Abstract
Background
Coinfection at various sites can complicate the clinical course of coronavirus disease of 2019 (COVID-19) patients leading to worse prognosis and increased mortality. We aimed to investigate the occurrence of coinfection in critically ill COVID-19 cases, and the predictive role of routinely tested biomarkers on admission for mortality.
Methods
This is a retrospective study of all SARS-CoV-2-infected cases, who were admitted to King Fahad Hospital of the University between March 2020 and December 2020. We reviewed the data in the electronic charts in the healthcare information management system including initial presentation, clinical course, radiological and laboratory findings and reported all significant microbiological cultures that indicated antimicrobial therapy. The mortality data were reviewed for severely ill patients who were admitted to critical care units.
Results
Of 1091 admitted patients, there were 70 fatalities (6.4%). 182 COVID-19 persons were admitted to the critical care service, of whom 114 patients (62.6%) survived. The in-hospital mortality was 13.4%. Coinfection was noted in 67/68 non-survivors, and Gram-negative pathogens (Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumanni) represented more than 50% of the etiological agents. We noted that the serum procalcitonin on admission was higher for non-survivors (Median = 1.6 ng/mL ± 4.7) than in survivors (Median = 0.2 ng/mL ± 4.2) (p ≤ 0.05).
Conclusion
Coinfection is a serious complication for COVID-19 especially in the presence of co-morbidities. High levels of procalcitonin on admission may predict non-survival in critically ill cases in whom bacterial or fungal co-infection is likely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel pathogen implicated in the large coronavirus disease of 2019 (COVID-19) pandemic receiving attention globally. The reported case–fatality rate (CFR) of COVID-19 is substantially heterogenous, and has been estimated between 0.15 and 1% in some studies [1, 2]. Factors that can influence the CFR in case of COVID-19 include the size of undiagnosed asymptomatic or mildly symptomatic cases, geographical location, healthcare system readiness, patient population and co-morbidities [3]. A meta-analysis of 27 age-stratified seroprevalence studies in high-resource settings estimated the CFR of COVID-19 based on age around 0.002, 0.01, 0.4, 1.4, 4.6, 15 and > 25% % at the age of 10, 25, 55, 65, 75, 85, and ≥ 90 years respectively [4].
However, mortality increases in hospitalized patients to around 24% as reported during the early waves in Europe, although it showed a decreasing trend over time [5]. A nation-wide surveillance study in the United Kingdom also showed reduced mortality among critically ill COVID-19 patients from 42 to 20% over a nine-month period, which was attributed to resource allocation [3]. Laboratory predictors of mortality include thrombocytopenia, lymphopenia, elevated biomarkers, such as serum ferritin and inflammatory cytokines like IL6, raised D-dimer or prothrombin time, abnormal liver or renal function tests, and elevated levels of troponin and/or creatine phosphokinase [6, 7]. In addition, there is an increasing body of evidence that high procalcitonin level on presentation may reflect severe illness although it can be normal in many cases of pneumonia [8]. Socioeconomic factors play a major role in the variability of reported in-hospital mortality rates, which are higher in case of resource-limited settings [9,10,11].
Bacterial and fungal coinfections at different body sites have been described in the initial COVID-19 studies from China in around 8% of the cases, where respiratory and bloodstream infections were the most common [12]. Fungal and bacterial infections have been especially in mechanically ventilated patients [12, 13]. Coinfection with influenza virus and other respiratory pathogens has been reported with a highly variable frequency across the studies [7, 14,15,16,17,18]. The pathogens described were heterogenous and may reflect the differences in testing protocols of COVID-19 patients in different studies. The age was not a significant risk factor for coinfection in one study by Kim et al. [16]. Less frequently recognized pathogens were shown in some cohorts including Mycoplasma pneumoniae, Orientia tsutsugamushi and dengue virus [18,19,20]. These pathogens may reflect the local endemicity of diseases and can be underestimated by laboratories that do not routinely perform molecular and serological detection. In our study, we aimed to calculate the in-hospital mortality and describe the clinical course and coinfection among non-survivor, critically ill COVID-19 patients in a university hospital in Saudi Arabia. In addition, the prognostic role of procalcitonin was assessed.
2 Materials and Methods
2.1 Research Settings
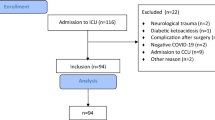
The retrospective study was conducted at King Fahad Hospital of the University, a 550-bed academic health institution. Cases of SARS-CoV-2 infection admitted to our hospital during the first wave of the pandemic in Saudi Arabia, between March 2020 and December 2020. We excluded asymptomatic watchers for patients who were detected by routine screening upon admission, cases diagnosed elsewhere and referred to us in a late status, and dead cases on arrival to the hospital. All cases suspected to have COVID-19 followed a respiratory pathway on presentation and throughout the admission. The data in the electronic charts in the healthcare information management system were systematically reviewed including initial presentation, clinical course, radiological and laboratory findings with significant microbiological cultures that indicated antimicrobial therapy. Coinfection was defined by significant growth of representative cultures in patients with clinical or radiological deterioration that indicated sample collection.
The severity of illness among COVID-19 confirmed patients was clinically determined based on the local national protocol [21]. The mortality-related data were collected for severely ill patients who were admitted to critical care units. Approval of the Institutional Review Board was obtained (IRB-2020-01-150).
2.2 Viral Assays
Nasopharyngeal swabs with viral transport media (Vircell, Granada, Spain) were used to screen for SARS-CoV-2 on admission and transported on ice pack to the laboratory for immediate processing. Reverse transcriptase polymerase chain reaction (RT-PCR) targeting SARS-CoV-2 was performed using the Xpert® Xpress SARS-CoV-2 (Cepheid, Sunnyvale, United States), which identifies the E gene and N2 gene of SARS-CoV-2 following the manufacturer’s recommendations and using positive and negative controls. All samples were processed under a biosafety cabinet Class II Type B to minimize the occupational risk, in a biosafety level 2 laboratory as no viral propagation or aerosol-generating procedure was performed.
2.3 Bacterial and Yeast Identification
Representative clinical specimens from the suspected sites of infections (sputum, tracheal aspirates, urine, soft tissues surrounding the vascular lines) were grown on the corresponding routine bacteriological media including Columbia Blood agar, chocolate agar, MacConkey agar plates (SPML, Riyadh, Saudi Arabia), and were incubated aerobically and anaerobically at 35 °C for 24–48 h. Any significant growth for the site was identified using the VITEK® MS (bioMe ́rieux Inc., Durham, NC, USA), an automated mass spectrometry microbial identification system based on matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) technology. Antimicrobial susceptibility testing was performed by VITEK 2 automated system (bioMe ́rieux Inc., Durham, NC, USA) as per the manufacturer’s recommendations.
2.4 Procalcitonin Measurement
The ADVIA Centaur® XPT Immunoassay System (Siemens, Munich, Germany) was utilized following the manufacturer’s instructions to measure quantitative levels of procalcitonin on admission as a part of the hospital policy for all cases suspected to have SARS-CoV-2 infection.
2.5 Statistical Analysis
The statistical analysis was performed using the Graphpad Prism Version 9.0. Kolmogorov–Smirnov test was measured to assess the difference of procalcitonin analyte levels between survivors and non-survivors. A significance value of p ≤ 0.05 was used.
3 Results
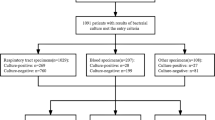
A total of 1091 patients were diagnosed, among whom there were 70 fatalities (6.4%). The median age for all patients was 48 years ± 4.3. The total number of COVID-19 cases who required admission was 521, with 70 deaths (in-hospital mortality rate = 13.4%). 182 cases required critical care admission, of whom 114 patients (62.6%) survived and 68 (37.4%) passed away. The non-survivors had a median body mass index (BMI) of 26.9 ± 3.1 and were 14 females (20.6%) and 54 (79.4%) males of whom 26 cases (38.3%) were Saudi and 42 (61.7%) were non-Saudi. The total case fatality rates in Saudi and non-Saudi patients were 28/639 (4.4%) and 42/452 (9.3%) (Odds Ratio = 2.1 95% CI 1.2–2.9). Figure 1 and Tables 1 and 2 summarize the initial clinical presentation for the 68 non-survivor patients.
Mortality was highest for patients aged 60 years or older; 47/70 (67.1%), followed by middle-aged cases between 31 and 59 years old 23/70 (32.9%). No mortality was encountered in patients between 0 and 30 years old.
The median time to intubation for the non-survivors was 4 days (0–32), with a median duration of intubation around 11 days (0–38). Invasive mechanical ventilation was used in 63/68 (92.6%) patients while non-invasive mechanical ventilation or oxygen supplementation were used in 36 (52.9%) and 40 (58.8%) patients respectively (Table 3).
Bacterial and fungal coinfections were noted among all but one non-survivor (67/68). Table 4 outlines the types of infection and pathogens identified in those cases. We noted that the serum procalcitonin on admission was higher for non-survivors (Median = 1.6 ng/mL ± 4.7) than in survivors (Median = 0.2 ng/mL ± 4.2) (p ≤ 0.05) as evident in Fig. 4. Other laboratory findings for the non-survivor group exhibited abnormal renal function tests in 32 (47.1%) raised LDH in 61 (89.7%), lymphopenia in 39 (57.4%), and neutropenia in only 1 case (1.5%).
4 Discussion
This study included all confirmed COVID-19 cases in an academic health institution with an overall case fatality rate of 6.4% during the first wave prior to the introduction of the COVID-19 vaccines. This rate is lower than in-hospital fatality in several countries [3, 5], which be attributed to several explanation, including the younger local patient population, healthcare infrastructure and virus-related factors. Nevertheless, the higher case fatality rate seen among other ethnic groups in our institution (Odds Ratio = 2.1 95% CI 1.2–2.9) raises a concern. Whether this phenomenon is related to host immune factors or social determinants leading to delayed presentation is unclear and worth further investigation. As in other studies, the mortality was increased proportionally with age in the described cohort [3].
Furthermore, the complications encountered in the described patient population were previously described, such as barotrauma, pneumomediastinum, bacteremia and other infections [22]. In our cohort, coinfection was a frequent event in non-survivors and Gram-negative pathogens, namely Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumanni, represented more than 50% of the etiological agents. The predominance of Gram-negative pathogens in our cohort can be interpreted by the inhabitance of hospital environments by these organisms which are known to harbor multidrug-resistance determinants adding to the complexity of management of COVID-19 cases [23]. Some studies reported lower incidence of coinfection because the study was designed to include co-infections in all COVID-19 patients, whereas our data were restricted to non-survivors [13, 24]. It is difficult to accurately estimate coinfection rate in all COVID-19 cases due to the fact that not all patients are being tested serially for coinfection, particularly during the early phase of the COVID-19 pandemic due to pressure on the healthcare systems. In a secondary-care study performed in England, 77% of COVID-19 cases had blood culture but 15% only had respiratory culture work-up, which can explain the heterogeneity in confection rates across the literature, although it is difficult to ascertain the true infection from colonization in some of those respiratory cultures [25]. Based on combined microbiological and clinical data, 25% and 57.4% of the non-survivors in our institution had bloodstream or respiratory infection respectively with 7.4% developing multiple infections mainly lower respiratory infections and bacteremia (Table 4). Post-viral bacterial pneumonia is thought to be mediated by complex interactions with the nasopharyngeal flora and the host immune system [26]. It was previously shown that influenza virus markedly reduced tracheal mucus velocity during the early phase of infection that continued for up to several weeks [27]. Thus, upper airway flora gets access to the lung parenchyma leading to secondary bacterial infections. Coinfection by influenza virus and other respiratory viruses in COVID-19 patients has been also described [14]. In our testing protocol, only influenza virus was routinely included and we did not detect any influenza coinfection among the first wave of COVID-19 cases (Figs. 2, 3).
Nearly all non-survivor patients in our study received mechanical ventilation. A previous study has shown 60% mortality among mechanically ventilated patients [5]. Among the potential prognostic factors in COVID-19 cases is the initial serum level of procalcitonin which may reflect disease severity [28, 29]. Our findings are consistent with a study by Liu et al. whose work suggested that mortality can be inferred from early measurements of procalcitonin levels (Fig. 4) [30]. Krause et al. have also found an association between early procalcitonin level > 0.1 ng/ml and duration of mechanical ventilation [31]. Procalcitonin has been used as a stewardship tool to distinguish bacterial from non-bacterial etiologies of inflammation especially in cases of lower respiratory tract infections [32]. Thus, high initial procalcitonin may reflect early bacterial coinfection in those cases since viral infections facilitate invasiveness by upper airways commensal opportunistic flora. Nevertheless, the rise in procalcitonin may be related to generic systemic inflammation seen in the later stages of illness that is not specific for secondary infections and so this phenomenon needs to be further examined [33]. Other promising prognostic markers in COVID-19 cases are under assessment, such as the neutrophil-to-lymphocyte ratio, although they are in need of further evaluation [34].
The main limitation of this study is its reliance on date representing a specific institutional experience rather than being a population-level study. In addition, the retrospective nature of the study did not allow standardizing the times of microbiological testing to investigate coinfection. Nevertheless, it contributes to the growing evidence of variable post-acute sequelae of the illness and mortality rates in relation to coinfections and other factors that represent challenges during the clinical course of COVID-19 infection.
5 Conclusion
This study showed that the in-hospital COVID-19 mortality is lower in case of high-resource settings and younger patient population. It also highlighted coinfection as a serious complication for SARS-CoV-2 leading to fatality, particularly in patients with other co-morbidities. High initial levels of procalcitonin on presentation may predict poor prognosis in critically ill patients who require admission to intensive care.
Availability of Data and Material
No supplementary files are enclosed. Raw data can be submitted on a justifiable request.
Abbreviations
- CFR:
-
Case–fatality rate (CFR)
- LDH:
-
Lactate dehydrogenase
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis. 2020;101:138–48. https://doi.org/10.1016/j.ijid.2020.09.1464 (Epub 2020 Sep 29).
Ioannidis JPA. Reconciling estimates of global spread and infection fatality rates of COVID-19: an overview of systematic evaluations. Eur J Clin Invest. 2021;51(5): e13554. https://doi.org/10.1111/eci.13554 (Epub 2021 Apr 9).
Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209–14. https://doi.org/10.1097/CCM.0000000000004747.
Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35(12):1123–38. https://doi.org/10.1007/s10654-020-00698-1 (Epub 2020 Dec 8).
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;22(369): m1966. https://doi.org/10.1136/bmj.m1966.
Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7(9):e671–8. https://doi.org/10.1016/S2352-3026(20)30217-9 (Epub 2020 Jul 10).
Harapan H, Fajar JK, Supriono S, Soegiarto G, Wulandari L, Seratin F, et al. The prevalence, predictors and outcomes of acute liver injury among patients with COVID-19: a systematic review and meta-analysis. Rev Med Virol. 2021;13: e2304. https://doi.org/10.1002/rmv.2304 (Epub ahead of print).
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5 (Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30).
African COVID-19 Critical Care Outcomes Study (ACCCOS) Investigators. Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): a multicentre, prospective, observational cohort study. Lancet. 2021;397(10288):1885–94. https://doi.org/10.1016/S0140-6736(21)00441-4 (Erratum in: Lancet. 2021 Jun 26;397(10293):2466).
Islam N, Shkolnikov VM, Acosta RJ, Klimkin I, Kawachi I, Irizarry RA, et al. Excess deaths associated with covid-19 pandemic in 2020: age and sex disaggregated time series analysis in 29 high income countries. BMJ. 2021;19(373): n1137. https://doi.org/10.1136/bmj.n1137.
Woolf SH, Chapman DA, Sabo RT, Zimmerman EB. Excess deaths from COVID-19 and other causes in the US, March 1, 2020, to January 2, 2021. JAMA. 2021;325(17):1786–9. https://doi.org/10.1001/jama.2021.5199 (Epub ahead of print).
Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–68. https://doi.org/10.1093/cid/ciaa530.
Kubin CJ, McConville TH, Dietz D, Zucker J, May M, Nelson B, Istorico E, Bartram L, et al. Characterization of bacterial and fungal infections in hospitalized patients with coronavirus disease 2019 and factors associated with health care-associated infections. Open Forum Infect Dis. 2021;8(6):ofab201. https://doi.org/10.1093/ofid/ofab201.
Wu X, Cai Y, Huang X, Yu X, Zhao L, Wang F, et al. Co-infection with SARS-CoV-2 and influenza A virus in patient with pneumonia, China. Emerg Infect Dis. 2020;26(6):1324–6. https://doi.org/10.3201/eid2606.200299.
Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;92(9):1549–55. https://doi.org/10.1002/jmv.25781 (Epub 2020 Mar 30).
Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–6. https://doi.org/10.1001/jama.2020.6266.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9. https://doi.org/10.1001/jama.2020.6775 (Erratum in: JAMA. 2020 May 26;323(20):2098).
Zha L, Shen J, Tefsen B, Wang Y, Lu W, Xu Q. Clinical features and outcomes of adult COVID-19 patients co-infected with Mycoplasma pneumoniae. J Infect. 2020;81(3):e12–5. https://doi.org/10.1016/j.jinf.2020.07.010 (Epub 2020 Jul 8).
Masyeni S, Santoso MS, Widyaningsih PD, Asmara DW, Nainu F, Harapan H, et al. Serological cross-reaction and coinfection of dengue and COVID-19 in Asia: experience from Indonesia. Int J Infect Dis. 2021;102:152–4. https://doi.org/10.1016/j.ijid.2020.10.043 (Epub 2020 Oct 25).
Bastola A, Sah R, Rajbhandari SK, Jha R, Fathah Z, Chalise BS, et al. SARS-CoV-2 and orientia tsutsugamushi co-infection in a young teen, Nepal: significant burden in limited-resource countries in Asia? Narra J. 2021;1(2): e34. https://doi.org/10.52225/narraj.v1i2.34.
Ministry of Health. Saudi MoH protocol for patients suspected of/ confirmed with COVID-19 version 2.1; 2020. Available from: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf. Accessed Nov 1, 2021.
McGuinness G, Zhan C, Rosenberg N, Azour L, Wickstrom M, Mason DM, et al. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020;297(2):E252–62. https://doi.org/10.1148/radiol.2020202352 (Epub 2020 Jul 2).
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–111. https://doi.org/10.1093/cid/ciw353 (Epub 2016 Jul 14. Erratum in: Clin Infect Dis. 2017 May 1;64(9):1298. Erratum in: Clin Infect Dis. 2017 Oct 15;65(8):1435. Erratum in: Clin Infect Dis. 2017 Nov 29;65(12):2161).
Sakka SA, Howse JP. Necrotic cutaneous arachnidism in the U.K. Br J Dermatol. 1994;130(4):542–3. https://doi.org/10.1111/j.1365-2133.1994.tb03398.x.
Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–9. https://doi.org/10.1016/j.cmi.2020.06.025 (Epub 2020 Jun 27).
Prasso JE, Deng JC. Postviral complications: bacterial pneumonia. Clin Chest Med. 2017;38(1):127–38. https://doi.org/10.1016/j.ccm.2016.11.006 (Epub 2016 Dec 13).
Pittet LA, Hall-Stoodley L, Rutkowski MR, Harmsen AG. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2010;42(4):450–60. https://doi.org/10.1165/rcmb.2007-0417OC (Epub 2009 Jun 11).
Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56(2): 106051. https://doi.org/10.1016/j.ijantimicag.2020.106051 (Epub 2020 Jun 10).
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 (Epub 2020 Mar 4).
Liu ZM, Li JP, Wang SP, Chen DY, Zeng W, Chen SC, et al. Association of procalcitonin levels with the progression and prognosis of hospitalized patients with COVID-19. Int J Med Sci. 2020;17(16):2468–76. https://doi.org/10.7150/ijms.48396.PMID:33029089;PMCID:PMC7532477.
Krause M, Douin DJ, Tran TT, Fernandez-Bustamante A, Aftab M, Bartels K. Association between procalcitonin levels and duration of mechanical ventilation in COVID-19 patients. PLoS One. 2020;15(9): e0239174. https://doi.org/10.1371/journal.pone.0239174.
Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, Bouadma L, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95–107. https://doi.org/10.1016/S1473-3099(17)30592-3 (Epub 2017 Oct 13 PMID: 29037960).
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. https://doi.org/10.1056/NEJMoa2002032 (Epub 2020 Feb 28).
Sarengat R, Islam MS, Ardhi MS. Correlation of neutrophil-to-lymphocyte ratio and clinical outcome of acute thrombotic stroke in patients with COVID-19. Narra J. 2021. https://doi.org/10.52225/narra.v1i3.50.
Acknowledgements
The authors would like to thank all the KFHU institution staff who were involved in routine handling of the cases included in the study.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
NB and SA carried out the data collection and participated to the analysis, contributed to the draft. SA participated in the data collection and contributed to the manuscript. SYA and AH participated in the design of the study and contributed to the data analysis. HB and MSA conceived of the study, and participated in its design and coordination and helped to draft the manuscript. AA performed the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no conflict of interest in this study.
Ethics Approval and Consent to Participate
Approval of the Institutional Review Board was obtained (IRB-2020-01-150).
Consent for Publication
Consent from participants was obtained as per the ethics code of the World Medical Association (Declaration of Helsinki).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alnimr, A.M., Alshahrani, M.S., Alwarthan, S. et al. Bacterial and Fungal Coinfection in Critically Ill COVID-19 Cases and Predictive Role of Procalcitonin During the First Wave at an Academic Health Center. J Epidemiol Glob Health 12, 188–195 (2022). https://doi.org/10.1007/s44197-022-00038-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44197-022-00038-4