Abstract
The main objective of this study is to evaluate the nutritional risk of stroke patients through deep learning and multimodal MRI images, and provide appropriate nutrition for patients in a timely manner to improve their recovery speed. The method of this article mainly solves the problem of incomplete understanding of patient nutritional risk through a deep learning (DL) and multimodal MRI image-based nutritional risk assessment (RA) model, and accurately provides corresponding countermeasures. The evaluation model based on DL and multimodal MRI images shows that 27 people in Group A are at nutritional risk, accounting for 90%. 26 people in Group B are at nutritional risk, accounting for 86.6%. Both groups of patients urgently need corresponding strategies to reduce risk. Therefore, this article also tested two sets of nutritional support methods, and the results showed that the nutritional support methods in Group B were more effective. The nutritional indicators are not only normal, but the infection rate and mortality rate of patients have also decreased. The results demonstrate that deep learning and multimodal MRI images can promote the recovery process of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the continuous development and progress of modern medicine, the mortality rate of stroke is lower than before, but the morbidity and disability rates are still high. This not only affects the patient's health level and quality of life but also adversely affects the patient's physical and mental health. Due to pain and heavy economic burden, the research and prevention of stroke have become a major public health problem in China [1]. Nutritional risk is a common complication in patients with severe stroke, which not only worsens the patient's state but also directly affects the patient's quality of life and even increases mortality. Therefore, in the treatment of severe stroke patients, in addition to paying attention to the treatment and nursing of the primary disease, nutritional RA can provide a scientific theoretical basis for nutritional support. However, the problem of how to detect malnutrition early and effectively implement nutritional support has always plagued the majority of clinical medical workers.
The incidence of stroke in China has been increasing in recent years. About 14.7% of the patients had dysphagia after stroke onset [2]. Dysphagia affects the intake of nutrients, which can easily lead to malnutrition or aggravate malnutrition, thereby affecting the recovery of stroke and increasing the incidence of complications. Therefore, nutritional screening and assessment for stroke patients with dysphagia, and timely and reasonable provision of nutritional support can improve the nutritional status of patients, enhance the immunity of patients, and promote the recovery of patients. This paper comprehensively evaluates the nutritional status of stroke patients with dysphagia based on DL and multimodal MRI images. To analyze the effects of different nutritional intervention methods on nutritional indicators, clinical indicators and prognosis, it provides an objective basis for how to select and provide nutritional support for stroke patients with dysphagia. The innovation of this paper is to propose a comprehensive assessment of nutritional risk in stroke patients based on DL and multimodal MRI images. It puts forward corresponding countermeasures in a timely manner to improve the quality of life and survival of patients.
2 Related Works
The risk of stroke is rising along with China's population aging. According to Sato et al. [3] senior stroke patients' functional recovery and post-discharge recovery are both impacted by malnutrition during hospitalization. He examined the link between changes in nutritional status and improvements in daily activities in elderly stroke patients hospitalized with acute stroke and identified the elements that contributed to these changes in nutritional status. The study by Lee et al. [4] was to determine how dietary risk affected stroke patients' ability to improve their balance and gait. Over the course of six weeks, thirty patients underwent standard therapy in five sessions of 60 min each. Someone has proposed a rehabilitation tool for accelerating post-stroke motor recovery. Fischer et al. [5] discovered the Brain Computer Interface (MI-BCI). For stroke patients, calibrating the BCI system is a tiresome and time-consuming operation, which cuts into the amount of time available for actual therapy engagement. Hoof et al. [6] discovered that patients with recent ischemic stroke had a higher risk of recurrence than patients with transient ischemic attack (TIA). To lower the danger of malnutrition, he used logistic regression for statistical analysis. According to research by Zaldivar et al. [7], stroke is the fourth most common cause of death in Europe. It is one of the most prevalent factors in adult patients' disabilities and is associated with high medical expenses. He looked at the connection between nutritional risk and quality of life. The condition of nutritional risk is one that is easy to appear. Scholars have realized that stroke has become more and more serious in recent years, and the probability of nutritional risk is also increasing. To reduce the risk of raw nutrition, it should be assessed, but scholars have not proposed an assessment method.
In clinical and research settings, the identification, localization, diagnosis, and classification of stroke are crucial. The early detection and identification of stroke disease, as well as the correct assessment of nutritional risk, have a decisive impact on the recovery of patients and prolongation of life. Michael and Arokia [8] introduced a novel segmentation algorithm for multimodal MRI images, which is also a technique for classifying stroke severity. Segmentation of preprocessed images can be done using multimodal MRI images. Galinsky et al. [9] presented a new method for integrating stroke datasets into magnetic resonance imaging analysis using DL. This approach allows for enhanced spatiotemporal localization of brain function and its correlation with morphological features and structural connectivity. Angulakshmi and Priya [10] found that assessing nutritional risk for stroke is challenging due to its diversity. DL-based methods are used to assess nutritional risk and find the best solution in time. Medical imaging research is one of the most crucial areas of study in the healthcare industry according to Alden et al. [11]. As a result, the speed and precision necessary for multimodal MRI images are at least as crucial as those required for data retrieval. The problems with medical pictures can be resolved with the aid of DL. He suggested a Convolutional NNs (CNN)-based analysis paradigm for enhancing medical image diagnosis. Scholars have found that the detection and judgment of lesions are very important in medicine, and the assessment of nutritional risk is also beneficial to prolong the survival of patients. Based on DL and multimodal MRI images, the accuracy of nutritional RA can be improved.
3 Evaluation Method Based on DL and Multimodal MRI Images
Stroke is a common disease in neurology. After stroke, according to the swallowing disorder, neurological disorder, intracranial hypertension, loss of body fluids, high-stress state of the body, neuroendocrine factors, and psychological factors, the diet and life of patients are also different. This also leads to an increased nutritional risk in patients [12]. Scholars have found that poor nutritional status in stroke patients may worsen brain damage. This leads to adverse outcomes, and specific nutritional interventions can improve neurocognitive recovery and prevent or treat complications caused by energy protein deficiency.
Clinically, it is often necessary to perform multiple image modalities or multiple image modalities of the same patient on the same patient. For a comprehensive analysis, it is necessary to acquire information from multiple images simultaneously. MRI imaging can also obtain different structural imaging of the same tissue [13]. There are also recent studies on multimodal image parsing methods using machine learning. By combining the brain images of various imaging modes to obtain various information about the brain, it realizes multi-information synthesis and plays the role of mutual learning.
3.1 Stroke Image Segmentation Based on Multimodal MRI Images
The main challenge of traditional segmentation algorithms lies in the large grayscale similarity between brain tissues in MRI images, as well as the differences between different cases. Multimodal MRI brain tumor image segmentation can make full use of the feature information of different modes in the MRI image to improve the effectiveness of segmentation. It is a research hotspot of brain tumor image processing in recent years. Images and voice are typical examples of two separate information modalities, though the term "multimodality" is used more broadly. Assuming that a person has now been identified using both voice and image data, recognition based on these two types of data is a use of multimodal data; the voice offers auditory traits and the image provides visual ones [14]. The proper rate of recognition would undoubtedly be substantially greater if these two types of information are combined. The distinction is that multiple MRI sequences are used to produce the multimodalities in MRI images [15].
Multi-modal MRI image segmentation can fully utilize the feature information of different modalities in MRI images, improve the effectiveness of segmentation, and has become a research hotspot in image processing in recent years. Picture preprocessing is frequently a crucial step that has an impact on how well a system performs in common image segmentation, recognition, or detection systems [16]. Grayscale normalization and contrast adjustment of multimodal MRI images are the main topics of this research. MRI images often have a gray level of 16 bits or higher, and the dispersed gray distribution and higher gray value have a certain detrimental effect on the accuracy of brain image segmentation. To do this, the grayscale normalization of the MRI data is carried out as in formula 1 before the segmentation process:
\(h\left( {a,b} \right)\) is the original histogram of the image and \(h_{\min }\) is its minimum gray level. \(BWM\) is the histogram of the image after normalization.
Even after normalization, MRI images still cannot adequately segment the brain because the contrast between various tissues may significantly weaken segmentation accuracy [17]. The multimodal MRI image's series of slices are extracted after the original MRI image has been preprocessed to make necessary adjustments. The method is depicted in Fig. 1:
As shown in Fig. 1, in the image segmentation, the model based on the level set method extends the evolution of the curve contour of the segmentation target into the three-dimensional space by describing the two-dimensional segmentation as the zero-level set of the three-dimensional level set function. Then it evolves or iterates the level set function using the evolution formula satisfied by the objective [18].
The contour curve evolution process of defining \(C = C\left( {a,t} \right)\) representation evolution in the image domain can be expressed as formula 2:
From the chain derivation rule, formula 3 can be obtained:
V represents the velocity \(V\left( {a,t} \right)\) defined on \(\Omega\), and formula 3 is the so-called level set formula, which describes the evolution and movement process of the level set. In the actual processing of the segmentation problem, it is not solved in the form of such a continuous function but expressed in a discrete form.
The differential multi-modal image obtained by differentiating the multi-modal MRI image is referred to as a global differential image (GDI) here because it is a differential operation for the entire image area [19]. The results obtained by GDI are used to locate the relative position of the brain in the MRI image. The purpose of using the segmentation results of GDI here is to improve the segmentation effectiveness of the entire segmentation method by making full use of the information about brain regions in multimodal images [20], as shown in Fig. 2:
As shown in Fig. 2, the obtained multimodal MRI image of the initial segmentation results of the brain still retains a lot of irrelevant segmentation results, so it is particularly important to deal with these irrelevant regions before the end of the segmentation. At this time, the segmentation results of GDI are particularly important, as shown in Fig. 2 to locate the location of the brain region. Then, the segmentation results of brain regions are obtained according to a weighted fusion rule and region screening [21, 22].
The multimodal intersection domain kernel matrix and the multimodal auxiliary domain kernel matrix are recalculated. Its multimodal synthesis kernel function is formula 4:
\(c_{m}\) is the weight of the kernel function on the mth mode.
Differential applications are common in computer vision. For example, in target tracking in a fixed scene, since the background does not change, to detect the moving target in the scene, the usual method is to use the difference to subtract the background.
The histograms of multimodal MRI differential images were used to obtain information about brain enhancement regions that were not visible in a single modality. Inspired by this, this section proposes a weighted difference operation on multimodal MRI images for the segmentation of the entire brain region, such as formula 5 and formula 6:
Among them, \(Sublmg\left( {i - j} \right)\) is the difference operation of images i and j, and \(\mu_{1}\) is used to control the gray intensity of the difference image. \({\text{WDI}}\) is used to obtain differential feature images of brain regions from multimodal MRI images, and \(\mu_{2}\) and \(\mu_{3}\) are control parameters to highlight the differential results of a modality. According to the grayscale mapping when extracting slices, it is necessary to make corresponding adjustments to obtain better visual differential feature information.
3.2 Physiological Data Feature Extraction Method Based on DL
Training in accordance with the parameters of the convolutional Neural Network (NN) model is the primary component of the feature learning process. Prior to preprocessing the original data, the data of various dimensions must be normalized to the same interval. The convolutional NN's parameters are trained in an unsupervised fashion using the training data and \(W^{{\left( {l,1} \right)}}\) is used to represent the network weight corresponding to the ith autoencoder. The algorithm is formula 7 and formula 8:
A set of ideal parameters is generated by continual iteration so that the features the network learns can more accurately replicate the original data. Finally, the upcoming nutritional RA is prepared using the learned network model. Its algorithm is expressed as the following formula:
\(a^{{\left( {n + l} \right)}}\) is the high-order feature of the original data that the model finally learns.
The NN algorithm known as Restricted Boltzmann Machine (RBM) uses unsupervised learning. The conventional NN makes use of artificially created data features, and it is simple for the network to settle on the neighborhood's best option. The classification impact degrades as the number of network layers rises. Figure 3 shows the RBM structural diagram.
It can be seen from Fig. 3 that the relevant characteristics are directly received once the generative model from the probabilistic graphical model is inserted into the Restricted Boltzmann Machine (RBM) network structure. It can effectively learn the NN's weights and resolve issues like manual feature extraction brought on by poorly thought out, subjectively personal considerations.
The RBM is essentially a NN model based on energy, and formula (11) expresses the energy function between the visible layer variable v and the hidden layer variable h.
The input layer and the hidden layer are the two layers of the RBM network. By minimizing the cost function, the weights of the network are calculated.
3.3 Nutritional RA Model Based on DL and Multimodal MRI Images
Convolutional NNs were discovered by two biologists studying the structure of the cat's visual system. The cells of the animal visual field are very complex structures, and the receptive field of these cells is very sensitive to a specific part of the visual input space. This is the source of the core concepts of local connections and weight sharing in convolutional neural networks, as shown in Fig. 4:
As seen in Fig. 4, the convolutional NN's weight sharing is comparable to lowering the number of network parameters, which significantly lowers the network's computational complexity and speeds up training. First, a data sample \(\left( {A,b_{p} } \right)\) is selected from the training set, and A is passed to the network as the input of the network and then changed to formula 12 through level-by-level learning:
The original data A is used as the input of the convolutional NN and \(H_{i}\) is used to represent the feature matrix of the ith layer of the convolutional NN. The calculation process of \(H_{i}\) in the convolutional layer can be expressed as formula 13:
W stands for the weight matrix of the convolution kernel of the ith convolutional NN layer.
A probability distribution of continuous probability variables is the gaussian distribution, sometimes referred to as the normal distribution. The data from the natural world, human society, psychology, education, and many other phenomena, such as student success quality and ability level, all fall under the normal distribution.
If the random variable a adapts to the Gaussian distribution \(a\sim N\left( {\mu ,\sigma^{2} } \right)\), its respective probability density feature can be represented as formula 14.
Among them, the existing data can be used to calculate the \(\mu\) and \(\sigma^{2}\) in the probability density function as follows:
The covariance matrix of the feature must be built in the multivariate Gaussian distribution model to calculate the \(p\left( a \right)\) of the feature, and the precise calculation method is formula 16:
Finally, the value of the probability of calculating the multivariate Gaussian distribution is formula 17.
The mean value of a row of data in the original matrix serves as each unit of the vector \(\mu\), which is one of them.
Because most patients' physiological parameters are in a safe state under normal circumstances, there are only a few times when the physiological data become abnormal. The thesis designs a method that belongs to a certain probability interval of feature points and the percentage of total features. Different characteristic nutritional risk intervals were divided, and a nutritional RA model was constructed. Its specific algorithm is expressed as formula 18:
Because the multivariate Gaussian distribution model frequently seeks to compare two characteristics simultaneously, it can come across a decision boundary \(\varepsilon_{n} = \left( {1,2,3,...,n} \right)\) that is quite huge. As a result, the model does not accurately match the feature distribution.
4 Experiment on Nutritional RA and Countermeasures for Stroke Patients
4.1 Nutritional RA Based on DL and Multimodal MRI Images
In this paper, 60 patients with severe strokes from August 2017 to August 2018 were selected to participate in the experiment, and SPSS13.0 software was used for statistical analysis. The basic conditions of the two groups of patients are shown in Table 1:
As shown in Table 1, the age range of group A and the age range of group B are not much different, there are 17 males and 13 females in group A. Group B had 16 males and 14 females, and there was no gender difference. The number of cerebral hemorrhage in group A was 6, and the number of cerebral hemorrhage in group B was 8. It can be seen that under the basic conditions, there is no difference between the two groups of patients.
The features found by convolution NN are applied to multivariate Gaussian distribution. The characteristic points in various probability intervals were classified into nutritional risk classes based on the size markers of the mean probability of the characteristic points in the interval, as shown in Table 2.
As shown in Table 2, the risk corresponding to A is a severe risk, the risk corresponding to B is moderate risk, the risk corresponding to C is a mild risk, and the risk corresponding to D is no risk.
At the same time, people also listed the segmentation prediction accuracy in the training process of 400 iterations, and compared the single-modal and multi-modal modes, as shown in Fig. 5:
As shown in Fig. 5, the predicted results are very close to the real results, and a better outline of the brain region can be effectively obtained when the grayscale performance is uneven. When the boundary is not very clear, ideal segmentation prediction is still obtained by using multimodal MRI images. It can be seen that the training accuracy of the multimodal model is higher than that of direct training. Based on this, it can be predicted that the RA model obtained by using multimodality can better adapt to the task of stroke image segmentation.
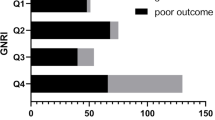
As shown in Fig. 6, it is important to consider whether the learned features follow a Gaussian distribution when providing them to the multivariate Gaussian model of nutritional RA.
The dataset test results demonstrate that the approach described in this research is capable of extracting the main feature representation of multi-dimensional physiological data, as shown in Fig. 6. The user's risk status level is then determined using the feature points within the same probability interval and their respective probabilities. Big data-based analysis can assist medical personnel and patients' families in comprehending the risk status of patients in this way. The model can be a useful tool for patients to utilize to measure their own nutritional condition over time and make corresponding measures in time so that patients can recover quickly.
The validity of the RA model proposed in this paper is verified, and then the nutritional risk of the two groups of patients should be assessed accordingly, so as to propose corresponding countermeasures. The evaluation results of the patients based on the evaluation model based on DL and multimodal MRI images are shown in Table 3
As shown in Table 3: In the evaluation results of patients based on DL and multimodal MRI images, there were 9 patients in group A who were at severe nutritional risk and 10 patients in group B. There were 12 patients at moderate nutritional risk in group A and 9 in group B. There were 6 patients at mild nutritional risk in group A and 7 in group B. There were only 3 patients in group A with no nutritional risk, and only 4 patients in group B. It can be seen that the majority of patients in the two groups are at nutritional risk, so it is necessary to implement nutritional countermeasures in a timely manner.
4.2 Influence of Two Nutritional Methods on Nutritional Indicators of Patients
After evaluating the nutritional risk of patients in the previous article, this paper proposes two groups of nutritional support methods and measures and compares the two methods. Group A used enteral nutrition support, and group B used enteral combined with parenteral nutrition. After 6 months of testing, this paper recorded the nutritional indicators of the two groups in these 6 months. The nutritional indicators were serum albumin and serum prealbumin, as shown in Fig. 7:
As shown in Fig. 7, serum albumin levels in the two groups gradually decreased after admission, and reached the lowest point on the 5th and 7th days in group A and group B, respectively. Serum albumin concentrations were higher in both groups than at the start of the observation. The serum albumin level of group B was generally higher than that of group A. On the 3rd day after admission, the serum prealbumin level in group A decreased to the lowest level, while that in the EN + PN group decreased to the lowest level on the 5th day. Serum prealbumin levels in group B were higher than those in group A.
4.3 Total Infection Incidence and Fatality Rate of Two Groups of Patients
To prove that the countermeasures proposed in this paper are useful, this paper investigates the total infection incidence and case fatality rate after six months of the two groups. The total infection incidence is shown in Table 4:
As shown in Table 4, there are 12 people with lung infection in group A and 7 people with a lung infection in group B. There were 8 people with urinary tract infection in group A and 3 people with urinary tract infections in group B. The number of intestinal infections in group A was 2, and that in group B was 0. The number of gastrointestinal bleeding in group A was 1, and that in group B was 1. The total infection rate of group A was 76.6%, and that of group B was 36.6%. It can be seen that the total infection rate of group B is half of that of group A, indicating that the countermeasures proposed in this paper are effective.
The total infection fatality rate after six months for the two groups is shown in Fig. 8:
As shown in Fig. 8, it can be observed that even if patients are given active nutritional support treatment in the early stage, the mortality rate of stroke patients is still higher. Therefore, to study the changes in nutritional status, endocrine metabolism and other indicators in stroke patients with dysphagia, as well as dynamic monitoring of clinical prognosis, aiming to detect the occurrence of nutritional disorders early and select appropriate nutritional pathways, this is of great significance for improving the clinical outcome and prognosis of patients, reducing infection rate and mortality.
5 Conclusion
Stroke is a disease with a high incidence in the elderly, which not only seriously affects the life safety of patients, but also causes nutritional risks due to the inability to eat normally in daily life. In actual clinical diagnosis, only one diagnostic method is difficult to diagnose and the misdiagnosis rate is high. Combining multiple methods can improve the sensitivity and specificity of diagnosis. Therefore, this paper proposes multimodal MRI images to segment medical images of stroke patients to assess whether there is a nutritional risk. However, multimodal MRI images alone cannot comprehensively assess the nutritional risk of patients, and it is necessary to combine DL to build a nutritional RA model based on DL convolutional NNs. This article uses mainstream image processing techniques in deep learning as a research tool, with the aim of improving segmentation efficiency and effectiveness, to improve the accuracy of patient nutritional risk assessment. The simultaneous use of both would greatly improve the accuracy of nutritional RA. Therefore, the experimental analysis is carried out in this paper, and the results show that the segmentation accuracy of multimodal MRI images is very high. This experiment demonstrates that the method proposed in this article not only reduces the infectivity of patients but also increases their serum prealbumin levels. The nutritional RA model based on DL has a high evaluation accuracy, and it can be applied to the nutritional RA of stroke, which can timely understand the nutritional status of patients and prescribe the right medicine. Due to the limited test site and materials, the experimental data may not be very accurate. The paper would always strive for excellence and do better.
Data availability statement
The experimental data used to support the findings of this study are available from the corresponding author upon request.
References
Chen, X.M., Wang, Y.F., Wang, Q., Zhou, Y.F.: Analysis of the trend of morbidity and mortality of stroke in Fuling District, Chongqing, From 2015 to 2019. Occup. Health Damage 36(5), 320–324 (2021)
Wang, W., Cheng, J.X., Yan, L.X., Chen, X.R., He, Y., Jie, W., Hou, L., Wang, Z.W., Wu, J.: Analysis of the incidence of stroke in the population at 10 monitoring points in China from 2015 to 2019. Modern Prevent. Med. 49(24), 4524–4528 (2022)
Sato, M., Ido, Y., Yoshimura, Y., Mutai, H.: Relationship of malnutrition during hospitalization with functional recovery and postdischarge destination in elderly stroke patients. J. Stroke Cerebrovasc. Dis. 28(7), 1866–1872 (2019)
Lee, H.J., Kim, Y.M., Lee, D.K.: The effects of action observation training and mirror therapy on gait and balance in stroke patients. J. Phys. Ther. Sci. 29(3), 523–526 (2017)
Fischer, U., Kaesmacher, J., Molina, C.A.: Primary thrombectomy in tPA (tissue-type plasminogen activator) eligible stroke patients with proximal intracranial occlusions. Stroke 49(1), 265–269 (2018)
Hoof, R., Schreuder, F., Nelemans, P., Truijman, M.T.B., Kooi, M.E.: Ischemic stroke patients demonstrate increased carotid plaque microvasculature compared to (ocular) transient ischemic attack patients. Cerebrovasc. Dis. 44(5–6), 297–303 (2017)
Zaldivar, J.N.C., Calvo, S., Bravo-Esteban, E., Ruiz, P.O., Santi-Cano, M.J., Herrero, P.: Effectiveness of dry needling for upper extremity spasticity, quality of life and function in subacute phase stroke patients. Acupunct. Med. 39(4), 299–308 (2021)
Michael, M.K., Arokia, R.J.: Deep Joint segmentation for the classification of severity-levels of glioma tumour using multimodal MRI images. IET Image Proc. 14(11), 2541–2552 (2020)
Galinsky, V.L., Martinez, A., Paulus, M.P., Frank, L.R.: Joint estimation of effective brainwave activation modes using EEG/MEG sensor arrays and multimodal MRI volumes. Neural Comput. 30(7), 1725–1749 (2018)
Angulakshmi, M., Priya, G.: Walsh hadamard transform for simple linear iterative clustering (SLIC) superpixel based spectral clustering of multimodal MRI brain tumor segmentation. Innov. Res. Biomed. 40(5), 253–262 (2019)
Alden, Z.S., Mohammed, A.H., Abboosh, M., Mousa, A.H.: An analyzer based DL framework for improving medical diagnosis in medical images case study Iraq Healthcare. Int. J. Adv. Sci. Technol 29(10S), 1192–1198 (2020)
Alyafeai Z, Ghouti L. A fully-automated DL pipeline for cervical cancer classification. Expert Systems with Application, 2020, 141(Mar.):112951.1–112951.19.
Dawud, A.M., Yurtkan, K., Oztoprak, H.: Application of deep learning in neuroradiology: brain haemorrhage classification using transfer learning. Comput. Intell. Neurosci. 2019(6), 1–12 (2019)
Dara, S., Tumma, P., Eluri, N.R., Kancharla, G.R.: Feature extraction in medical images by using DL approach. Indian J. Pure Appl. Math. 120(6), 305–312 (2018)
Maier, A., Syben, C., Lasser, T.: A gentle introduction to DL in medical image processing. Z. Med. Phys. 29(2), 86–101 (2019)
Aruna, G.: Application of DL in medical image processing—a comprehensive review. IJARCCE 9(6), 135–141 (2020)
Mehrtash, A., Ghafoorian, M., Pernelle, G.: Automatic needle segmentation and localization in MRI with 3-D convolutional NNs: application to MRI-targeted prostate biopsy. IEEE Trans. Med. Imaging 38(4), 1026–1036 (2019)
Patel, S.M., Dharwa, J.N.: Medical image enhancement through dl methods. Natl. J. Syst. Inform. Technol. 11(1), 35–44 (2018)
Yazan, G., David, P., Chaitanya, K., et al.: Coronary calcification segmentation in intravascular OCT images using DL: application to calcification scoring. J. Med. Imaging (Bellingham Wash) 6(4), 45002–45002 (2019)
Meng, S.W., Kuo, R.C., Yang, H.J., Lai, C.L., Hsieh, M.Y.: Recruiting an acute coronary team to perform emergent mechanical thrombectomy in acute ischemic stroke patients: a successful case and team model in a local hospital. Acta Cardiologica Sinica 34(1), 99–103 (2018)
Ganesh, A., King-Shier, K., Manns, B.J., Hill, M.D., Campbell, D.J.T.: Money is brain: financial barriers and consequences for canadian stroke patients. Can. J. Neurol. Sci. 44(02), 146–151 (2017)
Maruyama, K., Nakagawa, K., Satoshi, J., et al.: Malnutrition increases the incidence of death, cardiovascular events, and infections in patients with stroke after rehabilitation. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 27(3), 716–723 (2018)
Funding
This work was supported by the Binzhou Medical University "Clinical + X" Project (No.BY2021KYC + X47) and Shandong Traditional Chinese Medicine Science and Technology Development Plan Project (No.2021Q017).
Author information
Authors and Affiliations
Contributions
All authors have designed the study, developed the methodology, performed the analysis, and written the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
There are no potential competing interests in our manuscript.
Ethical Approval
'Not applicable' as the study did not require ethical approval.
Consent to Participate
'Not applicable' as the studies not involving humans.
Consent for Publication
As per the journal guidelines and norms.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, Y., Sun, J., Ren, X. et al. Nutritional Risk Assessment and Countermeasures for Stroke Patients Based on Deep Learning and Multimodal MRI Images. Int J Comput Intell Syst 16, 72 (2023). https://doi.org/10.1007/s44196-023-00258-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44196-023-00258-x