Abstract
Racemic albuterol is an equimolar mixture of two isomers, (R) and (S). Whether (R) and (S) isomers and the combination of both exert different effects in immune activation is not well defined. We analyzed the effects of (R+S)-albuterol, (R)-albuterol and (S)-albuterol in a murine model of allergic pulmonary inflammation and in activated T cells. Mice (C57BL/6) sensitized and aerosol challenged with the allergen ovalbumin (OVA) or phosphate buffered saline (PBS) were treated with (R)-albuterol, (S)-albuterol or (R+S)-albuterol. Following administration of (R)-albuterol, allergen induced bronchoalveolar lavage eosinophils and IgE showed a decrease, albeit not significantly by ANOVA. As T cells are important in allergic inflammation, we asked whether (R+S), (R) or (S)-albuterol might differ in effects on T cells and on the activity of the inflammatory transcription factor NF-κB. In activated T cells, (R)-albuterol administration decreased levels of inflammatory cytokines and NF-κB activity. These studies suggest that (R)-albuterol decreases cytokine secretion and NF-κB activity in T cells.
Similar content being viewed by others
Introduction
Allergic inflammation is characterized by enhanced T cell activation leading to the production of inflammatory cytokines and initiation of pathways such as tyrosine kinase Syk involving mast cells, eosinophils, and immunoglobulin E [1–4]. In asthma, this process leads to a phenotype characterized by bronchial inflammation and airway hyperresponsiveness. Activated T cells secrete cytokines that are pivotal in the pathogenesis of atopic asthma [5–7]. Further studies have elucidated the key role played by T cell costimulatory pathways [8, 9]
The cornerstone of asthma therapy is inhaled β2-adrenergic agonists in combination with inhaled and systemic steroids. Conventionally, inhaled beta agonists such as albuterol induce rapid bronchodilation, yet they also demonstrate anti-inflammatory properties [10, 11]. T cells possess surface β-adrenergic receptors [12] which upon stimulation activate protein kinase A (PKA) and induce cAMP, altering cytokine production. Whether beta agonists can impact allergic inflammation by regulating T cell activation remains undefined.
Beta agonists are commonly available as racemic mixtures composed of equimolar mixtures of (R)- and (S)- enantiomers. Interestingly, the pharmacokinetic properties and, at times, the biological effects of these isomers differ. (R)-albuterol binds to the β2-adrenergic receptor with high affinity, whereas (S)-albuterol exhibits weak binding to the β2-adrenergic receptor [13]. Studies of the pharmacokinetics of racemic albuterol have shown that elimination of (R)-albuterol is much more rapid than that of (S)-albuterol [14, 15]. Whereas the (R)-isomer induces bronchodilation [16], (S)-albuterol may induce airway hyperresponsiveness [17]. Also, (R)-albuterol demonstrates anti-inflammatory effects in both airway smooth muscle cells and T lymphocytes, while (S)-albuterol does not [18, 19]. Furthermore, β2 agonists may also augment surfactant secretion, decrease lung endothelial permeability, and decrease airway resistance [20].
In this study, we investigated whether albuterol isomers modulate effects on allergic responses in vivo in a murine model of allergic inflammation and, in vitro, in activated T cells. Additionally, we investigated whether activity of nuclear factor κ-B (NF-κB), which is an important transcription factor involved in the regulation of inflammatory processes including asthma, is regulated by albuterol isomers [21, 22].
Methods
Mice
Six to 8-wk-old C57BL/6 female mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The mice were maintained according to the guidelines of the committee on animals of the Harvard Medical School and the University of California, San Diego animal facility. Both institutions are accredited by the American Association for Accreditation of Laboratory Animal Care. All animal protocols received prior approval by the institutional review board.
Ovalbumin Sensitization and Challenge
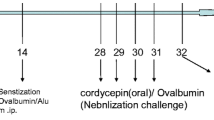
Mice were sensitized and challenged with the allergen ovalbumin (OVA) as previously described [9, 21, 23, 24]. OVA mice were sensitized via intraperitoneal injection with 10 μg of chicken OVA (Sigma, St. Louis, MO, USA) and 1 mg of A1(OH)2 (alum; Sigma) in 0.2 ml of phosphate-buffered saline (PBS; Sigma), followed by a boosting injection on day 7 with the identical reagents. PBS mice received 1 mg of alum in 0.2 ml of PBS without OVA. On days 14–20, mice received aerosolized challenge with 6% OVA or PBS, respectively, for 20 min/day via an ultrasonic nebulizer (Model 5000; DeVilbiss, Somerset, PA, USA). All groups were sacrificed at day 21 and analyzed for the allergic parameters described below.
Bronchoalveolar Lavage Analysis
Each mouse underwent bronchoalveolar lavage [25], as previously described [9, 21]. Cells were resuspended in RPMI (Sigma) (5 × 105 cells/ml). Slides for differential cells counts were prepared with cytospin (Shandon, Pittsburgh, PA, USA) and fixed and stained with Diff-Quik (Dade Behring, Newark, DE, USA).
Serum IgE
Blood was obtained by cardiac puncture on day 21. Total serum IgE levels were determined by ELISA as previously described [21]. Total serum IgE concentrations were calculated by using a standard curve generated with commercial IgE standard (BD PharMingen, San Diego, CA, USA).
Cytokine Assays
The cytokines were assayed from supernatant with LINCOplex mouse cytokine assays (LINCO Research, St Charles, Missouri) that are bead-based multiplex sandwich immunoassays with a limit detection of less than 5 pg/ml.
Histopathology
For histological analysis, tissue samples from the left lung were removed from the thoracic cavity and fixed in 4% paraformaldehyde and routinely processed into paraffin blocks. Paraffin sections, 5 μm thick, were cut and the tissues were screened with hematoxylin and eosin to verify the presence of at least three bronchioles per section.
Subcutaneous Insertion of Delivery Pump
On the first day of allergen challenge (day 14) a miniosmotic pump (ALZET Model 1007D, DURECT Corporation, ALZET Cupertino, CA, USA) containing (R+S)-albuterol (R)-albuterol, (S)-albuterol or PBS was inserted subcutaneously. After the mice were anesthetized (Ketamine 100 mg/kg & Xylazine 10 mg/kg), the area of pump implantation was shaved and cleaned with alcohol. An incision of 1 cm was made between the scapulae, and pumps were inserted subcutaneously. The pumps contained (R+S)- (100 μg of each isomer in a total volume of 100 μl of PBS), (R)-albuterol, (200 μg/100 μl), (S)-albuterol (200 μg/100 μl) or PBS (1×/100 μl) and delivered at a constant rate of 1 mg/kg/day.
Test Compounds
(R)- and (S)- albuterol were provided by Sepracor, Inc (Marlborough, MA, USA).
Measurement of Systemic Levels of (R)- and (S)- Albuterol
To determine concentrations of (R)-albuterol and (S)-albuterol in heparinized mouse plasma, 2.0 mL of ammonium acetate buffer (pH 8.7) was added to 0.2 mL of unknown sample spiked with 20 μL of 0.03 μg/mL n-methyl albuterol internal standard solution. The samples were vortexed and put through solid phase extraction using 3-mL PBA cartridges in a vacuum manifold. Cartridges were conditioned with 2 mL of methanolic glacial acetic acid, 2 mL of methanol, 2 mL of water, and 3 mL of 0.2 M ammonium acetate buffer (pH 8.7). The sample extracts were transferred to the cartridges which contained 1.0 mL of 0.2 M ammonium acetate buffer (pH 8.7). After the samples passed through the cartridges, they were rinsed with 2 mL of 0.1 M ammonium acetate buffer (pH 8.7), 2 mL of water, 1 mL of methanol : water (50 : 50), 2 mL of methanol :water : triethylamine : ammonium hydroxide (75 : 21 : 2 : 2), 1 mL of methanol : water (50 : 50) and 1 mL of methanol. Samples were eluted with 1.5 mL of methanolic glacial acetic acid. The samples were placed under vacuum and evaporated to dryness. After adding 0.150 mL of mobile phase, each tube was vortexed briefly and transferred to injection vials. The enantiomers were resolved and quantitated on a high performance liquid chromatographic system equipped with a fluorescence detector using an Astec chirobiotic T analytical column (25 cm × 4.6 mm) and a flow rate of 1.0 mL/minute. The mobile phase consisted of acetonitrile : methanol : glacial acetic acid : diethylamine (60 : 40 : 0.3 : 0.2). Analyzed concentrations were calculated using the peak height ratio of the compound of interest to internal standard using a linear (1/concentration2-weighted) calibration model.
Cells
EL4, a T cell cell line (American Type Culture Collection, Bethesda, MD) was established from a lymphoma induced in a C57BL/6 mouse by 9,10-dimethyl-1,2-benzanthracene. Murine splenocytes were isolated from C57BL/6 naïve mice and cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 50 μM 2-ME (complete medium). For activated samples, cells were cultured with Con A (5 μg/ml) and PMA (100 ng/ml) for 12 hours and then treated with (R)-albuterol (10-6 M), (S)-albuterol (10-6 M), or racemic albuterol [(R)-albuterol (10-6 M)+(S)-albuterol (10-6 M)] for the next 36 hours. For resting samples, cells were treated with Con A (5 μg/ml) and PMA (100 ng/ml), (R)-albuterol (10-6 M), (S)-albuterol (10-6 M), or racemic albuterol [(R)-albuterol(10-6 M) + (S)-albuterol (10-6 M)] for 24 hours.
Quantitative Real-time PCR
Total RNA was isolated from EL4 cells and splenocytes with TRI Reagent (Sigma-Aldrich, St. Louis, MO). Isolated RNA was reverse transcribed with SuperScript II RNAse reverse transcriptase (Life Technologies, Carlsbad, CA). Specific primer pairs for GAPDH (housekeeping gene), IL-2, IL-6, IL-13, and IFN-γ were designed with the Primer Express software (Applied Biosystems, Foster City, CA, USA). The sequences of the forward (FW) and reverse (RE) primer pairs used in the experiments were as follows: GAPDH: TTGTGGAAGGGCTCATGACC (FW), TCTTCTGGGTGGCAGTGATG (RE) (NM008084), IL-2: GTCAACAGCGCACCCACTT (FW), TGCTTCCGCTGTAGAGCTTG (RE) (NM008366), IL-6: TTCCATCCAGTTGCCTTCTTG (FW), GAAGGCCGTGGTTGTCACC (RE) (NM008355), IL-13: AATCTGTCTGCAGGTGGGCT (FW), GGCTTCTCACTTTCATTGGCAC (RE) (NM031168), IFN-γ: AGGTGTCACAACTGCTGCCA (FW), ACACCCGAATGAGCTGCTCT (RE) (NM008337). Direct detection of the PCR product was monitored by measuring the increase in fluorescence caused by the binding of SYBR Green to dsDNA. Using 5 μl of cDNA, 5 μl of primer, and 10 μl of SYBR Green Master Mix (Applied Biosystems) per well, the gene-specific PCR products were measured continuously by means of GeneAmp 5700 sequence detection system (Applied Biosystems) during 40 cycles. Non-template controls and dissociation curves were used to detect primer-dimer conformation and non-specific amplification. The threshold cycle (CT) of each target product was determined and set in relation to the amplification plot of GAPDH. The CT is the number of PCR cycles required for the fluorescence signal to exceed the detection threshold value. The detection threshold was set to the log linear range of the amplification curve and kept constant (0.3) for all data analysis. The difference in CT values of two genes was used to calculate the fold difference.
NF-κB Reporter Assay
The NF-κB promoter luciferase (pGL2) [26] and β-galactosidase reporter gene (pGK) [27] have been described previously. Each construct (1 μg) was added to EL4 cells (to 2 × 106) resuspended in nucleofector solution (Amaxa Biosystems) and electroporated using the C-9 program of the nucleofector. After 24 hours cells were treated Con A (5 μg/ml) and PMA (100 ng/ml), [(R)-albuterol(10-6 M) + (S)-albuterol (10-6 M)], (R)-albuterol (10-6 M) or (S)-albuterol (10-6 M), for 24 hours. For activated samples, cells were cultured with Con A (5 μg/ml) and PMA (100 ng/ml) for 12 hours and then treated with [(R)-albuterol (10-6 M)+(S)-albuterol (10-6 M)], (R)-albuterol (10-6 M) or (S)-albuterol (10-6 M), for the next 12 hours. Cells were lysed in reporter lysis buffer (Promega). Then, 10 μl of the cell lysate was mixed with 100 μl of luciferase assay reagent (Promega), and luciferase activity was measured by a luminometer (Turner Bio Systems). Luciferase activity was normalized for transfection efficiency by β-galactosidase activity measured with Galacto-light systems according to the manufacturer's instructions (Applied Biosystems, MA). Fold activation was calculated as the ratio of luciferase versus β-galactosidase activity in experimental samples compared to media alone.
Statistical Analysis
Analysis of variance (ANOVA) was performed by Sigma Stat software. Bonferroni correction for statistical adjustment of the p value for multiple comparisons was applied as a post-hoc analysis. Data are reported as means ± SEM. Statistical significance was defined by p < 0.05.
Results
Allergen-induced pulmonary inflammation is not influenced by the insertion of a miniosmotic pump
To analyze the effects of albuterol isomers in vivo in a murine model of allergic inflammation, we analyzed the potential immune effects of a delivery device for albuterol administration i.e. a miniosmotic pump inserted subcutaneously. We defined the effects of pump insertion alone on allergen-induced inflammation in a murine model. We measured allergen-induced BAL eosinophilia and total serum IgE in C57BL/6 mice following OVA sensitization and challenge and insertion of a subcutaneous miniosmotic pump containing PBS (Fig. 1). Consistent with our previous studies, OVA sensitized and challenged mice (OVA mice) demonstrated a significant increase in BAL eosinophilia and total serum IgE as compared with PBS mice (*p < 0.05, Fig 1A, B). Following the insertion of a pump containing PBS, OVA mice demonstrated a similar increase in BAL eosinophilia (†p < 0.01, Fig 1A) and total serum IgE (†p < 0.01, Fig 1B) compared to PBS mice. OVA mice compared to OVA mice that had a pump inserted (OVA+PBS) did not exhibit significant difference in BAL eosinophilia and total serum IgE.
Pump insertion does not alter allergic immune responses. Mice were sensitized and challenged with ovalbumin (OVA) as described in Methods. On the first day of OVA challenge (day 14, see Methods) a miniosmotic pump containing PBS (1×/100 ul) was inserted subcutaneously and delivered a constant dose of 25 ul/day. Cell counts were determined by differential staining of cells isolated from bronchoalveolar lavage (BAL) fluid. Total serum IgE and BAL cytokines (R&D Systems) were measured by ELISA. (A) BAL eosinoplhlia; (B) total serum IgE; (C) BAL IL-13. Data is shown as mean ± SEM (n = 8–10 per group). *PBS vs OVA p < 0.01, † PBS+PBS vs OVA+PBS p < 0.01.
Administration of albuterol isomers in a pulmonary allergic model
We next examined the effects of the albuterol isomers may influence immune responses. We measured BAL eosinophilia (Fig 2A), total serum IgE (Fig 2B) and pulmonary histology (Fig 3) in OVA mice following subcutaneous insertion of a pump containing either (R+S)-, (R)-, (S)- albuterol or PBS. As expected, OVA+PBS mice had significant increase in allergic responses compared with PBS+PBS mice (Fig 1A). OVA mice treated with (R)-albuterol demonstrated a decrease in eosinophilia (Fig. 2A) and IgE (Fig. 2B), albeit not significant by ANOVA analysis. A decrease in the pulmonary infiltrates (Fig. 3) is also observed. Serum levels of (R) and (S) albuterol were detected at day 1 and day 7 after pump insertion (not shown).
Analysis of allergic parameters following albuterol administration. (A) Mice were sensitized and challenged with OVA. On the first day of OVA challenge (day 14, see Methods) a miniosmotic pump containing (R+S), (R), (S)-albuterol or PBS was inserted subcutaneously (1 mg/kg/day). Cell counts were determined by differential staining of cells isolated from BAL fluid. Data is shown as mean ± SEM (n = 8–10 per group). (B) Total serum IgE levels were measured by ELISA. Data is shown as mean ± SEM (n = 8–10 per group).
(R)-Albuterol decreases pulmonary inflammation after OVA sensitization and aerosol challenge. On the first day of OVA challenge (day 14, see Methods) a miniosmotic pump (Alzet) containing (R)- or (S)- albuterol was inserted subcutaneously (200 μg/100 μl) and delivered a constant dose of 1 mg/kg/day (25 μl/day). A) PBS+pump, B) OVA+ pump, C) PBS+(R), D) OVA+(R), E) PBS+(S) and F) OVA+ (S). These pictures are representative of two mice examined in each group. Arrows show inflammatory cells. Magnification 10×.
Albuterol isomers exert a differential effect on cytokine levels following activation of splenocytes
We next determined whether albuterol effects on inflammation observed in vivo may be manifested in analysis of immune cells. Splenocytes were isolated from naïve C57BL/6 mice and activated with mitogens ConA and PMA. Activated splenocytes were incubated with (R+S), (R), (S) (10-6 M) for 24 hours. IL-2 and IL-13 cytokines were analyzed by real time PCR (Fig 4A, B). (R)-albuterol significantly decreased IL-2 and IL-13 mRNA levels. There was no difference in levels of IL-6 following administration of albuterol isomers in activated cells (not shown).
(R)-albuterol decreases cytokine levels in activated murine splenocytes. (R)-albuterol (R), (S)-albuterol (S) or racemic albuterol (R + S) at a dose (10-6 M) were added to murine splenocytes pre-activated with mitogens Concanavalin A (Con A, 5 μg/ml) and phorbol myristate acetate (PMA, 100 ng/ml) (CP), (Fig 3 A, B). RNA was isolated. IL-2 and IL-13 levels were measured by real time PCR. Fold change is the ratio of stimulated to untreated sample. Data are shown as mean ± SEM (n = 3). *p < 0.05 (CP vs R).
Albuterol isomers exert a differential effect on cytokine levels following activation of T Cells
T cells are critical for allergic inflammation [6–8, 24, 28]. We then investigated the effect of albuterol isomers on T cells using a T cell line (EL-4). We measured cytokine levels in both resting and activated T cells following the administration of (R+S)-,(R)- or (S)-albuterol (Figure 5A,B,C). Levels of IL-2, IL-13, IL-6 and IFN-γ were determined by real time PCR. When T cells were stimulated with mitogens ConA and PMA (CP) following by incubation with (R)-albuterol, there was a decrease in mRNA levels of IL-2, IL-13 and IL-6 (Fig. 5A, B, C). IL-2 and IL-13, but not IL-6 levels, were significantly decreased. There was no difference in levels of IFN-γ following administration of albuterol isomers in activated cells (not shown). In resting T (EL-4) cells there were no significant changes in IL-2, IL-6, IL-13 or IFN-γ levels following administration of albuterol isomers (data not shown). Thus, activated T cells demonstrate differential cytokine production when treated with albuterol isomers. We also examined cytokine secretion at the level of protein by bead-based multiplex sandwich immunoassays and found that (R)-albuterol significantly decreases IL-2 and IL-13 production (Figures 6A, 6B) (*p < 0.05).
(R)-albuterol decreases cytokine mRNA levels in activated T cells. (R)-albuterol, (S)-albuterol or racemic albuterol (R + S) at a dose (10-6 M) were added to T (EL-4) cells pre-activated with mitogens Concanavalin A (Con A, 5 μg/ml) and phorbol myristate acetate (PMA, 100 ng/ml) (CP). RNA was isolated. Levels of IL-2, IL-13 and IL-6 (A, B, C) were measured by real time PCR. Fold change is the ratio of stimulated to unstimulated sample. Data are shown as mean ± SEM (n = 3) *p < 0.05 (CP vs R).
(R)-albuterol decreases cytokine protein levels in activated T cells. (R)-albuterol, (S)-albuterol or racemic albuterol (R + S) at a dose (10-6 M) were added to T (EL-4) cells pre-activated with mitogens Concanavalin A (Con A, 5 μg/ml) and phorbol myristate acetate (PMA, 100 ng/ml) (CP). Supernatant was assayed for IL-2 and IL-13 cytokines by bead-based multiplex sandwich immunoassay. Data are shown as mean ± SEM (n = 3) *p < 0.05 (CP vs R).
(R)-Albuterol decreases NF-κB activity in activated T cells
We previously demonstrated a role for the transcription factor NF-κB in allergen-induced pulmonary inflammation and the modulation of T cell subtypes [21, 29]. In this study, we asked whether albuterol isomers would influence NF-κB activity in T cells. We measured NF-κB activity in resting and activated EL4 T cells by analysis of a NF-κB reporter luciferase gene construct following administration of albuterol isomers (Fig 7). Cells were activated with the T cell mitogens ConA +PMA (CP). T cells pre activated with CP, then treated with (R+S)- and (R)-albuterol display diminution of NF-κB activity when compared with cells treated with CP alone. In resting T cells, there were no changes in NF-κB activity after treatment with albuterol isomers (not shown).
(R+S) and (R)-albuterol decrease NF-κB activity. A NF-κB luciferase reporter construct (pGL2) was cotransfected with a β-galactosidase (pGK) reporter construct by electroporation into T (EL4) cells. After transfection, (R)-albuterol (R), (S)-albuterol (S), or racemic albuterol (R+ S) were added to cells pre-activated with (ConA, 5 μg/ml) and (PMA, 100 ng/ml) (CP) and then treated with (R)-, (S)-, or (R+S)-albuterol (Fig. 6). Relative light units (RLU) were normalized to β-gal (pGK) coreporter activity. Fold induction is the ratio of RLU of stimulated to unstimulated sample. Data are shown as mean ± SEM (n = 3).
Discussion
Short acting beta agonists, including albuterol, are a mainstay of asthma therapy due to their ability to promote bronchodilation; in addition they may display anti-inflammatory properties [10, 11, 30, 31]. Racemic albuterol contains equal concentrations of (R)- and (S)- enantiomers; yet, studies indicate that the (R)-and (S)-isomers may differ in their effects [19, 32–34]. In activated T cells, we show that (R)-albuterol exhibits anti-inflammatory effects that may be mediated by alterations in NF-κB activity.
The anti-inflammatory properties of beta agonists include a reduction in proliferation of airway smooth muscle cells [18, 35] as well as inhibition of cytokine-induced release of eotaxin, a potent eosinophil chemoattractant [36]. Beta agonists also inhibit the secretion of granular proteins [37] and the production of superoxide from eosinophils [32]. Prior studies suggest an anti-inflammatory effect of beta agonists on T lymphocytes. Beta agonists inhibit T cell receptor stimulated cytokine production in both human peripheral blood monocytes [38] and murine T cell clones [30] effects that may be mediated by β2-adrenergic receptor activation of protein kinase A (PKA) [10] and subsequent inhibition of TNF-α production and NF-κB activation [39].
(R)- and (S)- albuterol appear to differ in their pharmacological effects. While (R)- albuterol induces bronchodilation, the (S)- enantiomer shows biological effects including allergen-induced airway hyperresponsiveness in a guinea pig model and enhanced contractility in human bronchi [16]. Increases in intracellular calcium ions appear to underlie one mechanism by which the (S)-isomer may induce bronchoconstriction [20, 40]. (S)-albuterol also increases the expression of the pro-inflammatory mediators, PI3 and NF-κB, in human bronchial smooth muscle cells [20], while (R)-albuterol decreases proliferation of these cells via activation of PKA and inhibition of PI-3 and NF-κB [18].
Our study examined in vivo administration of (R) + (S) albuterol isomers. To exclude the possibility that introduction of a subcutaneous delivery device (miniosmotic pump) alters immune responses, we demonstrated that insertion of a pump does not alter parameters of allergic inflammation. Previous data in a murine allergic model indicate that both (R)- and (S)-albuterol may decrease allergen-induced pulmonary inflammation and goblet cell hyperplasia [34]. Our studies and Henderson et al. exhibit different protocols, timing and methods of allergen administration. Another variable is the time of drug exposure. In Henderson's studies, the time of drug exposure appears to be 3 times longer than in our protocol [34]. Also, our murine strain is C57BL/6 while Henderson's was Balb/c. As they also did not examine (R+S) albuteroI, no comparisons can be made with regards to analysis of the (R+S) group.
T cells play an important role in the pathophysiology of asthma by modulation of inflammatory cytokines and cells [6, 41, 42]. Our in vitro studies indicate that albuterol isomers display differential effects on activated but not resting T cells. Following activation by T cell mitogens, ConA and PMA (CP), T cells treated with (R)-albuterol demonstrated decreased levels of IL-2, IL-6 and IL-13 compared to cells treated with CP alone. These findings are consistent with previous data indicating differential effects of albuterol isomers on cell proliferation and cytokine production in human peripheral blood monocytes [19].
Our findings suggest possible mechanisms for the anti-inflammatory effects displayed by albuterol isomers that may occur via T cells. Allergen-induced pulmonary inflammation is a T cell dependent process mediated by key inflammatory cytokines which promote effector pathways involving eosinophils and IgE [43]. The isomers decrease parameters of allergic inflammation in a murine model [34]. Furthermore, in activated T cells, we show that (R)-albuterol reduces the inflammatory cytokines, IL-2 and IL-13. IL-2 is a reliable marker of T cell activation [44] and IL-13 is well known for its critical role in allergen-induced inflammation [45].
Finally, our data suggest that the effects of (R)-albuterol may be mediated by alterations in the activity of the inflammatory transcription factor NF-κB. NF-κB regulates the expression of a wide range of genes involved in immune and inflammatory responses [46, 47] and plays a role in the pathogenesis of asthma [21, 22, 48, 49]. Previous studies indicate that beta agonists exert anti-inflammatory effects on monocytic cells via generation of cyclic-AMP and activation of PKA leading to a decrease in TNF-α production and NF-κB activation [39]. Also, administration of albuterol isomers induces differential expression of NF-κB in airway smooth muscle cells [18, 20]. NF-κB can increase both IL-2 and IL-6 gene expression via binding to transcriptional promoter elements [25, 50]. Our study indicates that (R)-albuterol decreases cytokine production and NF-κB activity in activated T cells.
References
Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, Haddad el B, Lehr HA, Schmitt E, Bopp T: The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest 2005,115(2):313–325.
Costello PS, Turner M, Walters AE, Cunningham CN, Bauer PH, Downward J, Tybulewicz VL: Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene 1996,13(12):2595–2605.
Mekori YA, Metcalfe DD: Mast cell-T cell interactions. The Journal of allergy and clinical immunology 1999,104(3 Pt 1):517–523.
Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, Hasegawa A, Kohno Y, Nakayama T: Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci USA 2006,103(7):2286–2291.
Bodey KJ, Semper AE, Redington AE, Madden J, Teran LM, Holgate ST, Frew AJ: Cytokine profiles of BAL T cells and T-cell clones obtained from human asthmatic airways after local allergen challenge. Allergy 1999,54(10):1083–1093.
Robinson D, Hamid Q, Bentley A, Ying S, Kay AB, Durham SR: Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. The Journal of allergy and clinical immunology 1993,92(2):313–324.
Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB: Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992,326(5):298–304.
Krinzman SJ, De Sanctis GT, Cernadas M, Mark D, Wang Y, Listman J, Kobzik L, Donovan C, Nassr K, Katona I: Inhibition of T cell costimulation abrogates airway hyperresponsiveness in a murine model. J Clin Invest 1996,98(12):2693–2699.
Arestides RS, He H, Westlake RM, Chen AI, Sharpe AH, Perkins DL, Finn PW: Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol 2002,32(10):2874–2880.
Loza MJ, Foster S, Peters SP, Penn RB: Beta-agonists modulate T-cell functions via direct actions on type 1 and type 2 cells. Blood 2006,107(5):2052–2060.
Johnson M: Effects of beta2-agonists on resident and infiltrating inflammatory cells. J Allergy Clin Immunol 2002,110(6 Suppl):S282–290.
Williams LT, Snyderman R, Lefkowitz RJ: Identification of beta-adrenergic receptors in human lymphocytes by (-) (3H) alprenolol binding. The Journal of clinical investigation 1976,57(1):149–155.
Page CP, Morley J: Contrasting properties of albuterol stereoisomers. The Journal of allergy and clinical immunology 1999,104(2 Pt 2):S31–41.
Gumbhir-Shah K, Kellerman DJ, DeGraw S, Koch P, Jusko WJ: Pharmacokinetic and pharmacodynamic characteristics and safety of inhaled albuterol enantiomers in healthy volunteers. J Clin Pharmacol 1998,38(12):1096–1106.
Gumbhir-Shah K, Kellerman DJ, DeGraw S, Koch P, Jusko WJ: Pharmacokinetics and pharmacodynamics of cumulative single doses of inhaled salbutamol enantiomers in asthmatic subjects. Pulm Pharmacol Ther 1999,12(6):353–362.
Templeton AG, Chapman ID, Chilvers ER, Morley J, Handley DA: Effects of S-salbutamol on human isolated bronchus. Pulm Pharmacol Ther 1998,11(1):1–6.
Mazzoni L, Naef R, Chapman ID, Morley J: Hyperresponsiveness of the airways following exposure of guinea-pigs to racemic mixtures and distomers of beta 2-selective sympathomimetics. Pulm Pharmacol 1994,7(6):367–376.
Ibe BO, Portugal AM, Raj JU: Levalbuterol inhibits human airway smooth muscle cell proliferation: therapeutic implications in the management of asthma. Int Arch Allergy Immunol 2006,139(3):225–236.
Baramki D, Koester J, Anderson AJ, Borish L: Modulation of T-cell function by (R)- and (S)-isomers of albuterol: anti-inflammatory influences of (R)-isomers are negated in the presence of the (S)-isomer. J Allergy Clin Immunol 2002,109(3):449–454.
Berthiaume Y, Lesur O, Dagenais A: Treatment of adult respiratory distress syndrome: plea for rescue therapy of the alveolar epithelium. Thorax 1999,54(2):150–160.
Donovan CE, Mark DA, He HZ, Liou HC, Kobzik L, Wang Y, De Sanctis GT, Perkins DL, Finn PW: NF-kappa B/Rel transcription factors: c-Rel promotes airway hyperresponsiveness and allergic pulmonary inflammation. J Immunol 1999,163(12):6827–6833.
Birrell MA, Hardaker E, Wong S, McCluskie K, Catley M, De Alba J, Newton R, Haj-Yahia S, Pun KT, Watts CJ: Ikappa-B kinase-2 inhibitor blocks inflammation in human airway smooth muscle and a rat model of asthma. Am J Respir Crit Care Med 2005,172(8):962–971.
Velasco G, Campo M, Manrique OJ, Bellou A, He H, Arestides RS, Schaub B, Perkins DL, Finn PW: Toll-like receptor 4 or 2 agonists decrease allergic inflammation. Am J Respir Cell Mol Biol 2005,32(3):218–224.
Krinzman SJ, De Sanctis GT, Cernadas M, Kobzik L, Listman JA, Christiani DC, Perkins DL, Finn PW: T cell activation in a murine model of asthma. Am J Physiol 1996,271(3 Pt 1):L476–483.
Libermann TA, Baltimore D: Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Molecular and cellular biology 1990,10(5):2327–2334.
Devergne O, Cahir McFarland ED, Mosialos G, Izumi KM, Ware CF, Kieff E: Role of the TRAF binding site and NF-kappaB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J Virol 1998,72(10):7900–7908.
Mitchell T, Sugden B: Stimulation of NF-kappa B-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J Virol 1995,69(5):2968–2976.
Romagnani S: Regulation of the T cell response. Clin Exp Allergy 2006,36(11):1357–1366.
Aronica MA, Mora AL, Mitchell DB, Finn PW, Johnson JE, Sheller JR, Boothby MR: Preferential role for NF-kappa B/Rel signaling in the type 1 but not type 2 T cell-dependent immune response in vivo. J Immunol 1999,163(9):5116–5124.
Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE: Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol 1997,158(9):4200–4210.
Heijink IH, van den Berge M, Vellenga E, de Monchy JG, Postma DS, Kauffman HF: Altered beta2-adrenergic regulation of T cell activity after allergen challenge in asthma. Clin Exp Allergy 2004,34(9):1356–1363.
Volcheck GW, Kelkar P, Bartemes KR, Gleich GJ, Kita H: Effects of (R)- and (S)-isomers of beta-adrenergic agonists on eosinophil response to interleukin-5. Clin Exp Allergy 2005,35(10):1341–1346.
Abraha D, Cho SH, Agrawal DK, Park JM, Oh CK: (S, S)-formoterol increases the production of IL-4 in mast cells and the airways of a murine asthma model. Int Arch Allergy Immunol 2004,133(4):380–388.
Henderson WR Jr, Banerjee ER, Chi EY: Differential effects of (S)- and (R)-enantiomers of albuterol in a mouse asthma model. J Allergy Clin Immunol 2005,116(2):332–340.
Tomlinson PR, Wilson JW, Stewart AG: Salbutamol inhibits the proliferation of human airway smooth muscle cells grown in culture: relationship to elevated cAMP levels. Biochem Pharmacol 1995,49(12):1809–1819.
Pang L, Knox AJ: Regulation of TNF-alpha-induced eotaxin release from cultured human airway smooth muscle cells by beta2-agonists and corticosteroids. Faseb J 2001,15(1):261–269.
Leff AR, Herrnreiter A, Naclerio RM, Baroody FM, Handley DA, Munoz NM: Effect of enantiomeric forms of albuterol on stimulated secretion of granular protein from human eosinophils. Pulm Pharmacol Ther 1997,10(2):97–104.
Heijink IH, Vellenga E, Borger P, Postma DS, Monchy JG, Kauffman HF: Polarized Th1 and Th2 cells are less responsive to negative feedback by receptors coupled to the AC/cAMP system compared to freshly isolated T cells. Br J Pharmacol 2003,138(8):1441–1450.
Farmer P, Pugin J: beta-adrenergic agonists exert their "anti-inflammatory" effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am J Physiol Lung Cell Mol Physiol 2000,279(4):L675–682.
Mitra S, Ugur M, Ugur O, Goodman HM, McCullough JR, Yamaguchi H: (S)-Albuterol increases intracellular free calcium by muscarinic receptor activation and a phospholipase C-dependent mechanism in airway smooth muscle. Mol Pharmacol 1998,53(3):347–354.
Borgonovo B, Casorati G, Frittoli E, Gaffi D, Crimi E, Burastero SE: Recruitment of circulating allergen-specific T lymphocytes to the lung on allergen challenge in asthma. The Journal of allergy and clinical immunology 1997,100(5):669–678.
Lara-Marquez ML, Deykin A, Krinzman S, Listman J, Israel E, He H, Christiani DC, Perkins DL, Finn PW: Analysis of T-cell activation after bronchial allergen challenge in patients with atopic asthma. The Journal of allergy and clinical immunology 1998,101(5):699–708.
Nakajima H, Takatsu K: Role of Cytokines in Allergic Airway Inflammation. Int Arch Allergy Immunol 2006,142(4):265–273.
Kane LP, Lin J, Weiss A: It's all Rel-ative: NF-kappaB and CD28 costimulation of T-cell activation. Trends Immunol 2002,23(8):413–420.
Wills-Karp M: Interleukin-13 in asthma pathogenesis. Immunological reviews 2004, 202:175–190.
Baeuerle PA, Henkel T: Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 1994, 12:141–179.
Siebenlist U, Franzoso G, Brown K: Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol 1994, 10:405–455.
Ziegelbauer K, Gantner F, Lukacs NW, Berlin A, Fuchikami K, Niki T, Sakai K, Inbe H, Takeshita K, Ishimori M: A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. British journal of pharmacology 2005,145(2):178–192.
Hart LA, Krishnan VL, Adcock IM, Barnes PJ, Chung KF: Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. American journal of respiratory and critical care medicine 1998,158(5 Pt 1):1585–1592.
Verweij CL, Geerts M, Aarden LA: Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-kB-like response element. J Biol Chem 1991,266(22):14179–14182.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
This work was funded by a Sepracor investigator initiated study (PWF); Leesa M. Barone is an employee from Sepracor.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ferrada, M.A., Gordon, E.L., Jen, K.Y. et al. (R)-albuterol decreases immune responses: role of activated T cells. Respir Res 9, 3 (2008). https://doi.org/10.1186/1465-9921-9-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1465-9921-9-3