Abstract
Background
Smoking activates and recruits inflammatory cells and proteases to the airways. Matrix metalloproteinase (MMP)-12 may be a key mediator in smoke induced emphysema. However, the influence of smoking and its cessation on airway inflammation and MMP-12 expression during COPD is still unknown. We aimed to analyse airway inflammatory cell patterns in induced sputum (IS) and bronchoalveolar lavage (BAL) from COPD patients who are active smokers and who have ceased smoking >2 years ago.
Methods
39 COPD outpatients – smokers (n = 22) and ex-smokers (n = 17) were studied. 8 'healthy' smokers and 11 healthy never-smokers were tested as the control groups. IS and BAL samples were obtained for differential and MMP-12+-macrophages count analysis.
Results
The number of IS neutrophils was higher in both COPD groups compared to both controls. The amount of BAL neutrophils was higher in COPD smokers compared to healthy never-smokers. The number of BAL MMP-12+-macrophages was higher in COPD smokers (1.6 ± 0.3 × 106/ml) compared to COPD ex-smokers, 'healthy' smokers and healthy never-smokers (0.9 ± 0.4, 0.4 ± 0.2, 0.2 ± 0.1 × 106/ml respectively, p < 0.05).
Conclusion
The lower amount of BAL neutrophils in COPD ex-smokers, compared to COPD smokers, suggests positive alterations in alveolar compartment after smoking cessation. Smoking and disease itself may stimulate MMP-12 expression in airway compartments (IS and BAL) from COPD patients.
Similar content being viewed by others
Background
Smoking is the major known risk factor for the development of chronic obstructive pulmonary disease (COPD), which is characterized by progressive and not fully reversible airflow limitation [1]. The pathogenesis of COPD is multifactor, involving airway inflammation, associated with an infiltration of inflammatory cells and protease-antiprotease imbalance [2, 3].
Over 85% COPD patients have been regular smokers [4, 5]. It is well known, that inflammation initiated by smoking leads to a changes in both – airways and lung parenchyma. The main known contribution of smoking is activation and recruitment of inflammatory cells to the lungs [6–8]. We have previously observed a tendency of neutrophils to be increased in the airways of stable COPD patients [9]. Other studies have also shown that cigarette smoke produces an increase of neutrophils in bronchoalveolar lavage (BAL) and lung tissue [10–12]. Although, the major environmental risk factor – smoking, for COPD development is well known, the changes of COPD induced by inflammation after smoking cessation are less evaluated.
It was also suggested, that various metalloproteinases (MMPs), especially MMP-2 and MMP-9, mediate airway inflammation and remodelling [13–15]. Since, it is nearly impossible to investigate which individual MMP is the most important in COPD pathogenesis. MMP-12 was first detected as an elastolytic proteinase in alveolar macrophages of cigarette smokers [16]. Whilst, animal studies have shown that MMP-12 is important in cigarette smoke induced emphysema [17–19], the relevance of MMP-12 in human disease is controversial.
Thus, we aimed to analyse airway inflammatory cell patterns in smokers and ex-smokers with COPD and to compare whether it differs from 'healthy' smokers and never-smokers. Also, according to a previous study, showing an increase in MMP-12 in the induced sputum (IS) of COPD patients [20], we have assessed an expression of MMP-12 in IS and BAL cells from these COPD and healthy subjects groups. Furthermore, we analysed if the decline of pulmonary function in COPD patients is related to the smoking history and MMP-12 expression in airway cells.
Methods
Study population
We studied 39 outpatients with stable COPD, according to GOLD (stage II-III) [1]. All patients met following criteria: has not used inhaled and systemic steroids at least 1 month before the study and had more than 20 pack-years smoking history. None of the subjects showed signs of acute respiratory infection at least one month before the investigation. All patients were screened for deficiency of alfa-1 antitrypsin (AAT) by quantitative ELISA test (Eurodiagnosta, Sweden) and was established, that none of the patients had the Z allele, which may cause the deficiency of AAT. The patients were divided into 2 groups: COPD smokers (n = 22), who are currently smokers and COPD ex-smokers (n = 17), who ceased smoking at least 2 years before investigation (however, we did not test a cotinine level to ensure, if they have really ceased smoking).
8 smokers without airways obstruction ('healthy' smokers) and 11 healthy never-smokers with normal lung function were tested as control groups.
Smoking history was calculated in pack-years as the product of tobacco use (in years) and the average number of cigarettes smoked per day/20 (years × cig. per day/20).
The study was approved by the Regional Bioethics Committee in Kaunas University of Medicine and written informed consent was received from all participants.
Lung function testing
Pulmonary function was tested using a pneumotachometric spirometer "CustovitM" (Custo Med, Germany) with subjects in the sitting position, and the highest value of forced expiratory volume in 1 sec (FEV1) and forced vital capacity (FVC) from at least two technically satisfactory maneuvers differing by less than 5% was recorded. Normal values were characterized according to Quanjer and colleagues [21]. Subjects had to avoid the use of short-acting β2-agonists at least 8 h prior the test.
Sputum induction and processing
After lung function test, subjects inhaled 10 mL of sterile hypertonic saline solution (3%, 4% or 5% NaCl (Ivex Pharmaceuticals, USA)) at room temperature (RT) from an ultrasonic nebulizer (DeVilbiss Health Care, USA). The duration of each inhalation was 5 min and was stopped after expectoration an adequate amount of sputum. Spirometry was performed after each inhalation, in order to detect a possible decrease of FEV1. Sputum was poured into a Petri dish and separated from saliva. A fourfold volume of freshly prepared 0.1% dithiothreitol (DTT; Sigma-Aldrich, Germany) was added. The mixture was vortexed and placed on a bench rocker for 15 min. at RT. Next, an equal volume of phosphate-buffered saline (PBS; Sigma-Aldrich, Germany), solution was added to the DTT. The cell pellet was separated using 40 μm cell stainer (Becton Dickinson, USA). The mixture was centrifuged for 10 min at 4°C, the supernatant was aspirated and stored at -70°C for later assay.
The total cell counts, percentage of epithelial cells and cell viability were investigated using a Neubauer hemocytometer (Heinz-Herenz; Germany) by microscope (B5 Professional, Motic, China), using Trypan blue exclusion method. Cytospin samples of induced sputum were prepared using a cytofuge instrument (Shandon Southern Instruments, USA). The cytospin preparations for immunocytochemistry were air dried for 2 h and stored at -70°C until further investigation.
Bronchoscopy and BAL processing
Bronchoscopy was performed in a week after sputum induction procedure. Subjects were not allowed to drink or eat at least 4 h, to smoke at least 10 h before the procedure. To perform BAL, the local upper airways anesthesia with 5 mL of 2% lidocaine (Grindex, Latvia) was used. All bronchoscopic examinations were performed in the morning. The bronchoscope (Olympus, USA) was wedged into the segmental bronchus of the middle lobe and 20 mL × 7, a total 140 mL of sterile saline solution (0.9% NaCl) was infused. Fluid was gently aspirated immediately after the infusion has been completed and was collected into a sterile container. The fluid was immediately filtered using 40 μm cell stainer (Becton Dickinson, USA) and centrifuged at 4°C for 10 min. Supernatants were removed and frozen at -70°C for further investigation. Preparation of BAL cytospins was the same as the preparation of IS samples described above.
Cell analysis
Prepared IS and BAL cytospins were stained by the May-Grünwald-Giemsa method for differential cell counts. Cell differentiation was determined by counting approximately 400 cells in random fields of view under light microscope, excluding squamous epithelial cells. The cells were identified using standard morphological criteria, by nuclear morphology and cytoplasmic granulation. Cell counts were expressed as percentages of total cells and absolute values (106/ml).
MMP-12 immunocytochemistry (ICC)
MMP-12 expression in IS and BAL cytospin preparations was detected immunocytochemically. Cytospin preparations were fixed in 4% paraphormaldehyde (Merck, USA) in PBS for 20 min. and subsequently washed in PBS. All incubations were performed at RT. Non-specific binding sites were blocked with 5% normal blocking serum (Goat ABC Staining System, Santa Cruz, USA) for 35 min. The slides were incubated with optimum concentration of goat anti-human MMP-12 antibody (Santa Cruz, USA), which is raised against a peptide mapping near the C-terminus of MMP-12, and negative control (rabbit IgG, Santa Cruz, USA) for 30 min. After washings in PBS, the slides were incubated with biotinylated secondary antibody (Santa Cruz, USA) for 30 min. Followed by washings in PBS, slides were incubated with avidin-biotinylated peroxydase (Santa Cruz, USA) complex for 35 min. After washings, the staining with chromogenic substrate 3,3'diaminobenzidine system (Santa Cruz, USA) was developed for 10–15 min monitoring under light microscope. The slides were counterstained with Mayer's haematoxylin (Sigma-Aldrich, Germany) for 1–2 min and mounted in Crystal Mounting Medium (Santa Cruz, USA). All slides were evaluated under light microscope in random fields of view counting up to 300–400 cells. Morphologically, all MMP-12 expressing cells were macrophages. Macrophages with brown staining in cytoplasm were counted as MMP-12 positive macrophages (MMP-12+-macrophages) (Fig. 1A). Figure 1B represents the negative staining with rabbit IgG. The absolute amount of MMP-12+-macrophages (106/ml) was calculated according to the number of MMP-12+-macrophages and total inflammatory cell count. The intensity of staining was evaluated as: 0 – negative; +++ – very strong expression. The variations MMP-12+-macrophages were counted by two "blinded" researchers and the mean of their results was calculated. In most cases, the variation of cell count between examinators was less than 5%.
It is important to note, that we used DTT for preparation of IS samples, which may interfere with expression of MMP-12. Therefore, we have compared a preparation of few IS samples for MMP-12 and inflammatory cell count with DTT and without it, and we did not notice any significant differences.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences, version 12.0 for Windows (SPSS 12.0). Data was expressed as the mean of percentage or absolute value (106/ml) ± standard error of mean (SEM). Differences between all groups were explored using one-way ANOVA followed by Kruskal-Wallis test. Mann-Whitney U-test was used to assess the statistical significance of differences between the groups. A P-value < 0.05 was considered significant. Correlations between analysed parameters were assessed using Spearman's rank coefficient.
Results
Characteristics of subjects
The average age did not differ between investigated groups (Table 1). The number of pack-years did not significantly differ between COPD smokers, COPD ex-smokers and 'healthy' smokers. Lung function parameters did not differ between COPD groups, but were lower compared to controls.
Cellular composition of IS
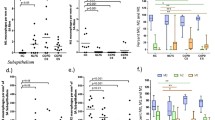
The total cell count of IS did not differ between all groups (Fig. 2A). The composition of inflammatory cells did not differ between COPD smokers and COPD ex-smokers. COPD groups showed a predominance of neutrophils, compared to both healthy subjects groups in percentages (Table 2). An absolute amount of these cells was higher in COPD smokers and COPD ex-smokers compared to healthy never-smokers, but not 'healthy' smokers (Fig. 2A).
Macrophages in IS were more obvious in 'healthy' smokers and healthy never-smokers, due to higher percentage of neutrophils in both COPD groups. The percentage of macrophages was significantly lower in COPD groups compared to both healthy subjects groups, and did not significantly differ between both COPD and between both controls groups. An absolute amount of macrophages in COPD smokers was lower compared to healthy never-smokers and did not significantly differ from 'healthy' smokers, however a tendency was seen (p = 0.06) (Fig. 2A).
Cellular composition of BAL
The total BAL cell number was higher in COPD groups, compared to healthy subjects groups, while it did not differ between COPD smokers and COPD ex-smokers and between 'healthy' smokers and healthy never-smokers (Fig. 2B). Also, the recovery of BAL was significantly higher in COPD ex-smokers, compared to COPD smokers (Table 2). While, this volume was significantly higher in both healthy subjects groups, than in COPD smokers and COPD ex-smokers. The recovery of BAL did not differ between both 'healthy' smokers and healthy never-smokers.
The percentage of neutrophils was increased in COPD smokers, compared to COPD ex-smokers and healthy subjects groups. Whereas, the percentage of these inflammatory cells in COPD ex-smokers was higher compared to healthy never-smokers, but did not differ from 'healthy' smokers. The percentage of BAL neutrophils in 'healthy' smokers was also higher than in healthy never-smokers. The absolute amount of neutrophils in COPD smokers was higher compared to all other groups (Fig. 2B).
Expression of MMP-12 in IS and BAL cells
An immunocytochemical staining of IS cells for MMP-12 did not show significant differences between COPD smokers and COPD ex-smokers neither in percentages (Fig. 3), nor in absolute values. The percentage of IS MMP-12+-macrophages was higher in COPD groups compared to healthy subjects groups. 'Healthy' smokers had higher percentage of these cells than healthy never-smokers (Fig. 3), but the absolute amount of MMP-12+-macrophages did not differ.
The amount of BAL MMP-12+-macrophages was also significantly higher in COPD groups than in controls in percentages and absolute values. Furthermore, the number of BAL MMP-12+-macrophages was higher in COPD smokers compared to COPD ex-smokers, and in 'healthy' smokers compared to healthy never-smokers (Fig. 3).
Analysing the BAL samples we have observed macrophages differentiating in size and granularity of cytoplasm, while we did not evaluate the relations of MMP-12 expression with their morphology.
Smoking history relation with cellular patterns, MMP-12 expression and lung function parameters
The number of pack-years correlated with FEV1 (%) in COPD smokers (R = -0.70, p < 0.05) and 'healthy' smokers (R = -0.61, p < 0.05). Also, the pack-years correlated with IS neutrophils in COPD ex-smokers (R = 0.66, p < 0.05). A correlation between pack-years and BAL neutrophils in COPD smokers, COPD ex-smokers and 'healthy' smokers groups (Fig. 4) was also obtained. Moreover, the pack-years correlated with BAL macrophages in COPD smokers (R = 0.87, p < 0.05) and 'healthy' smokers (R = 0.68, p < 0.05). These parameters did not correlate with IS inflammatory cells.
The number of IS macrophages negatively correlated with FEV1 (%) in COPD smokers (R = -0.53, p < 0.05) and COPD ex-smokers (R = -0.58, p < 0.05). The correlation between BAL macrophages and FEV1 (%) in all studied groups was also obtained (R = -0.88; -0.62; -0.67; -0.78, p < 0.05 respectively).
The number of pack-years correlated with IS MMP-12+-macrophages in COPD smokers (R = 0.54, p < 0.05), COPD ex-smokers (R = 0.64, p < 0.05) and 'healthy' smokers (R = 0.78, p < 0.05). Much stronger correlation between pack-years and BAL MMP-12+-macrophages was obtained (Fig. 5).
Discussion
We aimed to analyse the patterns of airway inflammation in COPD patients depending on their smoking status, and compare it to smokers without airways obstruction ('healthy' smokers) and healthy never-smokers. We have evaluated different tissue compartments (IS and BAL), as IS is thought to be a combination of resident mucus [22] and the composition of its cells may be influenced by inflammation in proximal airways [22]. While BAL cellular composition represents mainly the alveolar compartment [23–25], however this method usually is limited due invasiveness. We have analysed IS sputum and BAL, because differences in these patterns are still unclear. Also, we have analysed whether the possible differences in MMP-12 expression are influenced by smoking history and its cessation, as previous animal [17–19] and human [26, 27] studies showed, that smoking exposure may increase an expression of MMP-12.
The number and composition of IS inflammatory cells did not significantly differ between smokers and ex-smokers with COPD, while the number of neutrophils was increased compared to healthy subjects. These results are in agreement with major previous studies [23, 28], which have shown that cellular inflammatory response in COPD is characterized by an increase of total inflammatory cells, especially neutrophils, macrophages and lymphocytes in small and large airways [2, 3]. Thus, our results may indicate the similar inflammatory response in smokers and ex-smokers with COPD, which is associated not only with smoking, but also with systemic inflammation. Influence of smoking may explain an increased number of BAL neutrophils in COPD smokers, compared to COPD ex-smokers, 'healthy' smokers and healthy never-smokers. Interestingly, the number of BAL neutrophils did not differ between COPD ex-smokers and 'healthy' smokers, while the amount of these cells was increased compared to healthy never-smokers. This finding supports the hypothesis, that cigarette smoking may cause cellular alterations [3, 22], which may intensify an inflammation process, induced by disease itself.
Macrophage is predominant cell in IS from healthy never-smokers and 'healthy' smokers as well. The lower relative number of these cells obtained in COPD groups may indicate an ongoing inflammatory process. Also, the similar amount of BAL macrophages in COPD ex-smokers, 'healthy' smokers and never-smokers, suggests the possibility of positive alterations in the alveolar compartment after smoking cessation.
Also, we have obtained a higher recovery of BAL fluid in healthy subjects, compared to both COPD groups. According to Lofdahl et al. [29] suggestions, the extent of emphysema (measured as an emphysema index and the carbon monoxide diffusing capacity of the lung) may predict a low BAL recovery in patients with moderate-to-severe COPD. Moreover, the lower recovery of BAL fluid in COPD smokers than in COPD ex-smokers may indicate an increased inflammatory process in alveolar compartment strengthened by smoking. Furthermore, differences in BAL cell composition between COPD smokers and ex-smokers encouraged us to evaluate a correlation between smoking history, pulmonary function and inflammatory cells. We obtained, that smoking history (pack-years) positively correlates with number of BAL neutrophils in both COPD groups and 'healthy' smokers. Such relation once more supports the role of neutrophils recruitment in response to cigarette smoke and suggests that longer smoking history leads to more serious lung function damage. Smoking may have accumulative effect of inflammatory cells and may increase an inflammatory response in COPD and 'healthy' smokers as well. Also, we observed the positive correlation between BAL macrophages and smoking history in COPD smokers and 'healthy' smokers. It is known that cigarette smoke increases protease-antiprotease imbalance and alveolar macrophages, which are significant source of some MMPs [16, 18]. According to animal studies, MMP-12 deficiency protects against cigarette smoke induced emphysema [18, 19]. Though, most studies investigating MMP-12 were performed using animal models and exact role of MMP-12 in human COPD inflammation is not fully understood.
We analysed an expression of MMP-12 active form using immunocytochemistry.
The number of IS MMP-12+-macrophages did not differ between COPD groups, but it was higher compared to healthy subjects. Absence of significant differences in MMP-12 expression in IS may be explained by predominance of neutrophils, in COPD smokers and ex-smokers, which obviously do not express MMP-12. An expression of MMP-12 in IS from 'healthy' smokers was increased, compared to never-smokers, supporting the suggestion that smoking may increase an expression of this enzyme. Our results are in agreement to Demedts et al. [20], who found an increased sputum MMP-12 level in COPD patients, compared to healthy smokers, former smokers (>1 year) and never smokers, while they have not divided COPD patients into smokers and ex-smokers. Also, Molet et al., have reported an increase of MMP-12 in BAL and bronchial biopsies of COPD patients compared to controls [30], while they have not investigated an expression of MMP-12 according to smoking status.
One of the most interesting our findings was an increased number of MMP-12+-macrophages in BAL from COPD smokers compared to COPD ex-smokers. Also, the number of MMP-12+-macrophages was increased in both COPD groups, compared to controls. Nevertheless we observed a lower amount of BAL macrophages in COPD smokers, compared to COPD ex-smokers, the absolute and relative number of BAL MMP-12+-macrophages in COPD smokers was higher than in COPD ex-smokers. 'Healthy' smokers had higher number of BAL MMP-12+-macrophages, than never-smokers supporting the fact of smoking impact in MMP-12 expression. Actually, we did not evaluate the activity of macrophages in this study, thus we were not able to investigate the ratio of MMP-12 release and activated macrophages in this study.
Also, an increased number of BAL MMP-12+-macrophages in COPD ex-smokers, compared to 'healthy' smoking subjects, let us hypothesize that MMP-12 expression is induced not only by cigarette smoking, but may be an obligatory to the development of COPD.
Previous studies have shown that contribution of MMP-12 to smoke induced emphysema is probably enhanced by indirect effects, such as inactivation of AAT [31] and MMP-12 mediated recruitment of neutrophils to the lung [18]. Otherwise, our data suggests that MMP-12 may accumulate and do not rapidly decreases or inactivates after smoking cessation, exaggerating a persistent inflammation. An increased expression of MMP-12 in 'healthy' smokers, also may be a reason for COPD development in the future.
Conclusion
Smokers and ex-smokers with COPD had close to similar number and type of IS inflammatory cells, indicating an ongoing inflammation in proximal airways after smoking cessation. Although, the lower amount of BAL neutrophils in COPD ex-smokers, compared to COPD smokers suggests, that smoking cessation may cause positive alterations in alveolar compartment.
Also, a higher number of MMP-12+-macrophages in IS and BAL from COPD smokers and COPD ex-smokers, indicates that smoking, which is an initial step contributing to the development of COPD, may stimulate MMP-12 expression in airway cells. Moreover, it let as argue that MMP-12 expression may be induced not only by smoking, but by the disease itself. A lower amount of BAL MMP-12+-macrophages and other mentioned inflammatory cells, compared to COPD smokers, may indicate a decrease of alveolar inflammation after smoking cessation.
Abbreviations
- BAL:
-
bronchoalveolar lavage
- COPD:
-
chronic obstructive pulmonary disease
- DTT:
-
dithiothreitol
- FEV1 :
-
forced expiratory volume in 1 sec
- FVC:
-
forced vital capacity
- ICC:
-
immunocytochemistry
- IS:
-
induced sputum
- MMP-12:
-
matrix metalloproteinase
- PBS:
-
phosphate-buffered saline
- RT:
-
room temperature
References
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease [http://www.goldcopd.com] NHLBI/WHO Workshop Report 2006.
Barnes PJ, Shapiro SD, Pauwels RA: Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003, 22:672–88.
Domagala-Kulawik J, Maskey-Warzechowska M, Kraszewska I, Chazan R: The cellular composition and macrophage phenotype in induced sputum in smokers and ex-smokers with COPD. Chest 2003, 123:1054–1059.
Snider GL: Chronic obstructive pulmonary disease: a definition and implications of structural determinants of airflow obstruction for epidemiology. Am Rev Respir Dis 1989, 140:S3–8.
Fletcher C, Peto R: The natural history of chronic airflow obstruction. Br Med J 1977,1(6077):1645–1648.
Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD: The nature of small airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004, 350:2645–2653.
Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM: Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001, 163:1304–9.
Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, Mapp CE, Fabbri LM, Donner CF, Saetta M: Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med 1998, 158:1277–1285.
Sitkauskiene B, Sakalauskas R, Malakauskas K, Lotvall J: Reversibility to a β 2 -agonist in COPD: relationship to atopy and neutrophil activation. Respir Med 2003, 97:591–598.
Ekberg-Jansson A, Bake B, Andersson B, Skoogh BE, Lofdahl CG: Respiratory symptoms relate to physiological changes and inflammatory markers reflecting central but not peripheral airways. A study in 60-year-old 'healthy' smokers and never-smokers. Respir Med 2001, 95:40–7.
Finkelstein R, Fraser RS, Ghezzo H, Cosio MG: Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med 1995, 152:1666–72.
Eidelman D, Saetta MP, Ghezzo H, Wang NS, Hoidal JR, King M, Cosio MG: Cellularity of the alveolar walls in smokers and its relation to alveolar destruction. Functional implications. Am Rev Respir Dis 1990, 141:1547–52.
Russell RE, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, Fitzgerald M, Barnes PJ: Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. Am J Physiol Lung Cell Mol Physiol 2002, 283:L867-L873.
Maus UA, Koay MA, Delbeck T, Mack M, Ermert M, Ermert L, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J: Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. Am J Physiol Lung Cell Mol Physiol 2002, 282:L1245–52.
Parks WC, Shapiro SD: Matrix metalloproteinases in lung biology. Respir Res 2001, 2:10–9.
Shapiro SD, Kobayashi DK, Ley TJ: Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem 1993, 268:23824–9.
Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD: Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res 2006, 66:6149–55.
Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL: Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumour necrosis factor- release. Am J Respir Crit Care Med 2003, 167:1083–9.
Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD: Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997, 277:2002–4.
Demedts IK, Morel-Montero A, Lebecque S, Pacheco Y, Cataldo D, Joos GF, Pauwels RA, Brusselle GG: Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax 2006, 61:196–201.
Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC: Lung volumes and forced ventilatory flows. Report working party. Standartization of lung function tests. European Community for Steel and Coal. Official statement of the European Respiratory Society. Eur Respir J 1993, 6:5–40.
Thompson PB, Daughton D, Robbins GA, Ghafouri MA, Oehlerking M, Rennard SI: Intraluminal airway inflammation in chronic bronchitis. Characterization and correlation with clinical parameters. Am Rev Respir Dis 1989, 140:1527–37.
Gaga M, Zervas E, Loukides S: Inflammatory markers in monitoring response to treatment for asthma and chronic obstructive pulmonary disease. Pneumon 2004, 17:242–250.
Alexis NE, Hu SC, Zeman K, Alter T, Bennett WD: Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med 2001,164(10 Pt 1):1964–1970.
Pizzichini E, Pizzichini MM, Efthimiadis A, Hargreave FE, Dolovich J: Measurement of inflammatory indices in induced sputum: effects of selection of sputum to minimize salivary contamination. Eur Respir J 1996, 9:1174–1180.
Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M: Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 2000, 117:684–94.
Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, Kawakami Y: Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med 1999,159(6):1985–1991.
Willemse BWM, Hacken NHT, Rutgers B, Lesman-Leegte IGAT, Postma DS, Timens W: Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J 2005, 26:835–845.
Lofdahl JM, Cederlund K, Nathell L, Eklund A, Skold CM: Bronchoalveolar lavage in COPD: fluid recovery correlates with the degree of emphysema. Eur Respir J 2005, 25:275–281.
Molet S, Belleguic C, Lena H, Germain N, Bertrand CP, Shapiro SD, Planquois JM, Delaval P, Lagente V: Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary disease. Inflamm Res 2005, 54:31–36.
Gronski TJ Jr, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, Van Wart HE, Shapiro SD: Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem 1997, 272:12189–94.
Acknowledgements
We are grateful to Elvyra Draugeliene, MD and Vytis Dudzevicius, PhD for their invaluable help performing bronchoscopies; Kestutis Malakauskas, PhD for helpful discussions; Algirda Krisiukeniene, MDSandra Ragaisiene, MD, Irena Jakubanis, BSc and Inesa Jermalaviciene for their technical support. This study was in part supported by a Scientific Foundation of Kaunas University of Medicine (Project Grant PAR8), Lithuania.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
AB carried out the major part of cytological analysis and immunocytochemistry, participated in the writing of manuscript;
KS carried out screening and clinical evaluation of study subjects;
JJ participated in the study design, carried out the part of immunocytochemistry and performed some statistical analysis;
JL participated in the study design and in the sequence alignment
RS participated in the study design and in the sequence alignment
BS conceived and supervised the study and participated in its design, participated in the writing of the manuscript.
All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Babusyte, A., Stravinskaite, K., Jeroch, J. et al. Patterns of airway inflammation and MMP-12 expression in smokers and ex-smokers with COPD. Respir Res 8, 81 (2007). https://doi.org/10.1186/1465-9921-8-81
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1465-9921-8-81