Abstract
Background
Pulmonary veins (PVs) are the most important sources of ectopic beats with the initiation of paroxysmal atrial fibrillation, or the foci of ectopic atrial tachycardia and focal atrial fibrillation. Elimination of nitric oxide (NO) enhances cardiac triggered activity, and NO can decrease PV arrhythmogensis through mechano-electrical feedback. However, it is not clear whether NO may have direct electrophysiological effects on PV cardiomyocytes. This study is aimed to study the effects of nitroprusside (NO donor), on the ionic currents and arrhythmogenic activity of single cardiomyocytes from the PVs.
Methods
Single PV cardiomyocytes were isolated from the canine PVs. The action potential and ionic currents were investigated in isolated single canine PV cardiomyocytes before and after sodium nitroprusside (80 μM,) using the whole-cell patch clamp technique.
Results
Nitroprusside decreased PV cardiomyocytes spontaneous beating rates from 1.7 ± 0.3 Hz to 0.5 ± 0.4 Hz in 9 cells (P < 0.05); suppressed delayed afterdepolarization in 4 (80%) of 5 PV cardiomyocytes. Nitroprusside inhibited L-type calcium currents, transient outward currents and transient inward current, but increased delayed rectified potassium currents.
Conclusion
Nitroprusside regulates the electrical activity of PV cardiomyocytes, which suggests that NO may play a role in PV arrhythmogenesis.
Similar content being viewed by others
Background
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical medicine. The pulmonary veins (PVs) have been demonstrated to be an important source of the initiation of AF [1, 2] and also to have a role in the maintenance of AF [3]. Previous anatomical and electrophysiological study in isolated PVs specimen have demonstrated that PVs contain a mixture of pacemaker cells and working myocardium [4–9]. In canine PVs, we also demonstrated that PVs have arrhythmogenic activity through the enhancement of spontaneous activities or high frequency irregular rhythms [10, 11]. These findings confirmed the previous observation in embryological heart, whereas PVs were suggested to work as a subsidiary pacemaker [12]. Enhancement of automaticity and triggered activity in PV cardiomyocytes with pacemaker activity was suggested to play a critical role in the pathophysiology of AF [10, 11, 13, 14].
Previous studies have shown that nitric oxide (NO) has important regulatory effects on the cardiovascular system [15, 16]. NO has been shown to have a role in the development of triggered arrhythmias generated by Ca2+overload [17]. Our previous study in vivo also showed that NO could suppress trigged activity induced ventricular tachycardia [18]. It is known that PVs contain endothelium and smooth muscle which may produce NO through the enzyme of eNOS or iNOS. In addition, cardiac myocytes also express eNOS activity [19]. NO has been shown to regulate PV arrhythmogensis through mechano-electrical feedback [20]. Because PVs was known to induce atrial arrhythmia through the enhancement of triggered activity, it is possible that NO may play a critical role in the PV arrhythmogenic activity. Moreover, perioperative administration of nitroprusside (NO donor) during the rewarming period could prevent postoperative AF in patients undergoing myocardial revascularization, which suggests the anti-AF effects of nitroprusside [21]. However, it is not clear whether nitroprusside may have direct electrophysiological effects on PV cardiomyocytes. Therefore, the purpose of this study was to study the effects of nitroprusside on the ionic currents and arrhythmogenic activity of single PV cardiomyocytes.
Materials and methods
Isolation of single cardiomyocytes
The investigation conformed to the institutional Guide for the Care and Use of Laboratory Animals. Twenty-one mongrel dogs were used in this study. After the dogs were anesthetized with sodium pentobarbital (30 mg/kg, i.v.), the hearts were rapidly removed through a thoracotomy and dissected at room temperature in normal Tyrode solution with the composition (in mM) of 137 NaCl; 4 KCl; 15 NaHCO3; 0.5 NaH2PO4; 0.5 MgCl2; 2.7 CaCl2, and 11 dextrose. Tyrode solution was equilibrated with a gas mixture of 97% O2 -3% CO2, with a pH of around 7.4.
For dissection of the PVs, the left atrium was opened by an incision extending from the coronary sinus. The PVs were separated from the left atrium about 5 mm proximal to the junction between PVs and left atrium. The veins were separated from the lung parenchyma through the incisions about 20 mm distal to the ending of myocardial sleeve. The isolated PVs were perfused from the distal end with inside out of PVs through a polyethylene tubing. The other end of the polyethylene tubing was connected to a perfusion pump with a perfusion rate of 500 ml/hr. The proximal end and side branches of PVs were ligated with silk. The PVs were perfusated initially with oxygenated normal Tyrode solution and replaced with Ca2+-free Tyrode solution. The perfusate was replaced with oxygenated Ca2+-free Tyrode solution containing 3 units/ml collagenase (Sigma Type I) and 0.5 units/ml protease (Sigma, Type XIV). After softening of the PVs, the PVs were cut into fine pieces and gently shaken in 5-10 ml of Ca2+-free oxygenated Tyrode solution until single cardiomyocytes were obtained. The solution was then gradually changed to normal oxygenated Tyrode solution. Only cells showing clear cross striations were used. Experiments were carried out within the room temperature (34-36°C). The cells were allowed to stabilize in the bath for at least 30 min before experiments.
Electrophysiological and pharmacological study
Whole-cell patch-clamp was performed in cardiomyocytes by means of an Axopatch 1D amplifier (Axon Instruments, Calif, USA) at 35 ± 1°C. Borosilicate glass electrodes (o.d., 1.8 mm) were used, with tip resistances of 3-5 MΩ. Before formation of the membrane-pipette seal, tip potentials were zeroed in Tyrode solution. Junction potentials (9 mV) were corrected for action potentials (APs) recording. AP and transient inward currents were measured during superfusion with normal Tyrode solution, pipette solution contained (in mM): KCl 20, K aspartate 110, MgCl2 1, Mg2ATP 5, HEPES 10, EGTA 0.5, and LiGTP 0.1, Na2phosphocreatine 5, adjusted to pH 7.2 with 1 N KOH. The APs were recorded in current-clamp mode and ionic currents in voltage-clamp mode as described previously [11]. A small hyperpolarizing step from a holding potential of -50 mV to a testing potential of -55 mV for 80 ms was delivered at the beginning of each experiment. The area under the capacitative currents was divided by the applied voltage step to obtain the total cell capacitance of 36 ± 3 pF in 43 PV cardiomyocytes. Normally, 60% to 80% series resistance (Rs) was electronically compensated. AP measurements were begun at 5 minutes after cell rupture. The 50% (APD50) and 90% (APD90) of the AP duration were measured during 1 Hz electrical stimulation in the PV cardiomyocytes. Micropipettes were filled with a solution containing (in mM) CsCl 130, MgCl2 1, Mg2ATP 5, HEPES 10, EGTA 10, NaGTP 0.1, and Na2 phosphocreatine 5, titrated to a pH of 7.2 with CsOH for the experiments on the L-type calcium current (ICa-L). The micropipettes were filled with a solution containing (in mM) KCl 20, K aspartate 110, MgCl2 1, Mg2ATP 5, HEPES 10, EGTA 0.5, LiGTP 0.1, and Na2 phosphocreatine 5, titrated to a pH of 7.2 with KOH for the experiments on the APs, potassium currents, and transient inward currents. Voltage command pulses were generated by a 12-bit digital-to-analog converter controlled by pCLAMP software (Axon Instruments). Recordings were low pass-filtered at half the sampling frequency.
The ICa-L was measured as an inward current during depolarization from a holding potential of -50 mV to testing potentials ranging from -40 to +60 mV in 10-mV steps for 300 ms at a frequency of 0.1 Hz. The NaCl and KCl in the external solution were replaced by tetraethylammonium chloride and CsCl, respectively.
The transient outward current (Ito) was studied with a double-pulse protocol. A 30-ms pre-pulse from -80 to -40 mV was used to inactivate the sodium channels, followed by a 300-ms test pulse to +60 mV in 10-mV steps at a frequency of 0.1 Hz. CdCl2 (200 μM) was added to the bath solution to inhibit the ICa-L. The Ito was measured as the difference between the peak outward current and steady-state current. The sustained outward potassium currents (IKsus) were measured as the outward current density at the end of the steady state.
The delayed rectified outward potassium current (IK) was measured from the peak outward current at the end of 1 s and the depolarization from -40 to +60 mV in 10-mV steps at a frequency of 0.1 Hz during the infusion of CdCl2 (200 μM) and 4-aminopyridine (2 mM) in the bath solution.
Transient inward current was induced at clamped potentials from -40 to +40 mV for the duration of 2 sec and then repolarized to -40 mV. The amplitude of transient inward current was measured as difference between the peak of the transient current and the mean of current just before and after the transient current.
Statistics
All quantitative data are expressed as mean ± SE. The differences between before and after drugs administration were analyzed by Wilcoxon signed-rank test. A P value lower than 0.05 was considered to be statistically significant.
Results
Effects of nitroprusside on arrhythmogenic activity of PV cardiomyocytes
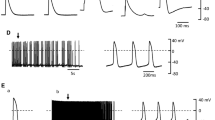
Nine PV cardiomyocytes with pacemaker activity received the administration of 80 μM nitroprusside. Nitroprusside decreased PV cardiomyocytes spontaneous beating rates from 1.7 ± 0.3 Hz to 0.5 ± 0.4 Hz in 9 cells (P < 0.05). Figure 1 shows the example that PV cardiomyocytes spontaneous activity was suppressed from to after the administration of nitroprusside. In addition, nitroprusside inhibited delayed afterdepolarization in 4 (80%) of 5 PV cardiomyocytes. The amplitude of delayed afterdepolarization was suppressed from 9 ± 2 to 4 ± 2 mV. Figure 2 shows the example of the changes of delayed afterdepolarization after nitroprusside.
Figure 3 shows the effects of nitroprusside on AP morphology in the PV cardiomyocytes, nitroprusside (80 μM) shortened the 90% and 50% of the AP duration. Figure 1 shows the example of AP before and after the administration of nitroprusside. However, nitroprusside did not change the resting membrane potential in these cardiomyocytes.
Effect of nitroprusside on the PV electrical activity. Action potential of PV cardiomyocyte before (indicated by black triangle) and after (indicated by blank triangle) the administration of nitroprusside. There were shorter action potentials after the administration of nitroprusside. *P < 0.05, versus before nitroprusside PV cardiomyocytes. (RMP: resting membrane potential, APA: action potential amplitude, APD50: 50% of action potential duration, APD90: 90% of action potential duration).
Effects of nitroprusside on ionic currents of PV cardiomyocytes
As the example shown in Figure 4, nitroprusside can decrease the ICa-L in PV cardiomyocytes. Moreover, nitroprusside can decrease the Ito in PV cardiomyocytes, but increase the IKsus (Figure 5). Figure 6 shows the current traces and I-V relationship of IK in PV cardiomyocytes before and after the administration of nitroprusside. Nitroprusside enhanced IK in PV cardiomyocytes. Figure 7 shows the tracing of transient inward current before and after the administration of nitroprusside, whereas nitroprusside inhibited transient inward current from 0.74 ± 0.13 pA/pF to 0.29 ± 0.09 pA/pF (n = 13, P < 0.001).
The current tracings and I-V relationship of the L-type calcium current (I Ca-L ) of the control and nitroprusside-treated PV cardiomyocytes (n = 6). The ICa-L in the nitroprusside-treated PV cardiomyocytes was smaller than that in the control PV cardiomyocytes. The insets of the current tracings show the various clamp protocols. *P < 0.05, **P < 0.01, versus before nitroprusside PV cardiomyocytes.
Effects of nitroprusside on the transient outward current (I to ) and sustained outward potassium currents (I Ksus ) in the PV cardiomyocytes (n = 6). Upper and lower panel shows the current tracings and I-V relationship of the Ito of before and after the administration of nitroprusside in PV cardiomyocytes. The Ito in the nitroprusside-treated PV cardiomyocytes was smaller than that in the control PV cardiomyocytes. Lower panel shows the current tracings and I-V relationship of the IKsus before and after the administration of nitroprusside in PV cardiomyocytes. The IKsus was larger in the nitroprusside-treated PV cardiomyocytes than that in the control PV cardiomyocytes. The insets of the current traces show the various clamp protocols. *P < 0.05, **P < 0.01, versus before nitroprusside PV cardiomyocytes.
Effect of nitroprusside on delayed rectified outward potassium current (I K ) in the PV cardiomyocytes (n = 11). Current traces (upper panel) and I-V relationship (lower panel) of IK in the PV cardiomyocytes. Insets show the various clamp protocols. *P < 0.05, **P < 0.01, ***P < 0.005, versus before nitroprusside PV cardiomyocytes.
Effect of nitroprusside on transient inward current (I ti ) in PV cardiomyocytes. Current traces of transient inward current indicated by blank triangle before (upper panel) and after (lower panel) the administration of nitroprusside in a PV cardiomyocyte. There was decreased transient inward current after nitroprusside. The transient inward current was induced from repolarization to -40 mV after a depolarizing pulse (from -40 to +40 mV for 3 s, see inset for clamp protocol).
Discussion
In this study, for the first time, we demonstrated that nitroprusside can directly suppress the spontaneous activity, inhibit delayed afterdepolarization with the decreases of transient inward currents in PV cardiomyocytes. NO has been shown to change automaticity in sinoatrial cell [22] and also been found to have lower plasma NO levels in the patients with AF than subjects with sinus rhythm [23]. These findings suggested that NO may be associated with the occurrence of AF. Previous studies have shown that PVs play a critical role in the genesis of atrial fibrillation. Our studies further showed that PVs have a high arrhythmogenic activity through the enhancement of automaticity and triggered activity [11, 13, 14]. In this study, we demonstrated that NO could inhibit PV cardiomyocytes spontaneous activity and also suppressed triggered activity from DAD. These findings confirmed that NO has a role in the PV arrhythmogenic activity.
NO have been shown to alter intracellular Ca2+ homeostasis or inhibit both L-type Ca2+ currents and the stimulation of L-type Ca2+ currents by β-adrenergic agonists [24–26]. Action of NOS3 has been shown to produce NO to enhance Ca2+ sensitivity of the slowly activating delayed rectified potassium current with the shortening of AP duration in guinea pig ventricular cardiomyocytes [27]. Similarly, this study found that direct administration of NO donor can decrease ICa-L, which may contribute to the shortening of AP duration and the decreases of PV spontaneous activity and triggered activity. Previous studies have indicated the importance of calcium homeostasis in PV electrical activity [28–30] and transient inward current plays an important role in the genesis of PV arrhythmogenesis [11, 13, 14]. In this study, we demonstrated that NO suppress transient inward currents, which may result in the suppression of triggered activity in PV cardiomyocytes. IKur has been found in atrial or PV cardiomyocytes. In this study, we measured the IKsus because the current density and I-V relationship of IKsus and IKur are quite similar [31], and found an increase of the IKsus after administration of nitroprusside, which may result in the shortening of AP duration in PV cardiomyocytes. Moreover, nitroprusside increase the IK also can shorten the AP duration in PV cardiomyocytes.
Potential limitations
The data in this study should be interpreted with caution due to the potential limitations. Only single dosage of nitroprusside was used in this study. It is not clear whether nitroprusside has concentration-dependent effects on the PVs. Additionally, although nitroprusside is considered to be NO donor, without the use of NO scavenger to reduce or reverse the effects of nitroprusside, we may not exclude the possibility that our findings may not be NO relevant. Moreover, NO signaling in cardiac muscle was reported to be caused by the stimulation of guanylate cyclase/cGMP production or nitrosylation of sulfhydryl groups on cystein residue of channel protein [32, 33]. However, this study did not investigate the molecular mechanisms underlying the ionic effects of nitroprusside on PV cardiomyocytes.
Conclusions
Nitroprusside has significant effects on regulating the arrhythmogenic activity of PV cardiomyocytes, which suggests that NO may play a role in PV arrhythmogenesis.
References
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Mouroux AL, Metayer PL, Clementy J: Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998, 339: 659-666. 10.1056/NEJM199809033391003.
Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, Hsu TL, Ding YA, Chang MS: Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999, 100: 1879-1886.
Jaïs P, Hocini M, Macle L, Choi KJ, Deisenhofer I, Weerasooriya R, Shah DC, Garrigue S, Raybaud F, Scavee C, Le Metayer P, Clémenty J, Haïssaguerre M: Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation. 2002, 106: 2479-2485. 10.1161/01.CIR.0000036744.39782.9F.
Masani F: Node-like cells in the myocardial layer of the pulmonary vein of rats: an ultrastructural study. J Anat. 1986, 145: 133-142.
Nathan H, Eliakim M: The junction between the left atrium and the pulmonary veins: an anatomic study of human hearts. Circulation. 1966, 34: 412-422.
Saito T, Waki K, Becker AE: Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol. 2000, 11: 888-894. 10.1111/j.1540-8167.2000.tb00068.x.
Cheung DW: Electrical activity of the pulmonary vein and its interaction with the right atrium in the guinea-pig. J Physiol (Lond). 1981, 314: 445-456.
Cheung DW: Pulmonary vein as an ectopic focus in digitalis-induced arrhythmia. Nature. 1981, 294: 582-584. 10.1038/294582a0.
Perez-Lugones A, McMahon JT, Ratliff NB, Saliba WI, Schweikert RA, Marrouche NF, Saad EB, Navia JL, McCarthy PM, Tchou P, Gillinov AM, Natale A: Evidence of specialized conduction cells in human pulmonary veins of patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2003, 14: 803-809. 10.1046/j.1540-8167.2003.03075.x.
Chen YJ, Chen SA, Chang MS, Lin CI: Arrhythmogenic activity of cardiac muscle in pulmonary Veins of the dog: Implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000, 48: 265-273. 10.1016/S0008-6363(00)00179-6.
Chen YJ, Chen SA, Chen YC, Yeh HI, Paul C, Chang MS, Lin CI: Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implication in initiation of atrial fibrillation. Circulation. 2001, 104: 2849-2854. 10.1161/hc4801.099736.
Blom NA, Gittenberger-de Groot AC, DeRuiter MC, Poelmann RE, Mentink MM, Ottenkamp J: Development of the cardiac conduction tissue in human embryos using HNK-1 antigen expression: possible relevance for understanding of abnormal atrial automaticity. Circulation. 1999, 99: 800-806.
Chen YJ, Chen SA, Chen YC, Yeh HI, Chang MS, Lin CI: Electrophysiology of single cardiomyocytes isolated from rabbit pulmonary veins: implication in initiation of focal atrial fibrillation. Basic Res Cardiol. 2002, 97: 26-34. 10.1007/s395-002-8384-6.
Chen YC, Chen SA, Chen YJ, Chang MS, Chan P, Lin CI: Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J Am Coll Cardiol. 2002, 39: 366-372. 10.1016/S0735-1097(01)01731-4.
Moncada S, Palmer RMJ, Higgs EA: Nitric oxide physiology, pathophysiology. Pharmacol Rev. 1991, 433: 109-142.
Kelly RA, Balligand JL, Smith TW: Nitric oxide and cardiac function. Circ Res. 1996, 79: 363-380.
Kubota I, Han X, Opel DJ, Zhao YY, Baliga R, Huang P, Fishman MC, Shannon RP, Michel T, Kelly RA: Increased susceptibility to development of triggered activity in myocytes from mice with raged disruption of endothelial nitric oxide synthase. J Mol Cell Cardiol. 2000, 32: 1239-1248. 10.1006/jmcc.2000.1158.
Chen YJ, Tsai CF, Chiou CW, Chan P, Chen SA: Effect of Nitric Oxide on Strophanthidin-induced Ventricular Tachycardia. Pharmacology. 2001, 62: 213-217. 10.1159/000056097.
Balligand JL, Ungurand D, Kelly RA, Kozaik L, Pimental D, Michel T, Smith TW: Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. J Clin Invest. 1992, 91: 2314-2319. 10.1172/JCI116461.
HU Y, Chen YC, Cheng CC, Higa S, Chen YJ, Chen SA: Fluvastatin reduces pulmonary vein spontaneous activity through nitric oxide pathway. J Cardiovasc Electrophysiol. 2009, 20: 200-206. 10.1111/j.1540-8167.2008.01281.x.
Cavolli R, Kaya K, Aslan A, Emiroglu O, Erturk S, Korkmaz O, Oguz M, Tasoz R, Ozyurda U: Dose sodium nitroprusside decrease the incidence of atrial fibrillation after myocardial revascularization? a pilot study. Circulation. 2008, 118: 476-481. 10.1161/CIRCULATIONAHA.107.719377.
Elvan A, Rubart M, Zipes DP: NO modulates autonomic effects on sinus discharge rate and AV nodal conduction in open-chest dogs. Am J Physiol. 1997, 272: H263-271.
Minamino T, Kitakaze M, Sanada S, Asanuama H, Kurotobi T, Koretsune Y, Fukunami M, Kuzuya T, Hoki N, Hori M: Increased expression of P-selectin on platelets is a risk factor for silent cerebral infarction in patients with atrial fibrillation: role of nitric oxide. Circulation. 1998, 98: 1721-1727.
Han X, Kobzik L, Balligand JL, Kelly RA, Smith TW: Nitric oxide synthase (NOS3)-mediated cholinergic modulation of Ca2+ current in adult rabbit atrioventricular nodal cells. Circ Res. 1996, 78: 998-1008.
Shimoni Y, Han X, Severson D, Giles WR: Mediation by nitric oxide of the indirect effects of adenosine on calcium current in rabbit heart pacemaker cells. Br J Pharmacol. 1996, 119: 1463-1469.
Wahler GM, Dollinger SJ: Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. Am J Physiol. 1995, 268: C45-54.
Bai CX, Namekata I, Kurokawa J, Tanaka H, Shigenobu K, furukawa T: Role of nitric oxide in Ca2+ sensitivity of the slowly activating delayed rectifier K+ current in cardiac myocytes. Circ Res. 2005, 96: 64-72. 10.1161/01.RES.0000151846.19788.E0.
Chang SH, Chen YC, Chiang SJ, Higa S, Cheng CC, Chen YJ, Chen SA: Increased Ca(2+) sparks and sarcoplasmic reticulum Ca(2+) stores potentially determine the spontaneous activity of pulmonary vein cardiomyocytes. Life Sci. 2008, 83: 284-292. 10.1016/j.lfs.2008.06.014.
Chen YJ, Chen YC, Wongcharoen W, Lin CI, Chen SA: K201, a novel antiarrhythmic drug on Calcium Handling and Arrhythmogenic Activity of Pulmonary Vein Cardiomyocytes. Br J Pharmacol. 2008, 153: 915-925. 10.1038/sj.bjp.0707564.
Wongcharoen W, Chen YC, Chen YJ, Chang CM, Yeh HI, Lin CI, Chen SA: Effects of a Na+/Ca2+ Exchanger Inhibitor on Pulmonary Vein Electrical Activity and Ouabain-induced Arrhythmogenicity. Cardiovasc Res. 2006, 70: 497-508. 10.1016/j.cardiores.2006.02.026.
Liu H, Jin MW, Huang Y, Sun HY, Chiu SW, Lau CP, Li GR: Raloxifene inhibits transient outward and ultra-rapid delayed rectifier potassium currents in human atrial myocytes. Eur J Pharmacol. 2007, 563: 61-68. 10.1016/j.ejphar.2007.01.072.
Fischmeister R, Castro L, Abi-Gerges A, Rochais F, Vandecasteele G: Species- and tissue-dependent effect of NO and cyclic GMP on cardiac ion channels. Comp Biochem Phys A. 2005, 142: 136-143. 10.1016/j.cbpb.2005.04.012.
Broillet MC: S-nitrosylation of proteins. Cellular Mol Life Sci. 1999, 55: 1036-1042. 10.1007/s000180050354.
Acknowledgements
The present work was supported by the Center of Excellence for Clinical Trial and Research in Neuroscience (DOH99-TD-B-111-003) and grants NSC 96-2628-B-038-012-MY3, NSC 96-2314-B-010-006, NSC 97-2314-B-038-030-MY3 and 98CM-TMU-10 from Chi-Mei Medical Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YKL and YYL interpreted the data and drafted the manuscript. YCC performed the experiments and revised it for scientific content. YJC and SAC conceived of this study, and participated in its design and coordination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lin, YK., Lu, YY., Chen, YC. et al. Nitroprusside modulates pulmonary vein arrhythmogenic activity. J Biomed Sci 17, 20 (2010). https://doi.org/10.1186/1423-0127-17-20
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1423-0127-17-20