Abstract
Background
No study has simultaneously investigated the impacts of migraine and anxiety disorders on painful physical symptoms (PPS) among patients with major depressive disorder (MDD). The study aimed to investigate this issue.
Methods
This open-label study enrolled 155 outpatients with MDD, who were then treated with venlafaxine 75 mg per day for four weeks. Eighty-five participants with good compliance completed the treatment. Migraine was diagnosed according to the International Classification of Headache Disorders. MDD and anxiety disorders were diagnosed using the Structured Clinical Interview for DSM-IV-TR. The visual analog scale (VAS) was used to evaluate the severity of eight PPS. Multiple linear and logistic regressions were used to investigate the impacts of migraine and anxiety disorders on PPS.
Results
Compared with patients without migraine, patients with migraine had a greater severity of PPS at baseline and post-treatment. After controlling for demographic variables and depressive severity, migraine independently predicted the intensities of eight PPS at baseline and four PPS post-treatment. Moreover, migraine independently predicted poorer treatment responses of chest pain and full remission of pains in the head, chest, neck and/or shoulder. Anxiety disorders predicted less full remission of pains in the abdomen and limbs.
Conclusion
Migraine and anxiety disorders have negative impacts on PPS among patients with MDD. Integrating the treatment of migraine and anxiety disorders into the management of depression might help to improve PPS and the prognosis of MDD.
Similar content being viewed by others
Background
Depression and painful physical symptoms (PPS) are closely related and interact [1, 2]. PPS are common among patients with major depressive disorder (MDD) [1–5]. MDD patients with PPS have a greater severity of depression [3, 5], a poorer quality of life [6, 7], and an increased suicidal risk [8]. Moreover, PPS also has negative impacts on the treatment response of depression [9, 10].
Migraine is common among patients with MDD and is related to other PPS [11–14]. MDD patients with migraine are associated with greater severities of depression and anxiety and a poorer health-related quality of life [11, 13, 15]. Migraine is an independent factor predicting somatic symptoms among patients with MDD [16]. Moreover, migraine also has negative impacts on the recovery of health-related quality of life among MDD patients [15].
Patients with depression are often comorbid with anxiety, which is related to a greater severity and a poorer treatment prognosis [17, 18]. Anxiety disorders also interact with PPS [19–21]; for example, a patient with generalized anxiety disorder (GAD) has a greater severity of PPS as compared with a control [21].
As described above, MDD, migraine, anxiety disorders, and PPS are closely related and interact [1, 2, 14, 21, 22]. Although many studies have investigated the impacts of migraine and anxiety disorders on patients with MDD [12, 16–18, 23], no study has reported the impacts of migraine and anxiety disorders on PPS among patients with MDD, to the best of our knowledge. Investigating the above issue is mandatory. If migraine and anxiety disorders are important factors related to PPS among patients with MDD, treatment of migraine and anxiety disorders might directly or indirectly improve PPS. There is a possibility that improvement of PPS might be helpful for improving the prognosis of depression, because PPS have a significantly negative impact on the prognosis of patients with MDD [3, 5–10]. Therefore, the purpose of this study was to investigate the impacts of migraine and anxiety disorders on PPS among patients with MDD. We hypothesized that migraine and anxiety disorders have negative impacts on PPS among patients with MDD.
Methods
Subject enrollment
This project was conducted from September 2005 to August 2007 in the psychiatric outpatient clinics of Chang Gung Memorial Hospital, a medical center in northern Taiwan. The project was approved by the Institutional Review Board of the same hospital. Study participants were recruited from consecutive outpatients aged 18–65 years who had not taken antidepressants or other psychotropic drugs within the previous four weeks. MDD and anxiety disorders were diagnosed using the Structured Clinical Interview for DSM-IV-text revision (TR) Axis I Disorders [24]. Patients who met the DSM-IV-TR criteria for MDD and were experiencing a current major depressive episode were considered eligible subjects [25]. To prevent depression and somatic symptoms from being confounded by other medical conditions, substance abuse, or psychotic symptoms, the following exclusion criteria were established: 1) a history of substance dependence or abuse without full remission in the previous month; 2) psychotic symptoms, catatonic features, or severe psychomotor retardation with obvious difficulty in being interviewed; and 3) chronic medical diseases such as hypertension, diabetes mellitus, and other medical diseases, except for headaches. Written informed consent, based on the guidelines regulated in the Declaration of Helsinki, was obtained from all subjects prior to study enrollment following an in-depth explanation of the procedures.
The study was designed as an open-label study and the eligible subjects were informed about the following issues: 1) Antidepressants used in the study have been proven for the treatment of depression; 2) The period of observation with a fixed-dosage antidepressant (venlafaxine extended-release, one 75 mg capsule per day) was four weeks; 3) Subjects were educated regarding depression and treatment issues. An information sheet was provided to ensure that all subjects received the same information.
Assessment of headaches
All patients completed a structured headache intake form, which was designed to meet the operational criteria of the International Classification of Headache Disorders, 2nd edition (ICHD-2) [26]. Questions regarding headache frequency, intensity, pattern, duration, location, aura, aggravation by physical activities, nausea, vomiting, photophobia, phonophobia, precipitating factors and painkiller use were included. An experienced headache specialist (SJW), blind to the psychiatric evaluation results, interviewed all patients after they had completed the headache intake form and made headache diagnoses. Then, the headache diagnoses were updated to ICHD-3 beta [27]. Subjects who fulfilled the criteria of migraine without aura and/or migraine with aura were categorized as the “migraine” group, while the other patients were classified as the “non-migraine” group.
Assessment of anxiety disorders
One psychiatrist, blind to the diagnoses of headaches and the psychometric assessment results, used the Structured Clinical Interview to diagnose the following anxiety comorbidities: panic disorder, agoraphobia, social phobia, specific phobia, post-traumatic stress disorder, obsessive–compulsive disorder, and GAD [24]. Subjects with any one of the anxiety comorbidities in a current episode or partial remission were categorized as the “anxiety disorders” group, while the others were categorized as the “non-anxiety disorders” group.
Instruments
The Hamilton Depression Rating Scale (HAMD) was used to evaluate the severity of depression [28]. Subjects evaluated their average pain intensity during the previous week using a visual analog scale (VAS), with 0 representing “no pain” and 10 representing “pain as severe as I can imagine.” The number of days suffered from each pain in the past week was recorded. Eight pains were evaluated, including pain in bone and/or joints (bone/joints), head, back, abdomen, chest, neck and/or shoulder (neck/shoulder), general muscle, and limbs. The improvement percentage (IP) of pain intensity was calculated by (VAS scores at baseline – VAS scores at the follow-up) × 100% / VAS scores at baseline. An IP of pain intensity ≥50 following the four-week treatment period was defined as “treatment response” and an IP =100 was defined as “full remission”.
Procedures
After the subjects were enrolled, they were treated for four weeks with venlafaxine extended-release, one 75 mg capsule per day. Because insomnia is a common complaint in patients with MDD, zolpidem (10 mg per tablet) was prescribed as needed at night only in the first 2 weeks of treatment, with a total amount ≤6 tablets. Four weeks later, the same scales were administered. The HAMD was re-evaluated by the same psychiatrist. Only subjects who complied with venlafaxine extended-release treatment ≥80% (total capsules that the subject took/total days) were included in the post-treatment analyses.
Statistical methods
All statistical analyses were performed using SPSS for Windows 12.0 (SPSS Inc., Chicago, IL, USA). The independent t test, paired t test, Pearson’s correlation, and Chi-square test were used in appropriate situations.
Four regression models were used to investigate the impacts of migraine and anxiety disorders on PPS after controlling for the HAMD scores and demographic variables. The first and second models, performed by multiple linear regressions with forward selection, investigated the impacts of migraine and anxiety disorders on pain intensities at baseline and post-treatment, respectively. The third and fourth models, performed by logistic regressions with forward selection, investigated the impacts of migraine and anxiety disorders on treatment response (IP ≥ 50) and full remission (IP = 100) of pain intensities, respectively. The first model investigated the impacts of migraine and anxiety disorders at baseline; therefore, the total subjects at baseline (N = 155) were used for analysis. The second to fourth models investigated the impacts of migraine and anxiety disorders post-treatment; therefore, only patients with good compliance (N = 85) were used. In the first and second models, the dependent variables were VAS scores (pain intensities of each pain) at baseline and post-treatment, respectively. In the third and fourth models, the dependent variables were treatment response and full remission, respectively. For the four regression models, the independent variables included five demographic variables (age, gender, educational years, marital status, and employment status), migraine, anxiety disorders, and the severity of depression (HAMD scores at baseline for the first, third, and fourth models and post-treatment for the second model). In all statistical analyses, a two-tailed P value <0.05 was considered statistically significant.
Results
Subjects
During the study period, 155 patients (49 men, 106 women) agreed to participate. Among them, 16 patients had been treated with antidepressants or other psychotropic drugs and 13 and 3 patients had quit these medications for at least three months and six weeks, respectively, before enrollment. Six patients had a history of alcohol abuse and all of them had achieved full remission for at least 3 months.
Table 1 shows their demographic variables. Among 155 patients, 85 (54.8%) subjects with good compliance (venlafaxine treatment ≥80% compliance) were included in the post-treatment analyses (treatment group). The mean amount of zolpidem taken during the first two-week treatment period was 2.1 ± 2.4 tablets. The other 70 subjects (withdrawal group) included 22 subjects who had poor compliance (compliance <80%) or were shifted to other antidepressants and 48 subjects who did not finish the treatment. There were no significant differences at baseline in the five demographic variables, the percentages of migraine and anxiety comorbidities, and the HAMD scores between the treatment and withdrawal groups (Table 1). In analyzing results post-treatment, only the treatment group was used.
Headache and psychiatric diagnoses
Among the 155 participants, 73 (47.1 %) had migraine, including 16 patients with chronic migraine (code 1.3), two with chronic migraine with medication overuse headache (codes 1.3 and 8.2), 49 with episodic migraine without aura (code 1.1), and 6 with episodic migraine both with and without aura (code 1.2). The other 82 subjects included 27 (17.4 %) with probable migraine without aura (code 1.5.1), one (0.6 %) with chronic tension-type headache (TTH) (code 2.3), 26 (16.8 %) with frequent episodic TTH (code 2.2), four (2.6 %) with infrequent episodic TTH (code 2.1), six (3.9%) with probable episodic TTH (code 2.4), nine (5.8 %) with headache unspecified (code 14.2), and nine (5.8%) without any headaches.
Among the 155 patients, 70 (45.2%) subjects had at least one anxiety comorbidity, including 9.7% (N = 15) with panic disorder and/or agoraphobia, 24.5% (N = 38) with social phobia, 18.1% (N = 28) with specific phobia, 11.6% (N = 18) with post-traumatic stress disorder, 6.5% (N = 10) with obsessive-compulsive disorder, and 7.1% (N = 11) with GAD.
Among the 85 patients in the treatment group, 44 (51.8%) patients had migraine, including 10 patients with chronic migraine (code 1.3), two with chronic migraine with medication overuse headache (codes 1.3 and 8.2), 27 with episodic migraine without aura (code 1.1), and 5 with episodic migraine both with and without aura (code 1.2), and 43 (50.6%) patients had any one of the anxiety disorders. There were no significant differences in the five demographic variables between those with and without migraine as well as between those with and without anxiety comorbidities.
Migraine and anxiety disorders are also comorbid with each other. Compared with MDD patients without migraine, MDD patients with migraine had a higher risk of comorbidity with any anxiety disorder (63.4% vs. 28.0%, odds ratio (OR) = 4.64, P <0.001 at baseline; 72.7% vs. 26.8%, OR = 7.27, P <0.001 post-treatment).
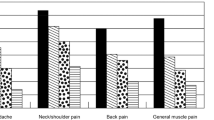
The percentages of PPS between groups
Table 2 shows the percentages of PPS in the treatment group between groups. Patients with migraine had significantly higher percentages in all PPS at baseline and post-treatment as compared with patients without migraine. Significant differences between patients with and without anxiety disorders were noted in terms of headache at baseline and pains in the neck/shoulder and limbs post-treatment.
Regarding the total number of PPS, patients with migraine had significantly higher numbers of PPS at baseline (6.3 ± 1.9 vs. 3.9 ± 2.1, P <0.001) and post-treatment (4.8 ± 2.4 vs. 2.3 ± 2.1, P <0.001) as compared with patients without migraine. Similarly, patients with anxiety disorders also tended to have a higher number of PPS at baseline (5.4 ± 2.3 vs. 4.8 ± 2.3, P = 0.21) and post-treatment (4.2 ± 2.5 vs. 3.0 ± 2.4, P = 0.02) as compared with patients without anxiety disorders.
Differences in the severity of depression and pains between groups
Table 3 shows that the patients with migraine had a significantly greater severity of depression and significantly higher pain intensities and frequencies in all PPS at baseline and post-treatment as compared with the patients without migraine, with the exception of chest pain at baseline.
In patients both with and without migraine, the severity of depression, the VAS scores and the frequencies of the eight PPS all showed significant (all P <0.05) improvement post-treatment, except for the intensities of pains in the head and limbs and the frequencies of pains in bone/joints, head, abdomen, and limbs among patients without migraine.
Comparing patients with and without anxiety disorders, significant differences were noted in only the severity of depression at baseline and the frequencies of pains in the head, abdomen, and neck/shoulder post-treatment.
For patients with and without any anxiety disorders, the HAMD scores, VAS scores, and pain frequencies at baseline showed significant improvement post-treatment, except for the intensities of pains in the head and limbs and the frequency of pain in the limbs among patients without anxiety disorders.
The correlation of pain intensities and depression
At baseline (N = 155), the HAMD score was significantly correlated to all pain intensities, with correlation co-efficiencies ranging from 0.39 (headache) to 0.20 (abdominal pain). Post-treatment (N = 85), the correlation co-efficiencies (all P <0.05) ranged from 0.35 (pain in bone/joint) to 0.23 (back pain). The correlations of age and educational levels with all pain intensities were not significant at baseline and post-treatment.
Differences in the percentages of treatment response and full remission between groups
Table 4 shows the trend that patients with migraine had lower percentages of treatment response and full remission in the PPS, except for pain in bone/joints. Statistically significant differences between patients with and without migraine were noted in chest pain for treatment response and in chest pain, headache, and neck/shoulder pain for full remission.
Patients with anxiety disorders also tended to have lower percentages of full remission post-treatment, and a significant difference was only noted in neck/ shoulder pain (Table 4).
The impacts of migraine on PPS at baseline and post-treatment
The first regression model demonstrated that migraine independently predicted the intensities of the eight PPS at baseline, after controlling for the demographic variables and HAMD scores (Table 5). Moreover, the impacts of migraine might be greater than the severity of depression, except for pains in the chest and limbs. The second regression model demonstrated that migraine independently predicted the intensities of pains in bone/joints, back, chest, and neck/shoulder post-treatment. In the two regression models, anxiety disorders were not entered.
Factors independently predicting the treatment response and full remission
The third and fourth (logistic) regression models demonstrated that migraine independently predicted the treatment response for chest pain (OR = 0.06, 95% CI = 0.01-0.50, P = 0.01) and full remission for pains in the head (OR = 0.15, 95% CI = 0.04-0.56, P = 0.01), chest (OR = 0.22, 95% CI = 0.06-0.84, P = 0.03), and neck/shoulder (OR = 0.12, 95% CI = 0.03-0.45, P <0.01) after controlling for the demographic variables and HAMD score at baseline. Anxiety disorders independently predicted full remission for pains in the abdomen (OR = 0.29, 95% CI = 0.09-0.96, P = 0.04) and limbs (OR = 0.22, 95% CI = 0.05-0.95, P = 0.04).
Discussion
Compared with patients without migraine, patients with migraine had a significantly higher number of PPS and higher pain intensities and frequencies in all PPS at baseline and post-treatment, with the exception of chest pain at baseline. After controlling for demographic variables, depression, and anxiety comorbidities, migraine independently predicted all PPS at baseline and four pains post-treatment (Table 5). These results implied two issues. 1) Migraine is an important factor related to PPS among patients with MDD. Although the severity of depression was significantly correlated to pain intensities, it could not explain all pain intensities. 2) The impacts of migraine on PPS might be greater than those of anxiety disorders among patients with MDD. However, this might need more evidence. Migraine and anxiety disorders are often comorbid with each other [29, 30]. Anxiety disorders are also comorbid with PPS [19–21]. Therefore, investigation of PPS among depressive and anxiety disorders should consider the impacts of migraine.
The result that the impacts of migraine on most PPS at baseline were greater than the severity of depression might be explained the following hypothesis. At baseline, subjects with migraine all suffered from headache (Table 2) and had a high headache intensity (VAS = 6.4 ± 2.6) and frequency (4.7 ± 2.4 days) in the past week (Table 3). Migraine is often accompanied with signs of increased intracranial and extracranial mechanical sensitivities and related to somatosensory amplification [31, 32]. Repeated migraine attacks might cause an effect similar to central sensitization, which is related to allodynia, hyperalgesia and spontaneous pain and might allow subjects to become more sensitive to other PPS [22, 31]. Moreover, headache intensity is related to muscloskeletal symptoms [33]. Post-treatment, the headache intensity and frequency of migraine were decreased. The effect of central sensitization resulting from headache might be decreased. Therefore, the impact of depression (HAMD scores post-treatment) on PPS might exceed the impact of migraine. In future studies, the impacts of headache attacks on other PPS might need to be further clarified. There is a possibility that treatment of migraine might help to improve headache attacks, then improve other PPS. The patients with migraine had significantly higher HAMD scores at baseline and post-treatment than the patients without migraine (Table 3). A trend was noted that the patients with migraine had a smaller reduction in the HAMD score post-treatment (9.4 points vs. 11.6 points for the migraine group vs. non-migraine group) as compared with the patients without migraine, and the difference in the HAMD scores between the migraine and non-migraine groups became greater (2.0 points at baseline and 4.2 points post-treatment) (Table 3). These results implied the possibility that migraine might be a factor related to a poorer prognosis of depression. However, more evidence is still needed. Moreover, the impacts of anxiety disorders should also be further studied.
After controlling for demographic variables and depression at baseline, migraine independently predicted a lower percentage of treatment response in chest pain and of full remission in pains in the chest, head, and neck/shoulder. This demonstrated that migraine also has negative impacts on the treatment prognosis of PPS. Patients with noncardiac chest pain are often comorbid with depression and anxiety disorders [19, 20]. Patients with migraine have a higher risk of chest pain, and a link between migraine and vasospastic disorders might be one of the possible reasons [34]. The possible reasons for which migraine has a negative impact on the recovery of chest pain should be investigated in future studies. Migraine was also an independent factor predicting a poor treatment prognosis of neck/shoulder pain. Headache is a strong factor associated with neck/shoulder soreness and musculoskeletal symptoms [33]. Moreover, patients with migraine have muscle hyperalgesia and decreased pressure pain thresholds in the neck/shoulder [35]. These might be the possible reasons for which migraine has a negative impact on the recovery of neck/shoulder pain post-treatment. Comorbidity with anxiety disorders was an independent factor related to poor treatment remission of abdominal pain. Recurrent abdominal pain is associated with anxiety disorders [36]. In fact, patients with migraine also have a higher risk of abdominal pain [37]. The interaction of abdominal pain, anxiety disorders, and migraine might need to be clarified.
Several methodological issues or limitations need to be addressed. 1) The analyses post-treatment only included patients with good compliance. Only 85 (54.8%) subjects with good compliance were included in the post-treatment analyses. Although there were no significant differences in the demographic variables and the HAMD scores between the treatment and withdrawal groups, possible bias was unable to be fully excluded, because patients with a poor treatment response might be more likely to drop out [38]. 2) This study had no placebo-controlled group because the major purpose was not to investigate the efficacy of venlafaxine. Therefore, the improvement of PPS and depression might be mixed with multiple effects. 3) The headache parameters in the past week were recalled by the patients. Collecting data prospectively using a headache diary might be more reliable. However, withholding pharmacological treatment while prospectively observing headache parameters might result in ethical problems owing to the suicidal risk. 4) This study only observed the four-week treatment response, because four-week treatment may be a milestone after which a treatment response can be observed [39]. 5) Although the total mean amount of zolpidem taken in the first two weeks was only 2.1 ± 2.4 tablets, possible bias resulting from zolpidem usage could not be excluded. 6) One previous study demonstrated that MDD patients with chronic migraine had the worst bodily pain on the SF-36, followed by episodic migraine [40]. Therefore, chronic migraine might have a greater contribution to influence PPS and treatment response than episodic migraine. In future study, a larger sample size should be used to compare the impacts of chronic migraine, episodic migraine, TTH, and other headache types on PPS and treatment response.
Conclusion
After controlling for depression, anxiety comorbidities, and demographic variables, migraine independently predicted the eight PPS at baseline and four PPS post-treatment. Moreover, migraine also independently predicted poor treatment prognosis of pains in the chest, head, and neck/shoulder. Anxiety disorders predicted poor treatment prognosis of pains in the abdomen and limbs. Depression, anxiety, migraine, and PPS might interact with each other. The impacts of migraine and anxiety disorders should be considered in investigating PPS among patients with MDD. Integration of the treatment for migraine and anxiety disorders into the treatment of depression might help improve PPS and the prognosis of MDD.
Abbreviations
- GAD:
-
Generalized anxiety disorder
- HAMD:
-
Hamilton Depression Rating Scale
- IP:
-
Improvement percentage
- ICHD-2:
-
International Classification of Headache Disorders 2nd edition
- MDD:
-
Major depressive disorder
- OR:
-
Odds ratio
- PPS:
-
Painful physical symptoms
- TTH:
-
Tension-type headache
- VAS:
-
visual analog scale.
References
Vaccarino AL, Sills TL, Evans KR, Kalali AH: Multiple pain complaints in patients with major depressive disorder. Psychosom Med 2009, 71: 159–162. 10.1097/PSY.0b013e3181906572
Ohayon MM, Schatzberg AF: Chronic pain and major depressive disorder in the general population. J Psychiatr Res 2010, 44: 454–461. 10.1016/j.jpsychires.2009.10.013
Brnabic A, Lin C, Monkul ES, Duenas H, Raskin J: Major depressive disorder severity and the frequency of painful physical symptoms: a pooled analysis of observational studies. Curr Med Res Opin 2012, 28: 1891–1897. 10.1185/03007995.2012.748654
Leuchter AF, Husain MM, Cook IA, Trivedi MH, Wisniewski SR, Gilmer WS, Luther JF, Fava M, Rush AJ: Painful physical symptoms and treatment outcome in major depressive disorder: a STAR*D (Sequenced Treatment Alternatives to Relieve Depression) report. Psychol Med 2010, 40: 239–251. 10.1017/S0033291709006035
Demyttenaere K, Reed C, Quail D, Bauer M, Dantchev N, Montejo AL, Monz B, Perahia D, Tylee A, Grassi L: Presence and predictors of pain in depression: results from the FINDER study. J Affect Disord 2010, 125: 53–60. 10.1016/j.jad.2010.02.106
Novick D, Montgomery W, Kadziola Z, Moneta V, Peng X, Brugnoli R, Haro JM: Do concomitant pain symptoms in patients with major depression affect quality of life even when taking into account baseline depression severity? Patient Prefer Adherence 2013, 7: 463–470.
Chung KF, Tso KC, Yeung WF, Li WH: Quality of life in major depressive disorder: the role of pain and pain catastrophizing cognition. Compr Psychiatry 2012, 53: 387–395. 10.1016/j.comppsych.2011.05.005
Bahk WM, Park S, Jon DI, Yoon BH, Min KJ, Hong JP: Relationship between painful physical symptoms and severity of depressive symptomatology and suicidality. Psychiatry Res 2011, 189: 357–361. 10.1016/j.psychres.2011.01.009
Novick D, Montgomery W, Aguado J, Kadziola Z, Peng X, Brugnoli R, Haro JM: Which somatic symptoms are associated with an unfavorable course in Asian patients with major depressive disorder? J Affect Disord 2013, 149: 182–188. 10.1016/j.jad.2013.01.020
Lin CH, Lane HY, Chen CC, Juo SH, Yen CF: Predictors of fluoxetine remission for hospitalized patients with major depressive disorder. Psychiatry Clin Neurosci 2011, 65: 510–517. 10.1111/j.1440-1819.2011.02235.x
Oedegaard KJ, Fasmer OB: Is migraine in unipolar depressed patients a bipolar spectrum trait? J Affect Disord 2005, 84: 233–242. 10.1016/j.jad.2003.11.007
Hung CI, Liu CY, Wang SJ: Migraine predicts physical and pain symptoms among psychiatric outpatients. J Headache Pain 2013, 14: 19. 10.1186/1129-2377-14-19
Hung CI, Wang SJ, Yang CH, Liu CY: The impacts of migraine, anxiety disorders, and chronic depression on quality of life in psychiatric outpatients with major depressive disorder. J Psychosom Res 2008, 65: 135–142. 10.1016/j.jpsychores.2008.04.011
Ligthart L, Gerrits MM, Boomsma DI, Penninx BW: Anxiety and depression are associated with migraine and pain in general: an investigation of the interrelationships. J Pain 2013, 14: 363–370. 10.1016/j.jpain.2012.12.006
Hung CI, Liu CY, Yang CH, Wang SJ: The negative impact of migraine on quality of life after four weeks of treatment in patients with major depressive disorder. Psychiatry Clin Neurosci 2012, 66: 8–16. 10.1111/j.1440-1819.2011.02286.x
Hung CI, Liu CY, Cheng TY, Wang SJ: Migraine: a missing link between somatic symptoms and major depressive disorder. J Affect Disord 2009, 117: 108–115. 10.1016/j.jad.2008.12.015
Ishak WW, Mirocha J, Christensen S, Wu F, Kwock R, Behjat J, Pi S, Akopyan A, Peselow ED, Cohen RM, Elashoff D: Patient-reported outcomes of quality of life, functioning, and depressive symptom severity in major depressive disorder comorbid with panic disorder before and after SSRI treatment in the STAR*D trial. Depress Anxiety 2014, 31(8):707–716. 10.1002/da.22152
Demyttenaere K, Verhaeghen A, Dantchev N, Grassi L, Montejo AL, Perahia DG, Quail D, Reed C, Tylee A, Bauer M: “Caseness” for depression and anxiety in a depressed outpatient population: symptomatic outcome as a function of baseline diagnostic categories. Prim Care Companion J Clin Psychiatry 2009, 11: 307–315. 10.4088/PCC.08m00748blu
Lipsitz JD, Hsu DT, Apfel HD, Marans ZS, Cooper RS, Albano AM, Gur M: Psychiatric disorders in youth with medically unexplained chest pain versus innocent heart murmur. J Pediatr 2012, 160: 320–324. 10.1016/j.jpeds.2011.07.011
Jonsbu E, Dammen T, Morken G, Lied A, Vik-Mo H, Martinsen EW: Cardiac and psychiatric diagnoses among patients referred for chest pain and palpitations. Scand Cardiovasc J 2009, 43: 256–259. 10.1080/14017430902946749
Romera I, Montejo Angel L, Caballero F, Caballero L, Arbesu J, Polavieja P, Desaiah D, Gilaberte I: Functional impairment related to painful physical symptoms in patients with generalized anxiety disorder with or without comorbid major depressive disorder: post hoc analysis of a cross-sectional study. BMC Psychiatry 2011, 11: 69. 10.1186/1471-244X-11-69
Tietjen GE, Brandes JL, Peterlin BL, Eloff A, Dafer RM, Stein MR, Drexler E, Martin VT, Hutchinson S, Aurora SK, Recober A, Herial NA, Utley C, White L, Khuder SA: Allodynia in migraine: association with comorbid pain conditions. Headache 2009, 49: 1333–1344. 10.1111/j.1526-4610.2009.01521.x
Howland RH, Rush AJ, Wisniewski SR, Trivedi MH, Warden D, Fava M, Davis LL, Balasubramani GK, McGrath PJ, Berman SR: Concurrent anxiety and substance use disorders among outpatients with major depression: clinical features and effect on treatment outcome. Drug Alcohol Depend 2009, 99: 248–260. 10.1016/j.drugalcdep.2008.08.010
First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York; 2002.
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IV-TR). American Psychiatric Association, Washington, DC; 2000.
Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders, 2nd ed. Cephalalgia 2004, 24: 1–160.
Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33: 629–808.
Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967, 6: 278–296. 10.1111/j.2044-8260.1967.tb00530.x
Beghi E, Bussone G, D'Amico D, Cortelli P, Cevoli S, Manzoni GC, Torelli P, Tonini MC, Allais G, De Simone R, D'Onofrio F, Genco S, Moschiano F, Beghi M, Salvi S: Headache, anxiety and depressive disorders: the HADAS study. J Headache Pain 2010, 11: 141–150. 10.1007/s10194-010-0187-2
Ratcliffe GE, Enns MW, Jacobi F, Belik SL, Sareen J: The relationship between migraine and mental disorders in a population-based sample. Gen Hosp Psychiatry 2009, 31: 14–19. 10.1016/j.genhosppsych.2008.09.006
Aguggia M, Saracco MG, Cavallini M, Bussone G, Cortelli P: Sensitization and pain. Neurol Sci 2013, 34: S37-S40. 10.1007/s10072-013-1382-0
Yavuz BG, Aydinlar EI, Dikmen PY, Incesu C: Association between somatic amplification, anxiety, depression, stress and migraine. J Headache Pain 2013, 14: 53. 10.1186/1129-2377-14-53
Blaschek A, Milde-Busch A, Straube A, Schankin C, Langhagen T, Jahn K, Schröder SA, Reiter K, von Kries R, Heinen F: Self-reported muscle pain in adolescents with migraine and tension-type headache. Cephalalgia 2012, 32: 241–249. 10.1177/0333102411434808
Rosamond W: Are migraine and coronary heart disease associated? An epidemiologic review. Headache 2004, 44: S5-S12. 10.1111/j.1526-4610.2004.04103.x
Fernandez-de-las-Penas C, Madeleine P, Caminero AB, Cuadrado ML, Arendt-Nielsen L, Pareja JA: Generalized neck-shoulder hyperalgesia in chronic tension-type headache and unilateral migraine assessed by pressure pain sensitivity topographical maps of the trapezius muscle. Cephalalgia 2010, 30: 77–86.
Dufton LM, Dunn MJ, Compas BE: Anxiety and somatic complaints in children with recurrent abdominal pain and anxiety disorders. J Pediatr Psychol 2009, 34: 176–186.
Roberts JE, Deshazo RD: Abdominal migraine, another cause of abdominal pain in adults. Am J Med 2012, 125: 1135–1139. 10.1016/j.amjmed.2012.06.008
Hung CI: Factors predicting adherence to antidepressant treatment. Curr Opin Psychiatry 2014, 27: 344–349. 10.1097/YCO.0000000000000086
Trivedi MH, Morris DW, Grannemann BD, Mahadi S: Symptom clusters as predictors of late response to antidepressant treatment. J Clin Psychiatry 2005, 66: 1064–1070. 10.4088/JCP.v66n0816
Hung CI, Liu CY, Fuh JL, Juang YY, Wang SJ: Comorbid migraine is associated with a negative impact on quality of life in patients with major depression. Cephalalgia 2006, 26: 26–32. 10.1111/j.1468-2982.2005.00985.x
Acknowledgement
This study was supported in part by National Science Council grants NSC 94-2314-B-182A-207 and NSC 95-2314-B-182A-188-MY2. The National Science Council had no further role in study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SJW and CIH designed the study and wrote the protocol. CIH, CYL, CYC, and CHY collected the data. CIH and SJW undertook the statistical analysis, and CIH wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hung, CI., Liu, CY., Chen, CY. et al. The impacts of migraine and anxiety disorders on painful physical symptoms among patients with major depressive disorder. J Headache Pain 15, 73 (2014). https://doi.org/10.1186/1129-2377-15-73
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1129-2377-15-73