Abstract
Aims
To assess the cross-sectional association between exercise capacity, gas exchange efficiency and endothelial function, as measured by flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NMD) of the brachial artery, in a large-scale population-based survey.
Methods
The study population was comprised of 1416 volunteers 25 to 85 years old. Oxygen uptake at anaerobic threshold (VO2@AT), peak exercise (peakVO2) and ventilatory efficiency (VE vs. VCO2 slope and VE/VCO2@AT) were assessed on a breath-by-breath basis during incremental symptom-limited cardiopulmonary exercise. FMD and NMD measurements at rest were performed using standardised ultrasound techniques.
Results
Multivariable logistic regression analyses revealed a significant association between FMD and ventilatory efficiency in current smokers but not in ex-smokers or non-smokers. There was no association between FMD and VO2@AT or peak VO2. In current smokers, for each one millimetre decrement in FMD, VE/VCO2@AT improved by -3.6 (95% CI -6.8, -0.4) in the overall population [VE vs. VCO2 slope -3.9 (-7.1, -0.6)]. These results remained robust after adjusting for all major influencing factors. Neither exercise capacity nor ventilatory efficiency was significantly associated with NMD.
Conclusion
In current smokers, FMD is significantly associated with ventilatory efficiency. This result may be interpreted as a potential clinical link between smoking and early pulmonary vasculopathy due to smoking.
Similar content being viewed by others
Introduction
Endothelial dysfunction represents an early, subclinical stage of vascular dysfunction that precedes the development of atherosclerosis [1] and predicts cardiovascular morbidity and mortality [2]. Its potential association with the functional capacity of the cardiovascular, pulmonary and muscular systems assessed by cardiopulmonary exercise testing (CPET) has been shown in small groups of young [3, 4] and old, healthy individuals [5, 6]. Usually, endothelial function is assessed by measuring flow-mediated dilation (FMD) using ultrasound. Occasionally, FMD is described in comparison to nitroglycerin-mediated dilation (NMD) as a surrogate of endothelial-independent vasoregulation. These measurements can be conducted in various vascular regions [7, 8]; however, for feasibility reasons, this vascular response is commonly assessed in forearm vessels [9, 10].
Dyspnoea, which is a symptomatic hallmark in patients with cardiovascular or pulmonary vascular diseases, can be quantified by gas exchange and ventilatory efficiency [11]. An impaired ventilatory efficiency is related to ventilation-perfusion inhomogeneities in patients with congestive heart failure [12, 13] and pulmonary hypertension [14, 15]. Thus, the ventilatory efficiency in eliminating carbon dioxide is considered a reliable measure for describing the relationship between pulmonary ventilation and perfusion [16]. Aside from the impact of cardiopulmonary diseases on ventilatory efficiency, previous studies have shown that smoking impairs ventilatory efficiency depending on the extent of cigarette exposure, which is possibly related to early airway dysfunction or, alternatively, pulmonary vasculopathy [17]. If endothelial function in the lungs mainly determines ventilatory efficiency, as assessed by gas exchange measurements, this would be a clinically accessible surrogate parameter to describe the functional integrity of pulmonary vessels and, hence, pulmonary perfusion. In normal pulmonary vessels, principal mediators of endothelial function, including nitric oxide (NO) and prostacyclin, regulate the maintenance of normal vascular tone and distribute the blood flow within the lung [18–20]. Correspondingly, diseases primarily affecting the pulmonary vascular bed, such as idiopathic pulmonary arterial hypertension, are associated with deficiencies in both mediators [21], which lead to diminished pulmonary endothelial function [22]. Previous data assessed within a small group of individuals suggest that the pulmonary vascular response to inhaled iloprost, a stable analogue of prostacyclin, is related positively to the extent of NO-dependent endothelial vasodilation, as assessed by FMD, in individuals with idiopathic pulmonary arterial hypertension (IPAH) [23]. It remains unknown whether FMD reflects endothelial dysfunction in pulmonary vessels in apparently healthy individuals as well.
Therefore, this investigation aimed to assess the potential link between peripheral endothelial function and gas exchange in a large-scale population-based study called the Study of Health in Pomerania (SHIP). The major hypothesis tested was that FMD is related to exercise capacity and ventilatory efficiency in a sample representing a wide age range of the general population.
Methods
Study population
The Study of Health in Pomerania (SHIP) is a population-based investigation in West Pomerania, a region in the northeastern part of Germany. The details of the study are given elsewhere [24, 25]. In brief, a sample from a population aged 20 - 79 years was recruited from 1997 to 2001 to be evaluated during baseline SHIP-0. Between March 2002 and September 2006, the 5-year follow-up examinations (SHIP-1) were performed, which comprised 3300 participants (1711 women). The study was reviewed by a board of independent scientists and approved by the Ethics Committee of the University of Greifswald (approval number Dec 12, 2001: IIIUV73/01). All participants provided written informed consent.
We offered all SHIP-1 participants the opportunity to take part in measurements of endothelial function (FMD and NMD), body plethysmography and CPET. Of the 3300 SHIP-1 participants, 1705 volunteered in both CPET and endothelial function determination. We excluded 278 subjects with non-readable ultrasound images and 11 subjects with missing data. The study population available for the present analyses consisted of 1416 (701 men, 715 women) volunteers. Table 1 summarises the details of the study population.
For sensitivity analyses, an apparently healthy population without factors possibly interfering with endothelial function and CPET was defined. For this purpose, subjects with the following characteristics were excluded (overlaps exist): past myocardial infarction, echocardiographic evidence of ventricular dysfunction or valvular disease, electrocardiographic signs of ischaemia, neuromuscular or musculoskeletal disorders based on neurological examination, malignancies, pulmonary diseases, chronic obstructive bronchitis, bronchial asthma, drugs against obstructive airway disease including inhaled steroids [(ATC) code R03], arterial hypertension according to the definition of the World Health Organization[26] or the use of antihypertensive medications at the time of enrolment, and diabetes. Thus, the apparently healthy study population comprised of 985 volunteers (472 men, 513 women).
Pre-exercise diagnostics and exclusion criteria
Sociodemographic and medical characteristics were assessed by computer-assisted personal interviews. Previous history of diseases was assessed based on self-reported physician's diagnosis. According to tobacco consumption, participants were categorised into current (one or more cigarettes per day), former, and non-smokers. Data on medication were collected using the anatomical therapeutic chemical (ATC) code [27]. Antihypotensives, antihypertensives, peripheral vasodilators, beta-blockers, calcium channel blockers, drugs acting on the renin-angiotensin system, statins, bronchodilators and nonsteroidal anti-inflammatory drugs (oral or inhaled), which could act as confounders, were included in the analyses. The diagnosis of arterial hypertension and diabetes mellitus was based on self-reported physician's diagnosis and physical examination [26].
Flow- and nitroglycerin-mediated dilation
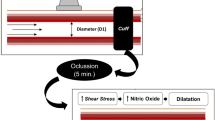
Endothelial function was assessed by FMD and endothelial-independent vasoregulation by NMD. Examinations were performed in a supine position by two observers. The subject's right arm was comfortably immobilised, and the brachial artery diameter was recorded 3-7 cm above the antecubital fossa using a 10-MHz linear array transducer ultrasound system (Cypress, Siemens AG, Erlangen, Germany). After the resting scan, a pneumatic cuff placed around the forearm 10 cm distal to the ultrasound location was inflated above a pressure of 220 mmHg for 5 min. Diameter measurements were repeated 60 s after cuff deflation. FMD was expressed as the post-occlusion brachial artery diameter corrected for baseline artery diameter (BAD) and as the ratio between brachial diameters before and after inflation of the pneumatic cuff. NMD was taken 3 min after sublingual administration of nitroglycerin (400 μg) in 1096 subjects (465 women). Examinations were performed and read by two observers. All ultrasound measurements in SHIP use strict quality management [28]. Intrareader, intraobserver, inter-reader, and interobserver variability were evaluated in certification procedures. Before data collection, 25 images were measured twice by each participating reader, and 12 volunteers were examined twice by each participating observer. During the data collection, observer certification procedures were repeated semiannually in six volunteers. At least 24 h was required between the two readings and examinations. Readers rated the quality of the digitally stored images as excellent, good, or adequate. The applied quality measures have been described elsewhere in detail [9].
Exercise testing
CPET was performed with a physician in attendance according to a modified Jones protocol [29] using a calibrated electromagnetically braked cycle ergometer (Ergoselect 100, Ergoline, Germany). Protocol details are given elsewhere [17, 30]. Gas exchange and ventilatory variables were analysed breath-by-breath using a VIASYS HEALTHCARE system (Oxycon Pro, Rudolph's mask), which had been recalibrated prior to each test. Twelve-lead ECGs were recorded at rest and every minute thereafter. Pulse oximetry was monitored continuously, and blood pressure was obtained by a cuff sphygmomanometer every two minutes. Prior to CPET, subjects were encouraged to reach maximal exhaustion. During exercise, no further motivation was utilised.
The minute ventilation, tidal volume, VO2 and VCO2 were acquired on a breath-by-breath basis and averaged over 10-second intervals. The peak oxygen uptake was defined as the highest 10-second average of VO2 in late exercise. The peak heart rate was averaged over that same period, and the peak O2 pulse was calculated as peak VO2 divided by peak heart rate. The peak respiratory exchange rate (RER) was calculated as the ratio of peak carbon dioxide output (VCO2) to peakVO2. The anaerobic threshold (AT) was determined according to Wasserman et al. [16]. The VE/VCO2@AT was averaged over a 30-second period. The VE/VCO2 ratio at rest was averaged over the last 30 seconds of a 3-minute resting period. The delta of the rest to anaerobic threshold VE/VCO2 ratio was calculated.
Statistical analysis
Continuous data are expressed as the mean (± SD), and nominal data are expressed as numbers (percentages) and 95% confidence intervals. For bivariate statistics, the Mann Whitney U test (continuous data) and χ2 test (nominal data) were applied to compare men and women. Multivariable linear regression models were performed to estimate the independent association of FMD or NMD with ventilatory efficiency and exercise capacity separately in current and non-/or ex-smokers in the overall and healthy populations for both sexes. Sensitivity analyses were performed to identify possible interfering factors. In the final model, we only considered those characteristics as confounders if inclusion in the model led to ≥ 10% change in the coefficient of interest. For this, clinical (medications against cardiopulmonary disorders, smoking, sex, age, height, weight, and arterial hypertension) and laboratory variables (diabetes and serum cholesterol) were included [9]. Thereafter, variables on the medications listed in Table 1 were entered into the model in various orders. Based on those analyses, the full models were adjusted for age, vascular baseline diameter, weight, and height. Statistical significance was defined by p < 0.05. All statistical analyses were performed with SAS software, version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
In the entire study population, the quality of FMD images was rated as excellent in 164 subjects (11.6%), good in 744 (52.5%), and adequate in 508 (35.9%). In the overall study population, the median RER at peak exercise was 1.10 (CI 1.05, 1.17) in men and 1.13 (CI 1.05, 1.19) in women. Thirty-five subjects reported a prior myocardial infarction, 20 had electrocardiographic evidence of myocardial ischaemia, 40 subjects had echocardiographic evidence of aortic dysfunction, 14 had echocardiographic evidence of mitral valve dysfunction, 30 echocardiographic had evidence of left ventricular dysfunction, 39 reported chronic obstructive pulmonary disease (COPD), 5 reported asthma and 37 reported other pulmonary diseases (overlaps existed). Use of drugs against cardiopulmonary diseases was reported by 187 subjects. None of the subjects revealed signs of pulmonary hypertension or had evidence of pulmonary embolism or clinically significant peripheral arterial vasculopathy.
Independent of smoking status in the healthy and overall population, FMD and NMD did not reveal any association with exercise capacity, as quantified by peakVO2 and VO2@AT, or with oxygen pulse.
In current smokers, FMD was inversely related to ventilatory efficiency (Table 2). In current smokers, for each one millimetre decrement in FMD, VE/VCO2@AT improved by -3.6 (95%CI -6.8; -0.4) in the overall population [VE vs. VCO2 slope -3.9 (-7.1, -0.6)] and -4.6 in apparently healthy volunteers (CI -8.2; -1.0) [VE vs. VCO2 slope -5.3 (-8.9, -1.7)]. In non- and ex-smokers FMD did not show any significant association with parameters of ventilatory efficiency (Table 3). NMD did not show a significant association to ventilatory efficiency. The decline in VE/VCO2 ratio from rest to exercise at the anaerobic threshold was not significantly associated with FMD. All effects were consistently reproducible through all reported or diagnosed comorbidities. There were no detectable differences between women and men.
Discussion
In terms of this study's hypotheses, neither in smokers nor non-smokers did endothelial function reveal any association with peak exercise capacity as verified by peak VO2 or aerobic exercise capacity, as judged by VO2@AT. Thus, it has to be postulated that NO-dependent endothelial function plays a minor, unverifiable role in muscle endurance and exercise capacity as assessed within a symptom-limited CPET in healthy volunteers.
Previous studies have suggested a potential interference of endothelial functioning with exercise capacity [3–6]. Palmieri et al. have shown a tight correlation between VO2@AT and peak VO2 in FMD in young adults [3], which is comparable to results that have been reported in older individuals by Rinder et al. and Rywik et al. [5, 6]. Furthermore, exercise training seems to influence endothelial function with corresponding increases in exercise capacity [31], and training status has been shown to influence exercise capacity and endothelial function [4]. However, all of these studies were based on small groups of volunteers and do not represent a general population. The suggested impact of NO-dependent endothelial function on exercise capacity is now challenged by our results. According to the data presented here, endothelial dysfunction quantified by FMD has no significant impact on exercise capacity as quantified by oxygen uptake at anaerobic threshold or peak exercise and is independent of smoking status and potentially confounding diseases. To what extent exercise endurance training may influence FMD parallel to exercise capacity could not be investigated by our study and has to be addressed by longitudinal and interventional studies.
This study shows that in current smokers, FMD is significantly correlated to ventilatory efficiency independent of sex and co-morbidities. This correlation is not verifiable in non- or ex-smokers. To the best of our knowledge, this is the first study describing a correlation between NO-dependent endothelial function and gas exchange efficiency and exercise capacity in a large-scale population-based study. Our previous work assessed the influence of smoking on exercise capacity and gas exchange efficiency in the same population-based study [17]. In that study, ventilatory efficiency correlated with the extent of smoking in individuals without apparent cardiovascular or pulmonary diseases and with normal lung function, body plethysmography and echocardiography [17]. One aspect of that study was to interpret changes in ventilatory efficiency as an early marker of parenchymal or vascular lung disease related to smoking independent of lung function abnormalities. Based on the inverse relationship between NO-dependent endothelial function and gas exchange efficiency, a vascular hypothesis might be supported. Endothelial dysfunction is related to several peripheral vascular diseases, such as arterial hypertension, diabetic vasculopathy [31–33] and pulmonary vascular diseases [18, 22, 23, 34]. However, ventilatory efficiency is impaired in patients with abnormal pulmonary circulation and reliably mirrors the severity of pulmonary vascular diseases, such as pulmonary arterial hypertension [35]. The significant correlation between ventilatory efficiency and FMD independent of health status may potentially suggest a sub-clinical smoking-related pulmonary vascular abnormality. Smoking has been proposed to potentially trigger pulmonary vascular disease in experimental studies in animals [36, 37]. In addition, smoking has been discussed as an important contributor to the development of pulmonary hypertension in COPD patients [38]. Pulmonary vascular abnormalities in patients with mild-to-moderate COPD mainly consist of the thickening of the intima in pulmonary muscular arteries, which interferes with lumen size [38]. Interestingly, studies conducted in smokers with normal lung function have also revealed intimal thickening in pulmonary muscular arteries [39]. In addition, ventilatory efficiency and gas exchange may be impacted by early airway disease as well. The potential link between smoking, early airway disease and pulmonary vasculopathy may be due to low-grade systemic inflammation. In early stages of chronic obstructive pulmonary disease, perfusion heterogeneity and low airflow obstruction have been observed, which suggests that in smokers, initially the smallest airways, parenchyma, and pulmonary vessels are affected [40]. In contrast to FMD, NMD is a marker of endothelium-independent vasodilation [41]. Although there was an association between smoking and FMD in our study, we did not find such an association for NMD. This result strengthens the hypothesis that smoking may affect endothelial function via the NO system.
Finally, our study has limitations. The SHIP project, as a large-scale observational population-based study, was not designed to test the hypotheses that vascular abnormalities are related to ventilatory inefficiency in smokers. However, to the best of our knowledge, this is the first study to describe the interaction of endothelial function, exercise capacity and ventilatory efficiency in a large population sample. Because this study is based on individual volunteering, as in any population-based cross-sectional survey, we cannot fully rule out selection bias. We observed that CPET volunteers were younger than non-participants, which might have led to a healthier study population [42].
Furthermore, due to ethical reasons the design of a population-based survey does not allow for histopathological investigations. Thus, the final proof of the hypotheses discussed here is pending.
Conclusions
In conclusion, in a general adult population, peripheral NO-dependent vasodilation assessed by FMD was not associated with exercise capacity and was independent of coexisting diseases. A significant, inverse association between FMD and ventilatory efficiency did exist in smokers, whereas this association was not verifiable in non- or ex-smokers. In current smokers, a decreased FMD was associated with impaired ventilatory efficiency. This association may be interpreted as a potential link between smoking and early pulmonary vasculopathy due to smoking exposure.
Abbreviations
- AT:
-
Anaerobic threshold
- ATC code:
-
Anatomical-technical-chemical code
- BAD:
-
Brachial artery diameter
- CPET:
-
Cardiopulmonary exercise testing
- ECG:
-
Electrocardiogram
- FMD:
-
Flow-mediated dilation
- IPAH:
-
Idiopathic pulmonary arterial hypertension
- NMD:
-
Nitrogen-mediated dilation
- NO:
-
Nitrate oxide
- peakVO2:
-
Peak oxygen uptake
- RER:
-
Respiratory exchange rate
- SHIP:
-
Study of Health in Pomerania
- VCO2:
-
Carbon dioxide output
- VE vs. VCO2 slope:
-
Slope of the regression of minute ventilation to carbon dioxide output
- VE/VCO2@AT:
-
Minute ventilation to carbon dioxide ratio at anaerobic threshold
- VO2:
-
Oxygen uptake
- VO2@AT:
-
Oxygen uptake at anaerobic threshold.
References
Faulx MD, Wright AT, Hoit BD: Detection of endothelial dysfunction with brachial artery ultrasound scanning. Am Heart J. 2003, 145 (6): 943-951. 10.1016/S0002-8703(03)00097-8.
Shimbo D, Grahame-Clarke C, Miyake Y, Rodriguez C, Sciacca R, Di Tullio M, Boden-Albala B, Sacco R, Homma S: The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis. 2007, 192 (1): 197-203. 10.1016/j.atherosclerosis.2006.05.005.
Palmieri EA, Palmieri V, Innelli P, Arezzi E, Ferrara LA, Celentano A, Fazio S: Aerobic exercise performance correlates with post-ischemic flow-mediated dilation of the brachial artery in young healthy men. Eur J Appl Physiol. 2005, 94 (1-2): 113-117. 10.1007/s00421-004-1285-0.
Moe IT, Hoven H, Hetland EV, Rognmo O, Slordahl SA: Endothelial function in highly endurance-trained and sedentary, healthy young women. Vasc Med. 2005, 10 (2): 97-102.
Rinder MR, Spina RJ, Ehsani AA: Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol. 2000, 88 (2): 761-766.
Rywik TM, Blackman MR, Yataco AR, Vaitkevicius PV, Zink RC, Cottrell EH, Wright JG, Katzel LI, Fleg JL: Enhanced endothelial vasoreactivity in endurance-trained older men. J Appl Physiol. 1999, 87 (6): 2136-2142.
Celermajer DS, Cullen S, Deanfield JE: Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation. 1993, 87 (2): 440-446.
Wensel R, Opitz CF, Kleber FX: Acetylcholine but not sodium nitroprusside exerts vasodilation in pulmonary hypertension secondary to chronic congestive heart failure. J Heart Lung Transplant. 1999, 18 (9): 877-883. 10.1016/S1053-2498(99)00041-8.
Volzke H, Robinson DM, Spielhagen T, Nauck M, Obst A, Ewert R, Wolff B, Wallaschofski H, Felix SB, Dorr M: Are serum thyrotropin levels within the reference range associated with endothelial function?. Eur Heart J. 2009, 30 (2): 217-224.
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al: Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002, 39 (2): 257-265. 10.1016/S0735-1097(01)01746-6.
Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M: Development of a ventilatory classification system in patients with heart failure. Circulation. 2007, 115 (18): 2410-2417. 10.1161/CIRCULATIONAHA.107.686576.
Guazzi M, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Arena R: The added prognostic value of ventilatory efficiency to the Weber classification system in patients with heart failure. Int J Cardiol. 2008, 129 (1): 86-92. 10.1016/j.ijcard.2007.05.028.
Kleber FX, Vietzke G, Wernecke KD, Bauer U, Opitz C, Wensel R, Sperfeld A, Glaser S: Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation. 2000, 101 (24): 2803-2809.
Wensel R, Opitz CF, Anker SD, Winkler J, Hoffken G, Kleber FX, Sharma R, Hummel M, Hetzer R, Ewert R: Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 2002, 106 (3): 319-324. 10.1161/01.CIR.0000022687.18568.2A.
Groepenhoff H, Vonk-Noordegraaf A, Boonstra A, Spreeuwenberg MD, Postmus PE, Bogaard HJ: Exercise testing to estimate survival in pulmonary hypertension. Med Sci Sports Exerc. 2008, 40 (10): 1725-1732. 10.1249/MSS.0b013e31817c92c0.
Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ: Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. 2004, Lippincott Williams and Wilkins, 4
Glaser S, Koch B, Ittermann T, Schaper C, Dorr M, Felix SB, Volzke H, Ewert R, Hansen JE: Influence of age, sex, body size, smoking, and beta blockade on key gas exchange exercise parameters in an adult population. Eur J Cardiovasc Prev Rehabil. 2010, 17 (4): 469-476. 10.1097/HJR.0b013e328336a124.
Cooper CJ, Landzberg MJ, Anderson TJ, Charbonneau F, Creager MA, Ganz P, Selwyn AP: Role of nitric oxide in the local regulation of pulmonary vascular resistance in humans. Circulation. 1996, 93 (2): 266-271.
Clement MG, Albertini M: Differential release of prostacyclin and nitric oxide evoked from pulmonary and systemic vascular beds of the pig by endothelin-1. Prostaglandins Leukot Essent Fatty Acids. 1996, 55 (4): 279-285. 10.1016/S0952-3278(96)90009-5.
Albertini M, Vanelli G, Clement MG: PGI2 and nitric oxide involvement in the regulation of systemic and pulmonary basal vascular tone in the pig. Prostaglandins Leukot Essent Fatty Acids. 1996, 54 (4): 273-278. 10.1016/S0952-3278(96)90058-7.
Giaid A, Saleh D: Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995, 333 (4): 214-221. 10.1056/NEJM199507273330403.
Peled N, Shitrit D, Fox BD, Shlomi D, Amital A, Bendayan D, Kramer MR: Peripheral arterial stiffness and endothelial dysfunction in idiopathic and scleroderma associated pulmonary arterial hypertension. J Rheumatol. 2009, 36 (5): 970-975. 10.3899/jrheum.081088.
Wolff B, Lodziewski S, Bollmann T, Opitz CF, Ewert R: Impaired peripheral endothelial function in severe idiopathic pulmonary hypertension correlates with the pulmonary vascular response to inhaled iloprost. Am Heart J. 2007, 153 (6): e1081-1087. 10.1016/j.ahj.2007.03.007. 1088
John U, Greiner B, Hensel E, Ludemann J, Piek M, Sauer S, Adam C, Born G, Alte D, Greiser E, et al: Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Soz Praventivmed. 2001, 46 (3): 186-194. 10.1007/BF01324255.
Volzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G, et al: Cohort Profile: The Study of Health in Pomerania. Int J Epidemiol. 2010
Whitworth JA: 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003, 21 (11): 1983-1992.
WHO: ATC index with DDDs annually guidelines for ATC classification and DDD assignment. 2008, Oslo, Norway: WHO Collaborating Centre for drug Statistics Methodology
Dorr M, Wolff B, Robinson DM, John U, Ludemann J, Meng W, Felix SB, Volzke H: The association of thyroid function with cardiac mass and left ventricular hypertrophy. J Clin Endocrinol Metab. 2005, 90 (2): 673-677.
Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N: Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985, 131 (5): 700-708.
Koch B, Schaper C, Ittermann T, Spielhagen T, Dorr M, Volzke H, Opitz CF, Ewert R, Glaser S: Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J. 2009, 33 (2): 389-397.
Clarkson P, Celermajer DS, Powe AJ, Donald AE, Henry RM, Deanfield JE: Endothelium-dependent dilatation is impaired in young healthy subjects with a family history of premature coronary disease. Circulation. 1997, 96 (10): 3378-3383.
Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Ronnemaa T, Raitakari OT: Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004, 110 (18): 2918-2923. 10.1161/01.CIR.0000147540.88559.00.
Kato T, Inoue T, Node K: Postprandial endothelial dysfunction in subjects with new-onset type 2 diabetes: an acarbose and nateglinide comparative study. Cardiovasc Diabetol. 2010, 9 (1): 12-10.1186/1475-2840-9-12.
Budhiraja R, Tuder RM, Hassoun PM: Endothelial dysfunction in pulmonary hypertension. Circulation. 2004, 109 (2): 159-165. 10.1161/01.CIR.0000102381.57477.50.
Arena R, Lavie CJ, Milani RV, Myers J, Guazzi M: Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: an evidence-based review. J Heart Lung Transplant. 2010, 29 (2): 159-73. 10.1016/j.healun.2009.09.003.
Lee SD, Lee JH, Kim EK, Choi KH, Oh YM, Shim TS: Effects of simvastatin on cigarette smoking-induced structural and functional changes in rat lungs. Chest. 2005, 128 (6 Suppl): 574S-
Wang T, Han SX, Zhang SF, Ning YY, Chen L, Chen YJ, He GM, Xu D, An J, Yang T, et al: Role of chymase in cigarette smoke-induced pulmonary artery remodeling and pulmonary hypertension in hamsters. Respir Res. 2010, 11: 36-10.1186/1465-9921-11-36.
Barbera JA, Peinado VI, Santos S: Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003, 21 (5): 892-905. 10.1183/09031936.03.00115402.
Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, Gimferrer JM, Rodriguez-Roisin R: Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol. 1998, 274 (6 Pt 1): L908-913.
Rodriguez-Roisin R, Drakulovic M, Rodriguez DA, Roca J, Barbera JA, Wagner PD: Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol. 2009, 106 (6): 1902-1908. 10.1152/japplphysiol.00085.2009.
Neunteufl T, Katzenschlager R, Abela C, Kostner K, Niederle B, Weidinger F, Stefenelli T: Impairment of endothelium-independent vasodilation in patients with hypercalcemia. Cardiovasc Res. 1998, 40 (2): 396-401. 10.1016/S0008-6363(98)00177-1.
Glaser S, Friedrich N, Ewert R, Schaper C, Krebs A, Dorr M, Volzke H, Felix SB, Nauck M, Wallaschofski H, et al: Association of circulating IGF-I and IGFBP-3 concentrations and exercise capacity in healthy volunteers: results of the Study of Health in Pomerania. Growth Horm IGF Res. 2010, 20 (6): 404-410. 10.1016/j.ghir.2010.09.002.
Acknowledgements and Funding
SHIP is part of the Community Medicine Net of the University of Greifswald, which is funded by grants from the German Federal Ministry of Education and Research for SHIP (BMBF, grant 01ZZ96030, 01ZZ0701) and the German Asthma and COPD Network (COSYCONET; BMBF grant 01GI0883); the Ministry for Education, Research, and Cultural Affairs and the Ministry for Social Affairs of the Federal State of Mecklenburg-West Pomerania. The contributions to the data collection made by all contributors are gratefully acknowledged.
All authors have significantly contributed to the conception and design of study, the analysis and interpretation of data, the drafting of the manuscript, the critical revisions for important intellectual content and the final approval of the manuscript submitted.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SG drafted the manuscript, made substantial contributions to the conception, design and acquisition of the data as well as the analysis and interpretation of the data and has given final approval for the version to be published. AO acted as one of the leading statisticians that analysed the data, made substantial contributions to the conception, design and acquisition of the data, was involved in drafting the manuscript and revising it critically for important intellectual content and has given final approval for the version to be published. CFO, SBF, KE, CS, RE, MD, HV and BK have made substantial contributions to the conception, design and acquisition of the data as well as the analysis and interpretation of the data, were involved in drafting the manuscript or revising it critically for important intellectual content and have given final approval for the version to be published.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gläser, S., Obst, A., Opitz, C.F. et al. Peripheral endothelial dysfunction is associated with gas exchange inefficiency in smokers. Respir Res 12, 53 (2011). https://doi.org/10.1186/1465-9921-12-53
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1465-9921-12-53