamber was connected to a smoking machine (D. Kobayashi, Washington University Medical Center, WA, USA). Exposures were performed four times a day, 5 days a week, with five Kentucky Reference cigarettes (2R4F, without filter) for six mice per exposure (Figure 1 – Smoke Protocol). The effects of this smoking protocol in mice at various other time points have been carefully characterized in earlier experiments by our laboratory[20]. Carboxyhemoglobin in the serum of mice exposed to this smoke protocol reached a non-toxic level of 8.3 ± 1.4% (compared with 1.0 ± 0.2% in air-exposed mice), which is similar to COHb blood concentrations of human smokers.
Combination Protocol 1
Smoke combined with long – term OVA aerosol exposure.
To evaluate the effect of cigarette smoke on the development of tolerance, four groups of mice (n = 8/group) were subjected to this tolerance induction protocol (OVA or PBS aerosols), combined with Smoke or Air. Smoke exposures were performed as described above in the Smoke protocol, with eight mice per exposure. Three times a week, smoke exposure was followed by OVA or PBS aerosols. The last smoke exposure took place 30 min before OVA or PBS aerosol. (Figure 1 – Combination Protocol 1).
Allergen Protocol 2
OVA and HEL aerosol exposures
To measure the immune responsiveness after chronic OVA exposure, OVA sensitized mice (n = 8–12/group), subjected to the tolerance protocol (8 wk OVA aerosols), were re-sensitized to a bystander allergen, Hen Egg Lysozyme (HEL, Sigma, St-Louis, MO; 10 μg HEL/alum i.p.) and re-challenged with daily HEL (1%) for 1 week ('OVA/HEL' group). OVA sensitized, PBS aerosol exposed mice were also re-immunized and challenged with HEL and were included as positive controls ('PBS/HEL' group). A negative control group ('PBS/PBS') was included as well (Figure 1 – Allergen Protocol 2).
Combination Protocol 2
Smoke combined with OVA and HEL aerosols
This experiment aimed to measure the immune responsiveness after chronic OVA and smoke exposure. OVA and Smoke or Air exposures were performed as in Combination Protocol 1 in two groups of mice (OVA/Smoke and OVA/Air groups; n = 8/group). The protocol was extended by subsequently re-sensitizing these mice to HEL (HEL/alum i. p.), and re-challenging them as described above. Meanwhile, the smoke exposures were continued in the test group ('OVA/HEL/Smoke' group), while remaining absent in the control group ('OVA/HEL/Air' group; Figure 1- Combination Protocol 2).
Combination Protocol 3
Smoke combined with short-term OVA aerosols
To evaluate effects of the smoke exposures on the time course of the tolerance induction, four groups of OVA sensitized mice (n = 8/group) were exposed to OVA or PBS aerosols for 2 and 4 weeks, combined with Smoke or Air. (Figure 1-Combination Protocols 3A and 3B respectively).
Broncho-alveolar lavage fluid (BALF): cellular analysis
Twenty-four hours after the last aerosol exposure, mice were sacrificed with i.p. pentobarbital (60 mg/kg; Sanofi, Libourne, France). BALF was taken by instillation of HBSS via a tracheal cannula. Three lavages with 0.3 ml HBSS followed by three lavages with 1 ml HBSS were performed. The recovered BALF of the first three fractions was centrifuged and the supernatant was used for cytokine detection. The cell pellet was then added to the rest of the lavage fluid, centrifuged, subjected to red blood cell lysis and resuspended for cell counts on cytospins (May-Grünwald/Giemsa).
Tissue processing and preparation of lung single-cell suspension for flow cytometric analysis
Following BALF, the pulmonary and systemic circulation was rinsed with saline-EDTA to remove the pulmonary intravascular pool of cells. The right lung was thoroughly minced and incubated in digestion medium at 37°C containing DNase I (grade II from bovine pancreas; Boehringer Ingelheim, Germany) and collagenase type II (Worthington Biochemical Corp., Lakewood, NJ). After obtaining a single-cell suspension, samples were centrifuged and resuspended in PBS containing 10 mM EDTA. Finally, cells were subjected to red blood cell lysis, washed and kept on ice until labelling. Cell counting was performed with a Z2 Beckman-Coulter particle counter (Beckman-Coulter, Ghent, Belgium). Flow cytometric analysis of dendritic cells and T-lymphocytes was defined as detailed previously[20]. Briefly, mouse dendritic cells populations were identified as low autofluorescent, CD11chigh MHCII+ cells. The maturation status of the dendritic cells was analysed by assessing the CD86 (B7.2) expression. Mouse T cell subpopulations were identified as CD3+ and CD4+ or CD8+ cells. CD69 expression was analysed to assess the activation status of the T cells. Monoclonal antibodies used to identify mouse dendritic cells populations were biotinylated anti-CD11c and phycoerythrin (PE)-conjugated anti-IAb and anti-CD86. Rat IgG2a-PE was used as isotype control. Markers used for mouse T-cell subpopulations staining were CD3-APC, CD4-FITC, CD8-FITC and CD69-PE (activation marker). All other monoclonal antibodies were obtained from Becton Dickinson Pharmingen (BD, Erembodegem, Belgium), except anti-CD11c (N418 hybridoma; gift from M. Moser, Brussels Free University, Belgium). Flow cytometry data acquisition was performed on a duallaser FACSCalibur™ flow cytometer running CELLQuest™ software (BD, Mountain View, CA, USA). FlowJo software (Treestar Inc., Ashland, OR) was used for data analysis.
Histology
After fixation of the left lung with 4% paraformaldehyde, slices from the left lobes were embedded in paraffin for histological analysis. Sections of 2 μm were stained with Congo Red, counter-stained with hematoxylin, to highlight eosinophils.
Semi-quantitative analysis of peribronchial inflammation
The slides were coded and the peribronchial (and perivascular) inflammation was graded in a blinded fashion using a reproducible scoring system [21]. A value from 0 to 3 was adjudged to each tissue section scored. A value of 0 was given when no inflammation was detectable, a value of 1 for occasional cuffing with inflammatory cells, a value of 2 when most bronchi were surrounded by a thin layer (1 to 5 cells) of inflammatory cells and a value of 3 when most bronchi were surrounded by a thick layer (>5 cells) of inflammatory cells. As 5–7 tissue sections per mouse were scored, inflammation scores could be expressed as a mean value per animal and could be compared between groups.
Lymph node cultures
Paratracheal and parathymic intrathoracic lymph nodes were removed. These mediastinal lung-draining lymph nodes were harvested into Tissue Culture Medium (TCM) containing tubes and digested enzymatically (collagenase/DNase) to obtain a single-cell suspension. Cells were cultured in TCM in a flat-bottom, 96 well plate (BD) alone or with 3 μg OVA or HEL, at a density of 8 × 105 cells per well. After 5 days of culture, supernatants were harvested and used for cytokine measurements.
IgE, cytokines and chemokines (ELISA)
Total, OVA- and HEL-specific serum IgE was measured with ELISA using coated microtiter plates and biotinylated polyclonal rabbit anti-mouse IgE (S. Florquin, ULB, Brussels, Belgium). OVA- and HEL- specific IgE are expressed in arbitrary units (U) per ml serum. ['OVA-IgE Units' (OVA-U) or 'HEL-IgE Units' (HEL-U)/ml]. One 'OVA-IgE Unit' was defined as a 1/100 dilution of an internal standard serum pool obtained from OVA (10 μg)/alum (1 mg) sensitized mice. One 'HEL-IgE Unit' was defined as a 1/100 dilution of an internal standard serum pool obtained from HEL (10 μg)/alum (1 mg) sensitized mice.
IL-13, TARC, IFN-γ, MCP-1, KC and eotaxin were determined on BALF fluid supernatant and IL-13, TARC, IFN-γ were measured on the supernatant of cultured lymph node cells using ELISA kits (R&D Systems, Abingdon, UK).
Statistical analysis
Data were analysed with the statistical packet SPSS 15.0 (SPSS Inc.; Chicago, IL). Values are expressed as mean +/- Standard Error of the Mean (SEM). Groups were compared using the Kruskall-Wallis test for screening significant differences between the groups. When p < 0.05, Mann-Whitney U-test with Bonferroni's conservative corrections were applied to compare the individual groups.
Results
1) Biological effects of repeated exposures to allergen or smoke
In a first experiment, sensitized C57BL/6 mice were challenged with OVA aerosol for 2 weeks to induce an asthma-like Th-2 driven inflammatory response in the airways (Figure 1 – Allergen Protocol 1A). Next, we prolonged the exposure to OVA aerosols up to 8 weeks (Figure 1- Allergen Protocol 1B). As previously described [3], this treatment made the allergen-induced eosinophilic airway inflammation disappear completely (data not shown).
Next, to document the biological effect of the applied smoke protocol for 8 weeks, C57BL/6 mice were exposed to either Smoke or Air for 8 weeks (Figure 1- Smoke Protocol). Analysis showed that BALF leukocytes, macrophages, neutrophils and lymphocytes were significantly increased in smoke exposed mice (Figure 2A). Furthermore, MCP-1 (Monocyte Chemotactic Protein-1 or CCL2) and KC (cytokine-induced neutrophil chemo-attractant or mouse IL-8, CXCL chemokine) were elevated in BALF supernatant (Figure 2B). Histopathology showed that the bronchi were infiltrated with mononuclear cells and neutrophils after 8 weeks exposure to cigarette smoke (not shown).
2) The fading of allergic inflammation by prolonged OVA aerosol challenge is not reversed by cigarette smoke
Next, we evaluated the effects of smoke exposure on the establishment of immune tolerance. Four groups of OVA-sensitized C57BL/6 mice (n = 8/group) were exposed to Smoke or Air combined with OVA or PBS aerosols for 8 weeks (Figure 1- Combination Protocol 1).
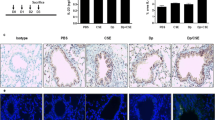
Airway inflammation
Mice exposed for 8 weeks to OVA inhalations only ('OVA/Air') displayed no detectable airway inflammation. Histology (Figure 3), cytology of BALF and lung (Figure 4A–D), and Th-1/Th-2 cytokines (Figure 4E–F) were comparable between PBS/Air and OVA/Air exposed mice. In contrast, mice exposed to smoke alone without OVA ('PBS/Smoke'), displayed a mild inflammatory response in the airways due to the effects of cigarette smoke, characterized by neutrophils, macrophages and dendritic cells in BALF and lung tissue (Figure 3 and 4). There was an increase in number and percentage of activated T-cells (CD4+CD69+ and CD8+CD69+ T-cells) in the lung tissue.
Results obtained from combining 8 weeks OVA or PBS aerosol exposure with Smoke or Air in sensitized C57BL/6 mice(Combination Protocol 1). (A.) BALF analysis of total cell counts and differentiation after prolonged exposure (8 weeks) to a combination of OVA or PBS aerosols and Smoke or Air. (B) Evaluation of the peribronchial inflammation via a semi-quantitative method (Mean score/airway). (C) Percentage of T – lymphocytes in lung that are CD4+CD69+ (as a percentage within the T cell population). (D) Percentage of dendritic cells in digested lung tissue (percentage of total lung cells). (E) Th-2 cytokine (IL-13 and TARC) profiles in BALF (pg/ml). (F) Cytokines MCP-1 and KC in BALF (pg/ml). n = 8 mice/group; bars indicate mean ± SEM. * p ≤ 0.05: all groups vs PBS/Air (Mann-Whitney U test); + p ≤ 0.05: PBS/Smoke vs OVA/Smoke (Mann-Whitney U test); # p ≤ 0.05: OVA/Smoke vs OVA/Air (Mann-Whitney U test); ND = Not Detectable (detection limit = 1–2 pg/ml)
In mice exposed to both OVA and smoke ('OVA/Smoke'), we did not observe a re-activation of Th-2 driven airway inflammation. In particular, there was no eosinophilic influx in BALF or in the airways as compared to the other groups (Figure 3 and 4A–B). The numbers and percentages of dendritic cells in BALF (data not shown) and lung and activated lung T-cells were comparable with mice exposed to cigarette smoke only (Figure 4C and 4D). Remarkably, the increase in BALF neutrophilia was significantly less pronounced as compared to the mice that were exposed to cigarette smoke only (PBS/Smoke vs OVA/Smoke, p < 0.05), which indicates an attenuated inflammatory response to the smoke in tolerized mice (Figure 4A). Airway histology showed infiltration of mononuclear cells and neutrophils in both smoke -exposed groups, while the infiltration was absent in mice exposed to PBS/Air or OVA/Air. Again this tissue inflammatory reaction was significantly less pronounced in the OVA/Smoke group as compared to the PBS/Smoke group (Figure 3 and 4B).
Cytokines
IL-13 in the BALF was around (PBS/Smoke group) or below (other groups) the detection limit (1–2 pg/ml) (Figure 4E). TARC was elevated in response to cigarette smoke, and this increase was less pronounced when OVA and smoke were combined (Figure 4E). MCP-1 and KC were elevated in both cigarette smoke exposed groups, but this increase tended to be lower in the group with combined exposures (For MCP-1: p = 0.083 PBS/Smoke vs OVA/Smoke; for KC: p = 0.065 PBS/Smoke vs OVA/Smoke; Figure 4F). Eotaxin in BALF was below the detection limit in all groups.
In the supernatant of cultured lymph node cells, higher constitutive levels of the Th-2 cytokine IL-13 were measured, but no differences between the groups were found (data not shown). IFN-γ was elevated in supernatant of cultured lymph node cells in response to smoke (data not shown). Again, this increase in IFN-γ was less pronounced in the group were cigarette smoke was combined with OVA aerosols (p < 0.05 PBS/Smoke vs OVA/Smoke).
Serum IgE
Total- and OVA-specific serum-IgE were comparably elevated in both OVA-exposed groups.(OVA-IgE: 25.75 ± 8.51 U/ml in OVA/Air vs 35.40 ± 9.47 U/ml in OVA/Smoke; p = 0.38).
3) Mainstream cigarette smoke exposure does not reverse the induction of functional bystander tolerance
Next, as combined OVA and mainstream cigarette smoke exposure for 8 weeks induced neither a persistent allergic airway inflammation nor an allergy exacerbation, we evaluated at the functional level in vivo whether the tolerance mechanisms effectively remained active. Therefore, we implemented Allergen protocol 2, but in the presence of cigarette smoke (Figure 1- Combination Protocol 2). The first group of mice was exposed to 8 weeks of OVA, then re-immunized and re-challenged with HEL ('OVA/HEL/Air' group); the second group was identically treated, but was concurrently exposed to cigarette smoke ('OVA/HEL/Smoke' group).
The inflammatory cells in BALF as well as the tissue inflammation and serum HEL-specific IgE (not shown) showed that there was no renewal of Th-2 driven eosinophilic airway disease in the OVA/HEL/Smoke group (Figure 5). This was also underscored by the measurements of Th-1/Th-2 cytokines in BALF (IL-13, TARC and eotaxin were below detection limit in both groups) and in supernatant of cultured lymph node cells (not shown). The only parameters that were different between both groups were neutrophils in BALF and neutrophils and mononuclear cell infiltrates around the airways (histology) in the OVA/HEL/Smoke group, to be attributed to the effects of the tobacco smoke.
Results obtained 8 weeks of OVA aerosols followed by re-sensitisation (i. p.) and re-challenge to HEL aerosols (Allergen Protocol 2) combined with concurrent Smoke or Air-exposures (Combination Protocol 2). (A) BALF cell differentiation. (B) Peribronchial inflammation (Mean score/airway). n = 8 mice/group; bars indicate mean ± SEM. * p ≤ 0.05: OVA/HEL/Smoke vs OVA/HEL/Air (Mann-Whitney U test);
4) Cigarette smoke exposure enhances the magnitude of an acute inflammatory response and slows down the time course of tolerance induction
Finally, we tested whether combined OVA aerosol and mainstream cigarette smoke exposure could have an influence on the time course of the tolerance induction. In order to investigate this, we concurrently exposed four groups of mice to Smoke or Air and OVA or PBS aerosols for 2 and 4 weeks (Figure 1- Combination Protocols 3A and 3B respectively).
Combined exposure for 2 weeks (Combination Protocol 3A) resulted in an augmented acute Th-2 mediated airway inflammation in OVA/Smoke exposed mice compared with OVA/Air exposed mice. This was evident from BALF analysis (Figure 6A), Th-2 cytokines (Figure 6B), histology (not shown) and lung dendritic cells and activated CD4+ T cells (Table 1). KC and MCP-1 were elevated in BALF of both smoke-exposed groups of mice, while eotaxin was only detectable in both OVA-exposed groups (not shown). CD86 expression on dendritic cells was increased in response to OVA exposure alone, as well as in response to combined OVA/Smoke exposure (Table 1). OVA-IgE tended to be higher in OVA/Smoke compared to OVA/Air exposed mice (Table 1; p = 0.074 OVA/Air vs OVA/Smoke).
BALF cell differentiation and cytokines (pg/ml) IL-13 and TARC after respectively 2 (Fig. 6A and 6B) and 4 (Fig. 6C and 6D) weeks of concurrent exposure to OVA/PBS and Smoke or Air in C57BL/6 mice (Combination Protocol 3A and 3B). n = 8 mice/group; bars indicate mean ± SEM. * p ≤ 0.05: all groups vs PBS/Air (Mann-Whitney U test); + p ≤ 0.05: OVA/Smoke vs. PBS/Smoke (Mann-Whitney U test); # p ≤ 0.05: OVA/Smoke vs. OVA/Air (Mann-Whitney U test); ND = Not Detectable (detection limit = 1–2 pg/ml)
Next, we investigated the influence of combined exposures on the time course of the tolerance induction by giving the mice both stimuli for 4 weeks (Combination Protocol 3B). The eosinophilic airway inflammation had almost completely disappeared in OVA/Air exposed mice. In contrast, a limited though significant airway eosinophilia remained present in OVA/Smoke mice (Figure 6C). IL-13 was below the detection limit in all groups. TARC (Figure 6D) was elevated in the OVA/Smoke exposed group compared to the OVA/Air group. The eosinophil chemo-attractant eotaxin in BALF was only detectable in the BALF of OVA/Smoke mice (5.20 ± 0.49 pg/ml), while being below the detection limit (<1–2 pg/ml) in the other groups. KC and MCP-1 were elevated in both smoke-exposed groups (data not shown). The observations in BALF were further underscored by measurements of IL-13 and TARC on supernatant of cultured lung-draining lymph node cells and by histology of the airways (not shown). The number of dendritic cells in the lung was elevated in both smoke- exposed groups, while activated CD4+ T cells were elevated in the OVA/Smoke group as compared to mice exposed to OVA alone (Table 1). The percentage of CD86 positive lung dendritic cells was significantly increased compared to PBS/Air mice in response to smoke alone and OVA/Smoke, but not to OVA alone (Table 1). Serum OVA-IgE remained elevated in both OVA challenged groups, but cigarette smoke exposure had, as for 2 weeks, no significant additive effect (Table 1; p = 0.40, OVA/Air vs OVA/Smoke).
Figure 7 represents a time curve to summarize these findings. We selected two important parameters, namely BALF eosinophilia and TARC. This figure illustrates the effects of combined cigarette smoke and OVA aerosol exposure for 2, 4 and 8 weeks. Figure 7 shows that cigarette smoke exposure initially augmented airway eosinophilia and BALF TARC levels and later on slowed down the onset of tolerance.
Discussion
The objective of this study was to evaluate the effects of mainstream cigarette smoke in a mouse model of inhalational tolerance. We show that a state of disease inhibition or 'tolerance', as we refer to this state of unresponsiveness to re-sensitization and re-challenge, can be induced in mice suffering allergic airway inflammation, despite concurrent exposure to both mainstream cigarette smoke and inhaled allergen. The smoke – induced inflammation even appeared attenuated in these tolerized mice. Importantly, mainstream cigarette smoke enhanced the acute allergic inflammation and slowed down the time course of tolerance induction.
We combined the 8 weeks OVA aerosol exposure protocol, sufficient to induce tolerance in sensitized C57BL/6 mice, with cigarette smoke (Combination Protocol 1) and found that cigarette smoke did not prevent the establishment of tolerance due to prolonged OVA aerosol challenge. In general, functional tolerance in vivo can be proven by re-immunization and challenge with the same or with bystander allergens. This experimental approach has been used to prove primary inhalational tolerance [22] or oral tolerance [23] in naïve mice. We previously also applied the same approach to this model of prolonged OVA aerosol challenge and we discovered that, after the eosinophilic inflammation had disappeared, re-immunization and short-term allergen re-challenge did not result in the re-appearance of allergic airway disease, and that this phenomenon had memory characteristics [3]. Here, re-immunization and re-challenge of the mice with the secondary antigen HEL in the presence of cigarette smoke (Combination Protocol 2) additionally showed that, also at the functional level, no Th-2 mediated immune responses could be mounted, despite the cigarette smoke. In line with these findings, one recent study also found no evidence of increased allergic inflammatory responses in a model of combined long-term OVA and tobacco smoke exposure, although another method of tobacco smoke exposure (nose-only) was used [24].
Remarkably, some parameters indicated that the inflammatory response to smoke was even attenuated in the mice concurrently subjected to the 8 weeks inhalational tolerance protocol. These findings suggest that the tolerance mechanisms could also dampen the airways' response to other pro-inflammatory stimuli or airway irritants. Since one of the proposed mechanisms is the induction of regulatory T cell activity, it is interesting to note that allergen-induced regulatory T cells can inhibit both Th-2 [25] and Th-1 responses in vivo, while they can also dampen the activation of the innate immune system [1]. However, in contrast with primary inhalational tolerance in naïve animals, where an important role for regulatory T cells has been documented [22, 26], we previously could not find any increases in the numbers of 'naturally occurring' CD4+CD25+Foxp3+ regulatory T cells in lung tissue upon chronic antigen inhalation [3]. Although this finding did not preclude the induction of other regulatory T cells subsets and gave no definite answer about the activity of these cells, it is tempting to speculate that regulatory T cell-independent mechanisms might be involved, such as induction of anergy, depletion of the T cells, and immunosuppressive activity of alveolar macrophages. In support of this hypothesis, inhibition of immune responses by repeated allergen challenge could also be obtained in one model in the absence of regulatory T cells, as shown by adoptive transfer experiments in RAG1-/- mice [27]. Recent data also showed the involvement of complement factor C5a in inhalational tolerance in naïve mice [28], although conflicting results have been published about its precise role during the course of an allergic inflammatory response [28–30]. Consequently, the role of the complement in inducing and/or maintaining inhalational tolerance through prolonged allergen challenge in sensitized mice has to be delineated further.
Although we had to reject our primary hypothesis that cigarette smoke could prevent tolerance establishment, we here documented that cigarette smoke exposure could influence the kinetics of the tolerance induction. Firstly, 2 weeks of concurrent exposure (Combination Protocol 3A) augmented the acute allergic airway inflammation in experimental asthma. This confirmed our previous observations of the aggravating effects of cigarette smoke on acute allergic inflammation in a BALB/c model of acute allergic airway inflammation [17]. In this model, a significant increase in OVA-IgE was also observed in response to concurrent short-term OVA and smoke exposure, while here only a trend was observed. These differences in response can be attributed to the fact that the BALB/c strain is naturally a high-IgE responder [31], in contrast to the C57BL/6 strain used here, which is lower-IgE responder to systemic sensitization. Secondly, combined exposures for 4 weeks (Combination Protocol 3B) showed that cigarette smoke significantly slowed down the onset of tolerance. We found persistence of airway eosinophilia and Th-2 mediators in OVA/Smoke mice but not in OVA/Air mice. This result clearly showed that tobacco smoke could influence the time course of the tolerance establishment.
A potential underlying mechanism involved in these phenomena is that mainstream tobacco smoke could exert pro-allergic effects by enhancing the maturation status and migration of the dendritic cells in the airways, either directly via its effects on the sub-epithelial dendritic cells [32, 33] or indirectly via stimulating the airway epithelium to release dendritic cell maturation stimuli such as TSLP and GM-CSF [34]. Over-expression of these growth factors in the airways have been shown to break inhalational tolerance [4, 35, 36]. Recently, a more mature dendritic cell phenotype has also been found in smokers [37]. On the other hand, inhibition of dendritic cell maturation in response to smoke has also been described in other studies using different exposure protocols [38], or in vitro conditioning of human dendritic cells with cigarette smoke extract [39], as well as in some human studies [40]. Although we here found a significant increase in the expression of CD86 on pulmonary dendritic cell after 4 weeks of OVA/Smoke exposure, the exact role of the dendritic cell maturation status and migration to the lymph nodes in the exacerbated acute inflammation and the delay in tolerance induction remains to be established.
It is also possible that other cigarette smoke -related factors can co-determine the immune responses in our model at the various time points, either directly or indirectly by influencing the dendritic cells. Lipopolysaccharide (LPS) is, among other danger signals, abundantly present in tobacco smoke. The effects of LPS on the immune system in general, and on the dendritic cell-T cell axis in particular, are dose dependent. As such, it was shown that low doses of LPS favour Th-2 responses whereas higher dosages usually suppress Th-2 responses in both humans and mice [41–45]. Concurrent allergen and cigarette smoke for longer time periods could thus have totally different effects on the eosinophilic inflammation than concurrent short-term exposures.
Relating to the clinic, it still remains to be established whether prolonged immune stimulation by the continuous 'therapeutic' administration of allergen in order to either exhaust allergen specific Th-2 memory cell populations or induce (regulatory T cell mediated) tolerance is a feasible approach [46]. Nevertheless, our data here indicate that cigarette smoke could interfere with such approaches.
Conclusion
This study shows that allergen-induced tolerance can be established in experimental asthma despite concurrent mainstream tobacco smoke exposure. Nevertheless, tobacco smoke exposure enhances the acute allergic airway inflammation and slows down the induction of tolerance, resulting in a more persistent eosinophil-rich airway inflammation.
Abbreviations
- OVA:
-
Ovalbumin
- HEL:
-
Hen Egg Lysozyme
- PBS:
-
Phosphate-Buffered Saline
- BALF:
-
Broncho-alveolar Lavage Fluid
- TCM:
-
Tissue Culture Medium
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- TARC:
-
Thymus-and Activation Regulated Chemokine
- MCP-1:
-
Monocyte Chemotactic Protein-1
- KC:
-
cytokine-induced neutrophil chemoattractant
- TSLP:
-
Thymic Stromal Lymphopoietin
- GM-CSF:
-
Granulocyte-Macrophage Colony Stimulating Factor
- LPS:
-
Lipopolysaccharide
References
O'Garra A, Vieira P: Regulatory T cells and mechanisms of immune system control. Nat Med 2004, 10:801–805.
Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, Akdis CA: IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol 2003, 33:1205–1214.
Van Hove CL, Maes T, Joos GF, Tournoy KG: Prolonged Inhaled Allergen Exposure Can Induce Persistent Tolerance. Am J Respir Cell Mol Biol 2007, 36:573–584.
Swirski FK, Sajic D, Robbins CS, Gajewska BU, Jordana M, Stampfli MR: Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J Immunol 2002, 169:3499–3506.
Swirski FK, D'Sa A, Kianpour S, Inman MD, Stampfli MR: Prolonged ovalbumin exposure attenuates airway hyperresponsiveness and T cell function in mice. Int Arch Allergy Immunol 2006, 141:130–140.
Schramm CM, Puddington L, Wu C, Guernsey L, Gharaee-Kermani M, Phan SH, Thrall RS: Chronic inhaled ovalbumin exposure induces antigen-dependent but not antigen-specific inhalational tolerance in a murine model of allergic airway disease. Am J Pathol 2004, 164:295–304.
Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M: Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med 2004, 169:378–385.
Wegmann M, Fehrenbach H, Fehrenbach A, Held T, Schramm C, Garn H, Renz H: Involvement of distal airways in a chronic model of experimental asthma. Clin Exp Allergy 2005, 35:1263–1271.
Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK: An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax 1998, 53:849–856.
Siegle JS, Hansbro N, Herbert C, Yang M, Foster PS, Kumar RK: Airway hyperreactivity in exacerbation of chronic asthma is independent of eosinophilic inflammation. Am J Respir Cell Mol Biol 2006, 35:565–570.
D'Amato G, Liccardi G, D'Amato M, Holgate S: Environmental risk factors and allergic bronchial asthma. Clin Exp Allergy 2005, 35:1113–1124.
Eder W, Ege MJ, von Mutius E: The asthma epidemic. N Engl J Med 2006, 355:2226–2235.
Moerloose KB, Robays LJ, Maes T, Brusselle GG, Tournoy KG, Joos GF: Cigarette smoke exposure facilitates allergic sensitization in mice. Respir Res 2006, 7:49.
Robbins CS, Pouladi MA, Fattouh R, Dawe DE, Vujicic N, Richards CD, Jordana M, Inman MD, Stampfli MR: Mainstream cigarette smoke exposure attenuates airway immune inflammatory responses to surrogate and common environmental allergens in mice, despite evidence of increased systemic sensitization. J Immunol 2005, 175:2834–2842.
Rumold R, Jyrala M, az-Sanchez D: Secondhand smoke induces allergic sensitization in mice. J Immunol 2001, 167:4765–4770.
Seymour BW, Pinkerton KE, Friebertshauser KE, Coffman RL, Gershwin LJ: Second-hand smoke is an adjuvant for T helper-2 responses in a murine model of allergy. J Immunol 1997, 159:6169–6175.
Moerloose KB, Pauwels RA, Joos GF: Short-term cigarette smoke exposure enhances allergic airway inflammation in mice. Am J Respir Crit Care Med 2005, 172(2):168–172.
Melgert BN, Postma DS, Geerlings M, Luinge MA, Klok PA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN: Short-term smoke exposure attenuates ovalbumin-induced airway inflammation in allergic mice. Am J Respir Cell Mol Biol 2004, 30:880–885.
Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, Deykin A, Dimango E, Fish JE, Ford JG, Israel E, Kiley J, Kraft M, Lemanske RF Jr., Leone FT, Martin RJ, Pesola GR, Peters SP, Sorkness CA, Szefler SJ, Wechsler ME, Fahy JV: Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med 2007, 175:783–790.
D'hulst AI, Vermaelen KY, Brusselle GG, Joos GF, Pauwels RA: Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur Respir J 2005, 26:204–213.
Curtis JL, Byrd PK, Warnock ML, Kaltreider HB: Requirement of CD4-positive T cells for cellular recruitment to the lungs of mice in response to a particulate intratracheal antigen. J Clin Invest 1991, 88:1244–1254.
Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A: Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest 2004, 114:28–38.
Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA: Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest 2005, 1923–1933.
Melgert BN, Timens W, Kerstjens HA, Geerlings M, Luinge MA, Schouten JP, Postma DS, Hylkema MN: Effects of 4 months of smoking in mice with ovalbumin-induced airway inflammation. Clin Exp Allergy 2007, 37(12):1798–1808.
Cottrez F, Hurst SD, Coffman RL, Groux H: T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol 2000, 165:4848–4853.
Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M: CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med 2005, 202:1549–1561.
Niu N, Le Goff MK, Li F, Rahman M, Homer RJ, Cohn L: A novel pathway that regulates inflammatory disease in the respiratory tract. J Immunol 2007, 178:3846–3855.
Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, Whitsett JA, Gerard C, Sfyroera G, Lambris JD, Wills-Karp M: A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest 2006, 116:783–796.
Drouin SM, Sinha M, Sfyroera G, Lambris JD, Wetsel RA: A protective role for the fifth complement component (c5) in allergic airway disease. Am J Respir Crit Care Med 2006, 173:852–857.
McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG: Allergens induce enhanced bronchoconstriction and leukotriene production in C5 deficient mice. Respir Res 2006, 7:129.
Hamelmann E, Tadeda K, Oshiba A, Gelfand EW: Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model. Allergy 1999, 54:297–305.
Nouri-Shirazi M, Tinajero R, Guinet E: Nicotine alters the biological activities of developing mouse bone marrow-derived dendritic cells (DCs). Immunol Lett 2007, 109:155–164.
Nouri-Shirazi M, Guinet E: A possible mechanism linking cigarette smoke to higher incidence of respiratory infection and asthma. Immunol Lett 2006, 103:167–176.
Holgate ST: The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol 2007, 28:248–251.
Zhou B, Comeau MR, De ST, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF: Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 2005, 6:1047–1053.
Swirski FK, Gajewska BU, Robbins CS, D'Sa A, Johnson JR, Pouladi MA, Inman MD, Stampfli MR: Concomitant airway expression of granulocyte-macrophage colony-stimulating factor and decorin, a natural inhibitor of transforming growth factor-beta, breaks established inhalation tolerance. Eur J Immunol 2004, 34:2375–2386.
Bratke K, Klug M, Bier A, Julius P, Kuepper M, Virchow JC, Lommatzsch M: Function-associated Surface Molecules on Airway Dendritic Cells in Cigarette Smokers. Am J Respir Cell Mol Biol 2008,175(8):783–790.
Robbins CS, Dawe DE, Goncharova SI, Pouladi MA, Drannik AG, Swirski FK, Cox G, Stampfli MR: Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol 2004, 30:202–211.
Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L: Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of th-2 priming. J Immunol 2005, 175:2684–2691.
Tsoumakidou M, Elston W, Zhu J, Wang Z, Gamble E, Siafakas NM, Barnes NC, Jeffery PK: Cigarette Smoking Alters Bronchial Mucosal Immunity in Asthma. Am J Respir Crit Care Med 2007, 175(9):919–925.
Alexis NE, Lay JC, Almond M, Peden DB: Inhalation of low-dose endotoxin favors local T(H)2 response and primes airway phagocytes in vivo. J Allergy Clin Immunol 2004, 114:1325–1331.
Alexis NE, Lay JC, Almond M, Bromberg PA, Patel DD, Peden DB: Acute LPS inhalation in healthy volunteers induces dendritic cell maturation in vivo. J Allergy Clin Immunol 2005, 115:345–350.
Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, Lauener RP, Schierl R, Renz H, Nowak D, von Mutius E: Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 2002, 347:869–877.
Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z: Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol 2007, 178:5375–5382.
Lundy SK, Berlin AA, Lukacs NW: Interleukin-12-independent down-modulation of cockroach antigen-induced asthma in mice by intranasal exposure to bacterial lipopolysaccharide. Am J Pathol 2003, 163:1961–1968.
Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL: Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol 2008, 8:142–152.
Acknowledgements
This project is supported by the Fund for Scientific Research – Flanders (FWO-Vlaanderen – Project G.0052.06) and by the Interuniversity Attraction Poles programme (IUAP) – Belgian state – Belgian Science Policy P6/35. Kurt G. Tournoy is a senior clinical investigator granted by FWO-Vlaanderen. The authors are indebted to Eliane Castrique, MR. Mouton, Ann Neessen, Indra De Borle, Christelle Snauwaert, Kathleen De Saedeleer for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CVH and KT were involved in the conception and design of the studies. CVH and KM carried out the laboratory experiments under supervision of TM. CVH, GJ and KT drafted the manuscript. All authors approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Van Hove, C.L., Moerloose, K., Maes, T. et al. Cigarette smoke enhances Th-2 driven airway inflammation and delays inhalational tolerance. Respir Res 9, 42 (2008). https://doi.org/10.1186/1465-9921-9-42
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1465-9921-9-42