Abstract
Maintenance of cell wall integrity is of great importance not only for plant growth and development, but also for the adaptation of plants to adverse environments. However, how the cell wall integrity is modulated under salt stress is still poorly understood. Here, we report that a nuclear-localized Agenet domain-containing protein SWO1 (SWOLLEN 1) is required for the maintenance of cell wall integrity in Arabidopsis under salt stress. Mutation in SWO1 gene results in swollen root tips, disordered root cell morphology, and root elongation inhibition under salt stress. The swo1 mutant accumulates less cellulose and pectin but more lignin under high salinity. RNA-seq and ChIP-seq assays reveal that SWO1 binds to the promoter of several cell wall-related genes and regulates their expression under saline conditions. Further study indicates that SWO1 interacts with importin ɑ IMPA1 and IMPA2, which are required for the import of nuclear-localized proteins. The impa1 impa2 double mutant also exhibits root growth inhibition under salt stress and mutations of these two genes aggravate the salt-hypersensitive phenotype of the swo1 mutant. Taken together, our data suggest that SWO1 functions together with importin ɑ to regulate the expression of cell wall-related genes, which enables plants to maintain cell wall integrity under high salinity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil salinity is one of the paramount factors that limit plant distribution, growth, and yield. Currently more than one-third of irrigated lands are affected by salinization in the world (FAO, 2011), and therefore improving the salt tolerance of crops is of great importance for global food security and sustainable agriculture. Upon exposure to high salinity, plants experience dramatic morphological, physiological, biochemical, and metabolic changes in order to adapt to adverse conditions. More and more evidences have shown that maintenance of plant cell wall integrity is critical for salt tolerance, especially for root elongation under high salinity (Shi et al., 2003; Zhu et al., 2010; Zhang et al., 2016; Zhao et al., 2018a; Zhao et al., 2019b), and cell wall biosynthesis is a dynamic process, which is regulated based on broad extracellular and intracellular contexts.
The plant cell wall consists of a polysaccharide network, in which cellulose microfibrils are crosslinked by hemicelluloses in a pectin matrix. Many glycoproteins and secreted peptides are also identified in the cell wall matrix (Somerville, 2006). Maintenance of cell wall integrity is critical for cell expansion during growth and development, and also allows plants to adapt to changing environments (Anderson & Kieber, 2020). Extensive studies have shown that perturbation of cell wall integrity affects stress tolerance in plants. For example, mutation in the SOS5 gene, which encodes an arabinogalactan protein with AGP-like and fasciclin-like domains, results in an arrested root elongation and a swollen root tip under salt stress (Shi et al., 2003). SOS6, encoding a cellulose synthase-like protein, is involved in the regulation of root elongation under salt stress (Zhu et al., 2010). Disruption of the key component in cellulose synthase complex, CESA6, leads to a salt-hypersensitive phenotype in Arabidopsis (Zhang et al., 2016). The companions of CESAs, including CC1 and CC2, are required for the elongation of hypocotyls under salt stress (Endler et al., 2015). A recent study showed that the receptor-like kinase FER, together with the cell wall-localized leucine-rich repeat extensins LRX3, LRX4, and LRX5, and the secretory peptides RALFs, function as a module to monitor cell wall integrity and regulate salt tolerance in Arabidopsis (Zhao et al., 2018a). MUR4 encodes a UDP-D-xylose 4-epimerase that is required for the the biosynthesis of UDP-arabinose (UDP-Ara), and UDP-Ara participates in the decorations of several cell wall polysaccharides and glycoproteins and thereby regulates cell wall integrity. Loss of function of the MUR4 gene leads to a reduced root elongation and abnormal cell adhesion under high salinity (Zhao et al., 2019b). Together, all these data indicate that maintenance of cell wall integrity is critical for salt tolerance in plants. However, the molecular mechanisms underlying the regulation of cell wall biosynthesis under salt stress are still largely unknown.
Importin ɑ, also known as karyopherin αs (KPNAs), act as adaptors to form import complexes with importin β and cargo proteins, and drive the nuclear localization signal (NLS)-containing cargo proteins to the nucleus. In this process, hydrolysis of GTP by GTPase Ran provides the energy for the transport of import complex, and the asymmetric distribution of Ran in its GTP and GDP-bound states across the nuclear envelope determines the formation and disassembly of the import complex (Quimby & Dasso, 2003; Goldfarb et al., 2004). Beyond their typical role as adaptors in import complex, importin ɑ proteins also exhibit unexpected functions in eukaryotic organisms (Miyamoto et al., 2016; Oka & Yoneda, 2018). Several studies have reported the involvement of importin ɑ in spindle assembly and nuclear envelope formation in Xenopus laevis (Gruss et al., 2001; Nachury et al., 2001; Ems-McClung et al., 2004; Hachet et al., 2004; Wilbur & Heald, 2013). A study showed that NUP-6, an importin ɑ in Neurospora, is required for heterochromatin targeting, but not for nuclear transport of the factors that catalyze H3K9 methylation, suggesting a role of importin ɑ in delivering chromatin modifiers to the final destination beyond NLS-mediated nuclear transport (Klocko et al., 2015). Although the versatile roles of importin ɑ have been unveiled in eukaryotic cells, only a few studies have reported their functions in plants. There are nine importin α genes in Arabidopsis, and the functions of most of these genes are still unclear. MOS6 (or IMPα3) is critical for plant innate immunity (Palma et al., 2005). Several importin α isoforms can interact with Agrobacterium tumefaciens virulence proteins VirD2 and VirE2, but only IMPA4 was documented to be involved in the transport of T-DNA into the nucleus (Bhattacharjee et al., 2008). So far, the roles of importin α in abiotic stress response are still unknown. As another core member of import complex, importin β, has been reported to participate in abiotic stress responses in Arabidopsis. As a homolog of human importin β1, AtKPNB1 is required for the regulation of ABA signaling pathway and drought stress response in Arabidopsis (Luo et al., 2013). SAD2 is another importin β-domain family protein involving in the nuclear import of an R2R3-type transcription repressor MYB4 in response to UV-B radiation (Zhao et al., 2007). The ABA hypersensitive phenotype of sad2 mutant also reveals its role in ABA response (Verslues et al., 2006).
Agenet domain, a plant-specific homolog of Tudor domain, belongs to the Royal family domains. This family also includes Chromo (chromatin-binding), PWWP (Pro-Trp-Trp-Pro), and MBT (malignant brain tumour) domains (Maurer-Stroh et al., 2003). In Arabidopsis, there are 71 Agenet/Tudor-like domains in 32 different proteins (Liu & Min, 2016). SHH1 is one of the Agenet/Tudor-like proteins participating in RNA-directed DNA methylation pathway. The tandem Tudor-like repeats in the SAWADEE domain of SHH1 is required for the recognition of H3K9me2 and the recruitment of RNA polymerase IV to target loci (Law et al., 2011; Law et al., 2013). The tandem Agenet domains in AGDP1 specifically bind to H3K9me2 mark, which is required for DNA methylation in heterochromatin regions (Zhang et al., 2018; Zhao et al., 2019a). AIP1 is an Agenet domain-containing protein that functions as a linker between DNA replication, transcription, and chromatin remodeling during flower development (Brasil et al., 2015). The Agenet domain of EML1 was originally identified as a H3K4me3 reader (Zhao et al., 2018b), and later it was reported that EML1 and EML3 bind to peptides containing H3K36 and encompassed multiple modifications, including methylation and acetylation (Coursey et al., 2018). The EML1 and EML3 also recognize the H3K36 of viral genome and participate in viral chromatin regulation (Coursey et al., 2018), suggesting a role of Agenet domain proteins in defense response. So far the functions of Agenet domain proteins in abiotic stress response have rarely been reported. Here, we report a previously uncharacterized Agenet domain-containing protein SWO1 (SWOLLEN 1), which interacts with importin ɑ and functions in salt stress response via the regulation of cell wall remodeling. Our results suggest that SWO1 may function as a scaffold protein to deliver some nuclear-localized proteins to their targeted DNA regulatory regions.
Results
SWO1 is required for root elongation under salt stress conditions
We collected a number of T-DNA insertion mutants that are disrupted in the genes encoding Agenet domain-containing proteins and analyzed their phenotypes under a variety of abiotic stresses. We found that a mutant (SAIL_236_A03), in which a T-DNA was inserted in the third intron of AT4G17330, showed a reduced root elongation when grown on MS solid medium supplemented with 100 mM NaCl (Fig. S1a, b). Another obvious phenotype observed in this mutant was the swollen root tip under high salinity, and thus we named this mutant as swo1 (swollen 1), and the SAIL_236_A03 mutant allele was designated as swo1–1. To figure out whether the swo1 mutant is also defective in the response to osmotic stress, we subjected the seedlings of the mutant to the MS medium supplemented with a high concentration (325 mM) of mannitol, which is often used to trigger osmotic stress. The result showed that the root elongation of the swo1–1 mutant was not affected under osmotic stress (Fig. S1c, d).
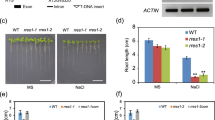
To further determine that the SWO1 gene is required for salt tolerance in Arabidopsis, we tested the phenotypes of other three independent swo1 T-DNA insertion mutant alleles, which were designated as swo1–2, swo1–3, and swo1–4 (Fig. S2a). In these three mutant alleles, a T-DNA was inserted in the first, eighth, and thirteenth exon of the SWO1 gene, respectively (Fig. S2a, b). Gene expression analysis showed that the transcript level of the SWO1 gene was dramatically reduced in all these three mutant alleles (Fig. S2c, d). Similar to the swo1–1, all these three mutant alleles exhibited defective root growth under high salinity, including reduced root elongation and swollen root tips (Fig. 1a, b). We transformed the full-length genomic DNA of SWO1 driven by its native promoter to the swo1–1 and swo1–2 mutant alleles. All complementation lines showed normal root elongation under high salinity compared with the wild type (Fig. 1c, d; Fig. S1e, f), demonstrating that SWO1 is required for salt tolerance in Arabidopsis. Since in the swo1–2, swo1–3, and swo1–4 mutant alleles, the T-DNAs are inserted in the exons of the SWO1 gene, so mainly these three mutant alleles were used for further study.
SWO1 is required for root growth under salt stress. (a) Four-day-old seedlings of the wild type and the three swo1 mutant alleles, swo1–2, swo1–3, and swo1–4, were transferred to Murashige and Skoog (MS) and MS + 100 mM NaCl media, respectively. The photographs were taken 7 days after transfer. (b) Quantification of the root lengths of the wild type and the swo1 mutants after being transferred to MS and MS + 100 mM NaCl media for 7 days. The values indicate the means ± SD (n = 10). Different letters represent significant differences between different genotypes under the same treatment, P < 0.01 (one-way ANOVA). (c) Phenotypes of the wild type, swo1–2, and complementation plants after being transferred to MS media supplemented with or without 100 mM NaCl. The seedlings were photographed 7 days after growth. (d) Comparison of the root length of the wild type, swo1–2, and complementation plants after being transferred to MS and MS + 100 mM NaCl media for 7 days. Values are means ± SD (n = 8). Different letters represent significant differences between different genotypes under the same treatment, P < 0.01 (one-way ANOVA). (e) Analysis of Na+ and K+ concentrations and Na+/K+ ratio in the roots and shoots of the wild type and the swo1 mutants after being transferred to agar-containing MS media with or without 100 mM NaCl for 10 days. Data are means ± SD (n = 3). Different letters indicate significant differences among the samples under the same treatment, P < 0.05 (one-way ANOVA). DW, dry weight
To investigate whether the increased sensitivity of the swo1 mutants to salt stress is due to abnormal ion homeostasis, we measured Na+ and K+ contents in both the roots and shoots of the wild type and swo1 mutants. After being exposed to 100 mM NaCl, the wild type and swo1 mutants did not show obvious difference in sodium concentration in both roots and shoots, but there was a slight decrease of potassium concentration in the shoots, but not in the roots of the swo1 mutants (Fig. 1e). As a result, the Na+/K+ ratio was not affected in the roots but was increased in the shoots of the swo1 mutants. These results suggested that the reduced root elongation of the swo1 mutant under salt stress is not caused by the over-accumulation of Na+ in the roots.
swo1 mutation leads to abnormal root cell morphology and altered cell wall contents under salt stress
The swollen root tip of the swo1 mutant under salt stress drove us to closely observe the root tip cells using differential interference contrast (DIC) microscopy. The morphology of root cells was almost identical between the wild type and swo1–2 under normal conditions (Fig. 2a-f). Under salt stress (100 mM NaCl), the wild type plants were able to maintain well-organized cell layers and defined cell shapes (Fig. 2g, i, k), whereas the swo1 mutants displayed enlarged and disordered root cells in both meristem and elongation zones (Fig. 2h, j, l). The enlargement of root cells suggested that the swo1 mutant loses the ability to tightly control cell expansion and growth under salt stress, which is likely caused by the disrupted cell wall integrity.
swo1 mutation results in an abnormal root cell morphology under salt stress. (a)-(f). Morphology of the root tips of the wild type ((a), (c), and (e)) and swo1–2 ((b), (d), and (f)) grown under normal conditions. (a) and (b), Scale bar = 100 μm. (c), (d), (e), and (f), Scale bar = 20 μm. (g)-(l). Morphology of the root tips of the wild type ((g), (i), and (k)) and swo1–2 ((h), (j), and (l)) transferred to MS + 100 mM NaCl medium for 7 days. (g) and (h), Scale bar = 100 μm. (i), (j), (k), and (l), Scale bar = 20 μm. (a), (b), (g), and (h) are the morphological views of root tips. (c), (d), (i) and (j) show the root cells in the meristem and transition zones, and (e), (f), (k), and (l) show the elongation zone of the roots
Cell wall is an important factor that determines cell expansion during growth and development, as well as in response to environmental stresses (Shi et al., 2003; Xu et al., 2008). To determine whether the abnormal root cell morphology of the swo1 mutant under salt stress is caused by the deficiency in cell wall integrity, we measured cell wall components, including cellulose, pectin, and lignin, in the wild type and the swo1 mutants. To assess cellulose content, the roots of the seedlings grown on the MS solid medium supplemented with or without 100 mM NaCl for 6 days were stained with calcofluor, a special fluorescent dye that strongly binds to cellulose. Compared with the wild type, deposition of cellulose in the roots of the swo1 mutant was markedly decreased after salt treatment (Fig. 3a). We then quantified the cellulose content in the wild type and swo1 mutants using anthrone-sulfuric acid colorimetric assay. Under normal conditions, the cellulose content in the swo1 mutants was comparable to that of the wild type. After salt treatment, however, the cellulose content was largely reduced in both the roots and shoots of the swo1 mutants (Fig. 3b). Specifically, in the roots of the swo1 mutants, the cellulose content was reduced by around 50% under salt stress. Salt stress also led to a dramatic decrease of pectin content in both the roots and shoots of the swo1 mutants (Fig. 3c). In contrast, lignin content was significantly increased in the swo1 mutants compared with the wild type in the presence of high salt, while the content of lignin was similar between the wild type and the swo1 mutants under normal conditions (Fig. 3d). Together, these results suggest that the abnormal root cell morphology and root elongation of the swo1 mutant under salt stress is largely caused by the compromised cellulose and pectin biosynthesis in the roots.
Cellulose and pectin contents are decreased in swo1 mutants under high salinity. (a) Confocal microscopic images of calcofluor-stained root tips of the wild type and swo1 mutant with or without NaCl treatment. The upper panel shows the images of the meristem zones and the lower panel shows the images of the maturation zones. Scale bar = 100 μm. (b) Quantitative analysis of cellulose content in the shoots and roots of the wild type and swo1 mutants with or without NaCl treatment. The cellulose content in the shoot of the wild type without NaCl treatment was set as 1. Values are means ± SD (n = 3). Different letters represent significant differences between different genotypes under the same treatment, P < 0.05 (one-way ANOVA). (c) Relative amount of total pectin in the shoots and roots of the wild type and swo1 mutants. The pectin content in the shoot of the wild type without NaCl treatment was set as 1. Values are means ± SD (n = 3). Different letters represent significant differences between different genotypes under the same treatment, P < 0.05 (one-way ANOVA). (d) Relative amount of lignin in the shoots and roots of the wild type and swo1 mutants. The lignin content in the non-treated wild type shoots was set as 1. Values are means ± SD (n = 3). Different letters represent significant differences between different genotypes under the same treatment, P < 0.05 (one-way ANOVA)
Reactive oxygen species accumulation is responsible for salt stress-triggered root cell swelling in swo1 mutant
Reactive oxygen species (ROS) have been proposed to be involved in the regulation of root growth and development, especially under stress conditions (Foreman et al., 2003; Tsukagoshi, 2016), and thus we examined ROS level in the roots of the swo1 mutant under salt stress. DAB staining was applied to detect hydrogen peroxide content. Under normal growth conditions, there was no difference in H2O2 levels between the roots of the wild type and the swo1 mutant (Fig. 4a, upper panel). However, after being suffered from high salinity, the swo1 mutant showed more H2O2 accumulation in the roots than the wild type (Fig. 4a, lower panel). By using a fluorescent molecular probe H2DCF-DA, the pronounced accumulation of ROS in the root of the swo1–2 mutant after salt stress was also observed (Fig. 4b).
ROS accumulation is responsible for salt stress-triggered root cell swelling in swo1 mutant. (a) DAB staining of H2O2 in the roots of the wild type and swo1–2 mutant. The upper panel shows the staining of the roots grown under normal conditions, and the lower panel shows the staining of the roots treated with 200 mM NaCl for 6 h. Scale bar = 50 μm. (b) Analysis of ROS level by H2DCF-DA staining. Four-day-old seedlings of the wild type and swo1–2 were transferred to MS media supplemented with or without 100 mM NaCl for 10 days. Roots were stained with H2DCF-DA and fluorescent signals were detected using an Olympus DP72 microscope. Scale bar = 100 μm. (c) Root phenotypes of the wild type and swo1 mutants after being transferred to MS, MS + NaCl (100 mM), and MS + NaCl+DPI (0.05 μM) media for 7 days. (d) Quantification of the root lengths of the wild type and swo1 mutants after being transferred to MS, MS + NaCl (100 mM), MS + NaCl+DPI (0.05 μM), and MS + NaCl+DPI (0.01 μM) media for 7 days. Data are means ± SD (n = 4). Different letters represent significant differences between different genotypes under the same treatment, P < 0.01 (one-way ANOVA). (e) Morphology of the root tips of the wild type and swo1–2 after being transferred to MS, MS + NaCl (100 mM), and MS + NaCl+DPI (0.05 μM) media. The left panel shows the images of the wild type and the right panel shows the roots of swo1–2. Scale bar = 200 μm. (f) DIC images show the root cell morphology of the wild type and swo1–2 grown on MS, MS + NaCl (100 mM), and MS + NaCl+DPI (0.05 μM) media for 7 days. Scale bar = 50 μm
To determine whether the over-accumulation of ROS is responsible for the salt-hypersensitive phenotype of the swo1 mutant under salt stress, the four-day-old seedlings of the wild type and swo1 mutants were transferred to NaCl media supplemented with NADPH-oxidase inhibitor diphenyleneiodonium chloride (DPI). After treatment of seedlings with 0.05 μM DPI, the root elongation of the wild type plants was inhibited, but the difference in root elongation between the wild type and swo1 mutants under high salinity was largely abolished (Fig. 4c, d). On the salt medium supplemented with a reduced concentration of DPI (0.01 μM), the root elongation of the wild type plants was not obviously affected by DPI, but the root elongation rate of the swo1 mutants was better than that on the salt medium without DPI (Fig. 4d). More importantly, application of DPI almost fully rescued the enlarged and disordered root cells of the swo1 mutants under high salinity (Fig. 4e, f). Together, these results indicated that the over-accumulation of ROS is responsible for the reduced root elongation and swollen root cells of the swo1 mutant under salt stress.
swo1 mutation affects the expression of ROS- and cell wall-related genes under salt stress
To investigate the mechanisms underlying the reduced root elongation and enlarged root cells of the swo1 mutant under high salinity, the roots of the wild type and swo1–2 mutant after NaCl treatment for 0 h, 2 h, and 14 h were collected for RNA-seq analysis (Table S2). RNA-seq data revealed that 310 (170 up-regulated and 140 down-regulated) genes were differentially expressed in the swo1–2 under normal conditions (fold change≥1.5, P-value<0.05). After salt treatment for 2 h, the number of the differentially expressed genes (DEGs) was still limited, with 137 genes up-regulated and 85 genes down-regulated in the swo1–2 mutant (fold change≥1.5, P-value<0.05). After salt treatment for 14 h, however, totally 1671 DEGs (fold change≥1.5, P-value<0.05) were identified, and among of them 1011 genes were up-regulated and 660 genes were down-regulated in the swo1–2 mutant (Fig. 5a).
SWO1 regulates cell wall-related gene expression under saline conditions. (a) Venn diagrams show the numbers of up-regulated (left panel) and down-regulated (right panel) genes in swo1–2 roots after salt treatment for 0, 2, and 14 h. (b) GO enrichment analysis of the differentially expressed genes (DEGs) in the swo1–2 after salt treatment for 14 h. (c) qRT-PCR analysis of DEGs involved in ROS generation and regulation, cell wall modification and pectin biosynthesis in the swo1–2 under salt treatment. PP2AA3 was used as the internal control. Values are means ± SD (n = 3). **P < 0.01 and *P < 0.05 (Student’s t test)
The up- and down-regulated DEG sets after salt treatment for 14 h were subjected to gene ontology (GO) enrichment analysis. The up-regulated genes in the swo1 mutant were significantly enriched in the categories “response to salt stress” (p-value = 2.10E-05), “response to ABA” (p-value = 1.52E-09), “cuticle development” (p-value = 1.00E-05), and “suberin biosynthetic process” (p-value = 1.92E-07) (Fig. 5b). Specifically, eight class III plant peroxidase family genes, which are supposed to be required for producing ROS and cell wall aromatic compounds oxidization, were up-regulated in the swo1 mutant compared with the wild type under salt stress (Table S3). The down-regulated genes in the swo1 mutant were classified into “root development” (p-value = 5.55E-04), “cell growth” (p-value = 2.70E-04), “pectin catabolic process” (p-value = 8.90E-06), “plant-type cell wall loosening” (p-value = 5.74E-05), and “phloem or xylem histogenesis” (p-value = 5.10E-04) (Fig. 5b). Among all the DEGs, 25 genes were identified to be involved in cell wall biogenesis and modification, and for most of them their expression was significantly reduced in the swo1 mutant under salt stress (Table S3). These genes include pectin biosynthesis genes (RGXT1, PGX3, and GATL2), cell wall network-forming genes (AGP30 and AGP31), cell wall modification genes (PME family members), and expansins (Table S3). Expansins are required for the regulation of cell expansion and growth via the modification of cell wall (Lee et al., 2001). Six members of the expansin family (EXPA1, EXPA4, EXPA11, EXPA14, EXPA20, and EXPB3) displayed decreased expression levels in the swo1 mutant under salt stress (Table S3). To verify our RNA-seq data, several genes involved in ROS homeostasis, cell wall loosening and modification, as well as pectin biosynthesis, were selected for qPCR analysis, and the results indicated that their expression patterns are consistent with that generated in RNA-seq assay (Fig. 5c). In our RNA-seq data, we found that the expression of MYB85, one of the key transcription factors participating in lignin biosynthesis, was up-regulated in the swo1 mutant root after salt treatment (Table S3), which is consistent with the increased lignin accumulation in the swo1 mutant under salt stress.
SWO1 directly binds to the genes involved in cell wall metabolism
It has been reported that the Agenet/Tudor-like domain is able to bind to histones (Nielsen et al., 2002; Min et al., 2003; Zhang et al., 2018; Zhao et al., 2019a). To identify the genomic binding sites of SWO1, the genome-wide ChIP-seq assay was performed. Two independent biological replicates were conducted (Table S4). The two replicates exhibited a high identity based on correlation analysis (r = 0.98) (Fig. 6a). The majority of the binding events occurred in the promoter regions (Fig. 6b), and the summits of the most binding sites were identified near the transcription start sites (TSS) or transcription end sites (TES) (Fig. 6c). Totally 2236 significant peaks, representing 2204 genes were identified in both replicates (Fig. 6d). To determine the biological functions of the target genes of SWO1, we performed GO enrichment analysis for all of the 2204 genes. The result revealed a significant enrichment of the terms involved in “regulation of response to osmotic stress” (p-value = 2.57E-04), “hyperosmotic salinity response” (p-value = 2.97E-04), “cellular response to extracellular stimulus” (p-value = 3.09E-03), suggesting that SWO1 is involved in the regulation of stress response. Importantly, the SWO1 binding sites were also enriched in the categories associated with plant cell wall metabolism, such as “xylem development” (p-value = 2.03E-03), “pectin catabolic process” (p-value = 4.11E-05), “cell wall biogenesis” (p-value = 2.81E-04), “xyloglucan metabolic process” (p-value = 3.40E-04), and “cell wall loosening” (p-value = 1.45E-06) (Fig. 6e), suggesting that SWO1 directly binds to the cell wall-related genes.
SWO1 binds to the promoters of cell wall metabolism-related genes. (a) Correlation analysis of SWO1 ChIP-seq data from two independent biological replicates. The X and Y axes represent read coverage normalized by total number of mapped reads in Log2 scale. Dot in each scatter plot shows the identified peaks in both replicates. (b) Genome-wide analysis of the binding preference of SWO1 in two ChIP-seq replicates. (c) Binding preference of SWO1 on gene bodies. TSS represents transcription start site and TES represents transcription end site of the nearest gene. (d) Analysis of the number of target genes that were identified in both biological replicates. (e) GO enrichment analysis of the SWO1-binding genes that were identified in both biological replicates. (f) Comparison of the genes generated from RNA-seq and ChIP-seq. Venn diagram shows the overlap of genes that are bound by SWO1 and are differentially expressed in the swo1–2 after salt treatment for 14 h. (g) Listed are the cell wall-related genes that are bound by SWO1 and are differentially expressed in the swo1–2 after salt treatment for 14 h
To determine whether the expression of the SWO1-binding genes was affected in the swo1 mutant, we compared the data generated from ChIP-seq and RNA-seq assays. The result showed that 238 SWO1-binding genes were differentially expressed in the swo1 mutant under high salinity, among of which 112 genes were up-regulated, and 126 genes were down-regulated (Fig. 6f). Among all the down-regulated genes, 19 are involved in plant cell wall metabolism (Fig. 6g, S3), including F8H, EXPAs, GH9B1, PGX3, and PMEs. F8H is required for the biogenesis of glucuronoxylan (Lee et al., 2009). Expansin genes (EXPA1, EXPA4, EXPA20, and EXPB3), as well as GH9B1, are involved in the regulation of plant growth and cell elongation (Shani et al., 2006). PGX3 is required for the maintenance of cell wall integrity under salt stress (Zheng et al., 2019). Cell wall biosynthesis gene PME3 contributes to the resistance of Arabidopsis to specific metal ion stresses (Weber et al., 2013) (Fig. 6g). Altogether, these results suggested that SWO1 regulates the expression of cell wall-related genes by directly binding to their promoters, and thus maintains root cell wall integrity under salt stress. For the ROS production-related genes that were differentially expressed in the swo1 mutant, no significant binding peaks were identified, which suggests that SWO1 may not directly regulate the expression of these ROS production-related genes.
The C-terminal domain of SWO1 is required for its nuclear localization
Based on the Pan-taxonomic Compara downloaded from Ensembl plants (https://plants.ensembl.org/index.html), we found that SWO1 and its homologs only exist in plant species but not in animals and bacteria, indicating that SWO1 is a plant-specific gene. We performed a BLASTP analysis of SWO1 protein against Arabidopsis database, and no paralogs of SWO1 were identified in Arabidopsis. The SWO1 is an extremely large protein (2037 aa), which consists of a tandem of conserved Agenet/Tudor-like domains and three predicted nuclear localization signal (NLS) motifs at the C-terminus, but no known motifs were identified at the N-terminus (Fig. 7a). Through the transient expression of SWO1-GFP driven by its native promoter in Nicotiana benthamiana, we found that SWO1 was localized exclusively in the nucleus, while as a control the free GFP was localized in both the cytosol and nucleus (Fig. 7b). We also checked the localization of SWO1 in Arabidopsis by using pSWO1:SWO1-GFP transgenic plants, which showed that SWO1 was localized in the nucleus in both leaves and roots (Fig. 7c, d).
Both N- and C-termini are required for the function of SWO1 in the regulation of salt tolerance. (a) Schematic diagram shows the N-terminal and C-terminal domains of SWO1 protein. Black rectangles and red dot represent plant Agenet and Agenet-like domains, respectively, with the corresponding amino acid positions in the parentheses. The positions of the three NLS motifs are marked in purple. The fragments of SWO1-N and SWO1-C are also indicated in the diagram. (b) Subcellular localization of SWO1 in N. benthamiana leaves. The leaves expressing free GFP were used as control. Scale bar = 20 μm. (c) Subcellular localization of SWO1 in Arabidopsis roots (upper panel) and leaves (bottom panel). Scale bar = 20 μm. (d) Overlay between SWO1-GFP and DAPI (4′,6-diamidino-2-phenylindole) staining in Arabidopsis roots. Scale bar = 10 μm. (e) Subcellular localizations of the full-length and truncated SWO1 proteins in Arabidopsis roots were detected by confocal microscopy. Scale bar = 20 μm. (f) Four-day-old seedlings of the wild type and transgenic plants expressing the N-terminal or C-terminal domain of SWO1 in swo1–2 mutant background were transferred to MS and MS + NaCl (100 mM) media. The photographs were taken 7 days after transfer. (g) Quantification of the root lengths of the wild type and transgenic plants expressing truncated SWO1 after being transferred to MS and MS + 100 mM NaCl media. Data are means ± SD (n = 6). Different letters represent significant differences between different genotypes under the same treatment, P < 0.01 (one-way ANOVA)
To uncover which domain is required for the nuclear localization of SWO1, we generated stable transgenic Arabidopsis plants expressing the N-terminal or C-terminal part of SWO1 in the swo1–2 background. Similar to that of the full-length protein, the C-terminal domain that contains the NLS motifs was still localized in the nucleus (Fig. 7e), illustrating that the C-terminal domain is required for the nuclear localization of SWO1. However, in contrast to the full-length SWO1 that was distributed in the nucleoplasmic region close to the nuclear envelope, the C-terminal domain of SWO1 was relatively evenly distributed in the nucleoplasm (Fig. 7e, Fig. S4a), suggesting that the N-terminus is probably required for the attachment of SWO1 to nuclear envelope. Surprisingly, in the transgenic plants expressing the N-terminal domain, no fluorescence signals could be detected (Fig. 7e). To exclude the possibility that the low protein abundance of the N-terminal domain of SWO1 was caused by the abnormal expression of this truncated fragment, both semi- and quantitative reverse transcription-PCRs (RT-PCRs) were performed, and the results showed that the N-terminal domain of SWO1 was transcribed normally in the transgenic plants (Fig. S4b, c). To understand the biological functions of the N-terminal and C-terminal domains of SWO1, the transgenic plants expressing the N-terminus and C-terminus of SWO1 in the swo1–2 mutant background were subjected to NaCl treatment, and neither of these transgenic plants could complement the reduced root elongation and swollen root tips of the swo1–2 mutant under high salinity (Fig. 7f, g), suggesting that both the N-terminal and C-terminal domains are essential for the function of SWO1 protein. To test whether salt stress affects the subcellular localization of SWO1, we treated the pSWO1:SWO1-GFP complementation plants with 100 mM NaCl in a time-course manner, which showed that the localization of SWO1 was not obviously changed under salt stress (Fig. S5).
SWO1 interacts with importins IMPA1 and IMPA2
To further understand the functions of SWO1 in salt tolerance, affinity purification assay was performed using the transgenic plants expressing pSWO1:SWO1g-Myc, and mass spectrometric (MS) analysis was conducted to identify co-purified proteins. Totally four independent experiments were performed, and the co-purified proteins were analyzed. Among these proteins, several members of importins, including IMPA1, IMPA2, IMPA4, MOS6, KPNB1, and XPO1A, were identified in two or more independent replicates (Fig. 8a). Since IMPA1 and IMPA2 were identified in all of the four independent replicates, their interactions with SWO1 were further examined by split luciferase (split-LUC) assay in N. benthamiana, which showed that SWO1 interacted with both IMPA1 and IMPA2. Truncation assay indicated that the C-terminal part of SWO1 is sufficient for the interactions (Fig. 8b). We also performed IP-MS analysis for the transgenic Arabidopsis plants expressing pIMPA1:IMPA1-Myc or pIMPA2:IMPA2-Myc, and SWO1 was co-purified in both samples (Fig. 8c). These results strongly indicated that SWO1 interacts with IMPA1 and IMPA2 and other importins.
SWO1 interacts with importin ɑ IMPA1 and IMPA2. (a) Mass spectrometric analysis of SWO1-interacting proteins. The co-purified proteins, including IMPA1, IMPA2, IMPA4, MOS6, KPNB1, and XPO1A are listed. NA means that the corresponding protein was not identified in the IP-MS data. (b) Split luciferase complementation assay was performed in N. Benthamiana leaves. The combinations of the indicated plasmids were co-transformed to N. Benthamiana leaves through Agrobacterium infiltration. Luciferase activity was determined 48 h after infiltration. nLUC represents the N-terminal fragment of firefly luciferase; cLUC represents the C-terminal fragment of firefly luciferase. (c) Mass spectrometry analysis of the proteins co-purified with IMPA1 and IMPA2. The SWO1 that was co-purified with IMPA1 and IMPA2 is shown. NA means that the corresponding protein was not identified in the IP-MS data
Apart from importins, several nuclear pore complex (NPC) components, including the cytoplasmic member LOS4, the outer and inner ring members Nup96, RAE1, and Nup155, and the central FG factors Nup98, Nup54, and Nup35, were co-purified with SWO1. In addition, LINC4, SUN1, and WIT1 that are localized within the nuclear envelope were also identified as the co-purified proteins of SWO1 (Table S5). These results suggested that SWO1 may directly attach to nuclear envelope.
Mutations of IMPA genes enhance the salt-hypersensitivity of swo1 mutant
As SWO1 physically interacts with importins, we investigated the biological significance of these interactions and the roles of importin ɑ in salt tolerance. We obtained T-DNA insertion mutant lines of IMPA1 and IMPA2, namely impa1–1 and impa2–1, respectively. The double mutants swo1–2 impa1–1, swo1–2 impa2–1, and impa1–1 impa2–1, and the triple mutant swo1–2 impa1–1 impa2–1 were generated by crossing and the homozygotes were identified. qRT-PCR results verified the absence of the transcripts of IMPA1, IMPA2, and SWO1 in the corresponding mutants (Fig. S6). Both impa1–1 and impa2–1 single mutants showed similar phenotypes as the wild type under salt stress. However, the root elongation of the impa1–1 impa2–1 double mutant was arrested under high salinity (Fig. 9a, b). Moreover, mutation in either the IMPA1 or IMPA2 gene enhanced the root growth inhibition of the swo1–2 mutant under high salinity, and the triple mutant swo1–2 impa1–1 impa2–1 showed even more severe root growth retardation and more serious root cell abnormality under salt stress (Fig. 9a, b, c). These results suggested that IMPA1 and IMPA2 are functionally redundant and work together with SWO1 in the regulation of root elongation under high salinity.
IMPA1 and IMPA2 are required for salt tolerance in Arabidopsis. (a) Phenotypes of the wild type, swo1–2, impa1–1, impa2–1, swo1–2 impa1–1, swo1–2 impa2–1, impa1–1 impa2–1, and swo1–2 impa1–1 impa2–1 seedlings grown on MS and MS + 100 mM NaCl media. Photographs were taken 7 days after growth. (b) Root lengths of the wild type, swo1–2, impa1–1, impa2–1, swo1–2 impa1–1, swo1–2 impa2–1, impa1–1 impa2–1, and swo1–2 impa1–1 impa2–1 seedlings grown on MS and MS + 100 mM NaCl media for 7 days. Data are means ± SD (n = 6). Different letters represent significant differences between different genotypes under the same treatment, P < 0.01 (one-way ANOVA). (c) Root cell morphology of the wild type, swo1–2, impa1–1 impa2–1, and swo1–2 impa1–1 impa2–1 grown on MS and MS + NaCl (100 mM) media for 7 days. Scale bar = 50 μm
SWO1 is not required for the NLS-mediated nuclear protein import
It is well-known that importins function in NLS-mediated nuclear protein import, and we tested whether SWO1 acts as a component of importin complex. To this end, the GFP-NLS-CHS-NES(−)Rev construct, which expresses a cytosolic chalcone synthase (CHS) that is tagged with GFP-NLS and an inactivated nuclear export signal [NES(−)Rev], was transformed to the protoplasts of wild type, impa1–1 impa2–1, swo1–2, and kpnb1 mutant. This construct has been used to elucidate the function of AtKPNB1 in the regulation of the shuttling of nuclear proteins (Luo et al., 2013). In the protoplasts derived from the wild type plants, the nuclear-localized GFP signal was detected in more than 50% of protoplasts (Fig. S7a). As expected, in the protoplasts of kpnb1 mutant, less than 30% of protoplasts displayed GFP fluorescence signal in the nucleus (Fig. S7a). In the protoplasts generated from impa1–1 impa2–1, around 31% of protoplasts showed GFP fluorescence signal in the nucleus (Fig. S7a), indicating that mutations of both IMPA1 and IMPA2 affect the NLS-mediated import of nuclear proteins. However, in the protoplasts of the swo1–2 mutant, the ratio of GFP signal in the nucleus was similar to that in the wild type, suggesting that SWO1 mutation alone does not affect the NLS-mediated import of nuclear proteins (Fig. S7b).
Since IMPA1 and IMPA2 are required for NLS-mediated nuclear import (Fig. S7a), and SWO1 contains three NLS motifs, we were wondering whether SWO1 is the substrate of IMPA1 and IMPA2. We transformed pSWO1:SWO1-GFP to swo1–2 and impa1–1 impa2–1 mutants and compared the localization of SWO1 in these two transgenic plants. The results showed that the localization of SWO1 was indistinguishable in these two plant materials (Fig. S7c), suggesting that IMPA1 and IMPA2 are not required for the nuclear localization of SWO1. To test whether SWO1 functions in the recycling of IMPA1 and IMPA2 between the cytoplasm and nucleus, we also detected the subcellular localization of IMPA1 and IMPA2 in the swo1 mutant, which showed that the localization of IMPA1 and IMPA2 was not changed in the swo1–2 mutant (Fig. S7d), suggesting that SWO1 is not required for the regulation of the nucleocytoplasmic shuttling of IMPA1 and IMPA2.
Discussion
In this study, we found that SWO1, a previously uncharacterized Agenet domain-containing protein, plays an important role in salt stress response via its associations with importin ɑ IMPA1 and IMPA2 in the nucleus. Both swo1 and impa1 impa2 mutants displayed enhanced root elongation inhibition under salt stress, and pyramiding these three mutations resulted in a more severe deficiency in root growth under high salinity. The swo1 mutant exhibited root tip swelling under salt stress, which was largely caused by the disruption of cell wall integrity and increased accumulation of ROS. The role of SWO1 in the regulation of cell wall integrity was supported by the data showing that SWO1 binds to the promoters of many cell wall-related genes and regulates their expression under salt stress. Considering that SWO1 is a large protein that is physically associated with both importin ɑ and nuclear pore proteins and also binds to the promoters of the targeted genes, we speculate that SWO1 may act as an adaptor that links specific DNA regions to the NPC and thereby mediates the rapid and accurate delivery of the nuclear-imported proteins to their target genes (Fig. S8).
Cell wall is a specific structure that distinguishes plants from animals, and it fulfills diverse functions throughout the plant life. In addition to providing mechanical support and maintaining structural integrity, the wall is also the forefront to perceive a variety of environmental stimuli (Scheller & Ulvskov, 2010; Hamann, 2012). It has been well documented that cell wall integrity contributes to salt tolerance in plants. Under stress conditions, plants remodel the cell wall to adapt to unfavorable environments. Mutants that fail to maintain cell wall integrity usually exhibit a salt-hypersensitive phenotype, and typical phenotypes of cell wall-deficient mutants under salt stress are reduced root elongation and enlarged root cells (Shi et al., 2003; Zhu et al., 2010; Endler et al., 2015; Zhang et al., 2016; Zhao et al., 2018a; Zhao et al., 2019b). Here, mutation in the SWO1 gene resulted in root cell swelling and reduced root elongation, and the cellulose and pectin contents were significantly reduced in the swo1 mutant under salt stress, suggesting that SWO1 is required for the maintenance of cell wall integrity under high salinity. Notably, in most of the cell wall-deficient mutants, the root swelling under high salinity is usually restricted to the elongation zone, but in swo1 mutant the root cell swelling extends to the meristem region. RNA-seq and ChIP-seq data indicated that SWO1 modulates cell wall remodeling by regulating the expression of many cell wall-associated genes, including pectin biosynthesis genes and cell wall modification genes, suggesting that the cell wall deficiency in the swo1 mutant under salt stress is caused by the disrupted expression of cell wall-associated genes, but the molecular mechanism underlying the gene expression regulation by SWO1 needs to be further investigated.
In our study, we found that SWO1 directly interacted with importin ɑ IMPA1 and IMPA2, and importin β KPNB1. Combining with a previous study showing that SWO1 was co-purified with importin ɑ protein MOS6 (Roth et al., 2017), we can conclude that SWO1 is associated with the import complex. SWO1 harbors three NLS motifs, and whether these motifs are required for the interaction of SWO1 with import complex remains to be determined. The functional association of SWO1 with IMPA1 and IMPA2 was also genetically demonstrated in our study. Phenotypic analysis indicated that impa1–1 impa2–1 double mutant exhibited reduced root elongation under salt stress, although the phenotype was weaker than the swo1 mutant. Mutation of either IMPA1 or IMPA2 aggravated the short root phenotype of the swo1 under salt stress conditions, and the triple mutant swo1–2 impa1–1 impa2–1 showed even more retardation of root elongation and more severe root cell abnormality under high salinity, illustrating that SWO1 and IMPA1/IMPA2 function together to regulate salt tolerance in Arabidopsis. Since SWO1 also interacts with importin α members IMPA4 and MOS6, the relatively weak phenotype of the impa1–1 impa2–1 double mutant compared with the swo1 mutant could be explained by the functional redundancy of these importin α proteins. However, we cannot rule out the possibility that SWO1 may regulate salt tolerance via both importin α-dependent and -independent pathways.
Although the interaction of SWO1 with import complex is clear, the biological significance of their association is still elusive. One possibility is that SWO1 is a cargo of the import complex, because SWO1 contains three NLS motifs, but we did not detect the cytosolic retention of SWO1 in impa1–1 impa2–1 mutant background, suggesting that the association of SWO1 with IMPA1 and IMPA2 is not critical for the nuclear import of SWO1. However, whether other importin α proteins function redundantly to regulate the transportation of SWO1 to the nucleus still needs to be investigated. Another possibility is that SWO1 functions as a member of the import complex to facilitate the import of other nuclear-localized proteins, but our results showed that swo1 mutation did not affect the nuclear import of GFP driven by the NLS signal. Our data also showed that the nuclear localization of IMPA1 and IMPA2 was not affected in the swo1 mutant. These results suggest that SWO1 does not participate in the process of the import of nuclear proteins. Since SWO1 is a large protein that interacts with several members of NPC and nuclear envelope, and is also capable of binding to the specific genomic regions, it is possible that SWO1 serves as a bridge between the nuclear envelope and chromatin, and the SWO1 protein may capture the imported nuclear proteins via its associations with the importin complex and then deliver the nuclear proteins to their target sites to regulate gene expression in response to salt stress.
The SWO1 protein contains Agenet domains that bind to histones, but it does not possess a transcriptional activity to directly promote gene expression, so it is likely that SWO1 acts as a harbor to deliver some unknown transcription factors or epigenetic regulators to the promoters of cell wall-associated genes. In our IP-MS data, we found a number of transcription factors, including zinc finger and bZIP families, that were co-purified with SWO1 (Table S6). Among these transcription factors, AtNFXL1 is required for plant growth under salt stress (Lisso et al., 2006), and OXS2 functions in salt tolerance (Jing et al., 2019). Roles of bZIP transcription factors in abiotic stress response have already been elucidated in previous studies. bZIP68 and VIP1 are involved in oxidative stress and mechanical stress response, respectively (Li et al., 2019; Tsugama et al., 2019), and bZIP59 regulates anthocyanin accumulation under salt stress in Arabidopsis (Van Oosten et al., 2013). In the future, the roles of these transcription factors in the regulation of cell wall-related genes expression need to be investigated. Agenet domain proteins have been documented as readers of histone modifications and function in epigenetic regulation (Nielsen et al., 2002; Min et al., 2003; Zhang et al., 2018; Zhao et al., 2019a). We searched for the candidate upstream regulators of the SWO1-targeted genes obtained from ChIP-seq using Plant Regulomics (Ran et al., 2020), and found a high enrichment of H2AK121ub in these genes. The histone modification H2AK121ub is written by H2A E3 ubiquitin ligase PRC1 and removed by ubiquitin-specific proteases UBP12 and UBP13 (Cao et al., 2005; Derkacheva et al., 2016). H2AK121ub is associated with a less accessible but still permissive chromatin at transcriptional regulation hotspots (Yin et al., 2021). H2AK121ub modification can repress gene expression via PRC2-mediated H3K27me3 deposition, while at the same time, it has an anti-repressive role possibly due to the recruitment of the H3K27-demethylase REF6 (Kralemann et al., 2020). In our IP-MS assay, UBP12 and UBP13 were co-purified with SWO1 (Table S5). Whether SWO1 transcriptionally regulates its target genes through binding and modifying H2AK121ub under salt stress remains to be studied. Since SWO1 also pulled down the chromatin remodeling protein CHR11 (Table S5) in the IP-MS assay, whether SWO1 is involved in chromatin remodeling and nucleosome patterning under salt stress needs further investigation. In brief, our study reveals a new component that is important for the regulation of cell wall integrity under salt stress and provides novel insights into the mechanisms that plants use to transduce environmental stimuli to the nucleus to control the expression of cell wall-associated genes under salt stress.
Materials and methods
Plant materials, growth conditions and genetic analysis
The Columbia ecotype of Arabidopsis thaliana was used in this study. The T-DNA insertion mutants swo1–1 (SAIL_236_A03), swo1–2 (SALK_201050C), swo1–3 (SALK_206899C), swo1–4 (SAIL_1284_D06), impa1–1 (SALK_082616C), and impa2–1 (SALK_149000C) were ordered from Arabidopsis Biological Resource Center (ABRC). The seeds were germinated and grown on half-strength Murashige & Skoog (MS) media for 7–10 days, and then the seedlings were transferred into soil and grown at 23 °C with a long-day light cycle of 16 h light/8 h dark.
Double and triple mutants were generated via hybridization, and the homozygous mutants were identified by PCR in the F2 population. Genomic DNAs isolated from 0.1 g of leaves were used for PCR. The primers used for PCR are listed in Table S1.
Generation of transgenic plants
For complementation and subcellular localization analysis, we generated SWO1pro:SWO1-GFP construct, in which the expression of SWO1-GFP is driven by the native promoter of SWO1 (2168 bp upstream region). The full-length genomic SWO1 sequence was amplified using the wild type genomic DNA as a template. For the truncated SWO1 proteins fused with GFP, the promoter fragment was amplified from the wild type genomic DNA, and the coding sequences of the N (aa 1–1000) and C (aa 1001–2037) terminus were amplified using the wild type cDNA as a template. The fragments were cloned into pCAMBIA1300 vector with a GFP tag at the C terminus. These constructs were transformed into swo1–2 plants. A similar procedure was applied for cloning the full-length genomic DNA of SWO1 to pCAMBIA1305 vector with a 3 × Myc tag at the C terminus. The resulting SWO1pro:SWO1g-Myc construct was transformed to swo1–1 mutant. The entire coding region of IMPA1 or IMPA2 together with 2 kb of the upstream fragments were amplified using the wild type genomic DNA as a template, and cloned into pCAMBIA1300-GFP and pCAMBIA1305–3 × Myc vectors, respectively. The primers used for constructs are listed in Table S1.
All clones were confirmed by sanger sequencing and the recombinant constructs were transformed into A. tumefaciens strain GV3101. Floral dip method (Clough & Bent, 1998) was used for transformation, and the transgenic plants were screened on the media containing 50 mg/L hygromycin.
RNA-seq and gene expression analysis
For transcriptome analysis, seven-day-old wild type and swo1–2 seedlings grown on vertical MS media were transferred to liquid MS media supplemented with 250 mM NaCl for 0, 2, and 14 h. Roots were collected and total RNAs were extracted using the RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions. Sequencing was carried out by using Illumina NovaSeq 6000 platform. After processing of the raw data, clean reads were mapped to Arabidopsis genome sequences downloaded from TAIR 10 database. Differentially expressed genes (DEGs) were identified according to the published method (Robinson et al., 2010), with a criteria of fold change≥1.5 and P-value<0.05.
For gene expression analysis, total RNA was isolated from seven-day-old seedlings and 1 μg of the total RNA was reversely transcribed to cDNA using TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech). Quantitative RT-PCR was carried out on a CFX96 real-time system (Bio-Rad) using SYBR Premix Ex-Taq (Takara) with the specific primers listed in Table S1.
Abiotic stress treatment
For growth under stress conditions, Arabidopsis seeds were surface sterilized with 5% (v/v) NaClO containing 0.01% Triton-X-100 (v/v) for 10 min, and then washed 5 times before being sown on MS media. Four-day-old seedlings were transferred to vertical media supplemented with NaCl, mannitol, or chemicals under indicated concentrations and grown for the indicated times. The length of primary roots was measured using Image J software. One-way ANOVA or Student’s t test was used for statistical analysis.
Quantification of Na+ and K+
Four-day-old seedlings of the wild type, swo1–2, swo1–3, and swo1–4 were transferred to 100 mM NaCl media for 10 days. For comparison of Na+ and K+ accumulation, the roots and shoots of the treated plants were harvested, respectively, and rinsed five times with distilled water. All samples were fully dried at 65 °C for 4 days and weighed, followed by digestion with HNO3/HClO4 (85:15, v/v) at 135 °C for 4 h. The concentrations of Na+ and K+ were determined using Inductively Coupled Plasma Mass Spectrometry (ICP-MS, PerkinElmer NexION300D).
Determination of cell wall components
To observe cellulose accumulation in roots after salt treatment, four-day-old seedlings were transferred to MS media with or without 100 mM NaCl. After growth for additional 6 days, roots were fixed in 2.5% glutaraldehyde in 0.2 M sodium phosphate buffer (PH 7.0) for 24 h at 4 °C, followed by staining with 0.2% calcofluor (Sigma-Aldrich). Images were taken using a confocal laser microscope (TCS SP8, Leica) at 405 nm.
To quantify the contents of cell wall polymers, the seedlings treated with NaCl for 14 days were harvested. Shoots and roots of the seedlings were detached and measured separately. For the quantification of cellulose and lignin contents, alcohol insoluble residue (AIR) was prepared and starch was removed according to Foster et al., (2010a). After sequential removal of lipids, proteins, and non-cellulosic polysaccharides, cellulose content was determined based on the quantification of glucose via the anthrone-sulfuric acid colorimetric assay (Scott & Melvin, 1953). Total lignin content was quantified via acetyl bromide soluble lignin (ABSL) assay (Foster et al., 2010b). To quantify the total pectin content, samples were fine powdered with liquid nitrogen and washed twice with 96% ethanol. The dried sediment was re-suspended with ddH2O and vacuum freeze dried. Pectin was finally dissolved and isolated in sulfuric acid overnight, and the content was determined colorimetrically according to a previous method (Blumenkr & Asboehan, 1973).
Detection of reactive oxygen species
For H2O2 staining, seedlings were incubated in 1 mg mL− 1 3, 3′-Diaminobenzidine (DAB) (Sigma) dissolved in 50 mM Tris-acetate buffer (PH 5.0) for 30 min in dark at room temperature. The seedlings stained with DAB were transferred to de-staining buffer (acetic acid: glycerol: ethanol = 1:1:3, v/v) and boiled for 10 min. Once chlorophyll was completely removed, the de-staining buffer was replaced with 95% ethanol (v/v), followed by visual score for color intensity. The 2′,7′- dichlorofluorescein diacetate (H2DCF-DA) staining assay for ROS estimation was performed as described previously (Foreman et al., 2003). In brief, seedling roots were incubated in 20 μM H2DCF-DA (Invitrogen) for 1 h at 4 °C and then washed with PBS buffer for 3 times. Fluorescent signals of ROS production were detected using microscope (DP72, Olympus).
Microscopic observation
To observe root cell morphology of Arabidopsis under salt stress, four-day-old seedlings were transferred to 100 mM NaCl medium for 7 days. Roots were then excised and root tips were observed with differential interference contrast (DIC) under bright field using Axio Imager upright microscope (Zeiss). For the detection of subcellular localization, the roots of eight-day-old transgenic Arabidopsis, or the Nicotiana Benthamiana leaves that were transformed with plasmids for 2 days, were observed using confocal laser scanning microscopy (TCS SP8, Leica).
Split-LUC complementation assay
The open reading frames of SWO1, IMPA1, and IMPA2 were amplified from cDNA of the wild type Arabidopsis plants and cloned into pCAMBIA1300-nLUC and pCAMBIA1300-cLUC, respectively. To generate the truncated SWO1 proteins fused with LUC, the corresponding CDSs were cloned into the LUC vector to form SWO1-N (aa 1–1000) and SWO1-C (aa 1001–2038) constructs. The recombinant plasmids were transformed into Agrobacterium stain GV3101. After cultivation with 220 rpm at 28 °C overnight, Agrobacteria were harvested and re-suspended with infiltration buffer (10 mM MgCl2, 10 mM MES pH 5.6, and 100 μM acetosyringone) to a final concentration of OD600 = 0.5. Corresponding combinations of plasmids were infiltrated into Nicotiana benthamiana leaves after 2 h incubation at room temperature in dark. Followed by 48 h growth in chamber, tobacco leaves were sprayed with luciferin, and signals were detected using a CCD camera (PoLoN 1300B).
Immunoprecipitation and mass spectrometry (IP-MS)
Total protein extraction and IP were performed as described previously (Law et al., 2010). In brief, epitope-tagged transgenic seedlings were powdered and suspended in lysis buffer (50 mM Tris pH 7.6, 150 mM NaCl, 5 mM MgCl2, 10% glycerol, 0.1% NP-40, 0.5 mM DTT, 1 mM PMSF, and protease inhibitor cocktail). Samples were completely homogenized, followed by centrifugation at 4 °C. Supernatants were incubated with pre-equilibrated anti-Myc agarose beads (Sigma). After incubation at 4 °C with rotation for 2 h, the beads were washed for 6 times with 5 min each time using lysis buffer, and 3 times with PBS buffer. The agarose beads were finally re-suspended in PBS buffer and used for mass spectrometry according to a previous publication (Wang et al., 2015).
Arabidopsis protoplast assay
Arabidopsis protoplasts were isolated from three- to four-week-old wild type, swo1–2, impa1–1 impa2–1, and kpnb1 plants grown under short day (10 h light/14 h dark) conditions. Well-expanded leaves were stripped with abaxial epidermis and submerged into the enzyme solution (1.5% cellulose R10, 0.4% macrozyme R10, 0.4 M mannitol, 20 mM KCl, 20 mM MES, 10 mM CaCl2, and 1% BSA). After digestion for 1–2 h in dark using a shaker at 50 rpm, an equal volume of W5 solution (0.1% Glucose, 0.08% KCl, 0.9% NaCl, 1.84% CaCl2·2H2O, and 2 mM MES) was added and the solutions containing protoplasts were filtered with a 55 μm nylon mesh. Followed by washing with W5 solution, the protoplasts were re-suspended in W5 solution, and kept on ice for 30 min in dark. After centrifugation, the concentration of cells was adjusted to 1–2 × 105 mL− 1 using MMg solution (0.4 M mannitol, 15 mM MgCl2, and 4 mM MES) for transformation. PEG-mediated transformation was performed according to a previous method (Yoo et al., 2007), and fluorescence signals were observed using confocal laser scanning microscopy (TCS SP8, Leica) after transformation for 16 h.
ChIP-seq assay
Chromatin immunoprecipitation (ChIP) assay was performed according to a previously described protocol with some modifications (Saleh et al., 2008). 5 g of the fourteen-day-old wild type and SWO1pro:SWO1-GFP seedlings were collected for cross-linking with 1% formaldehyde in PBS buffer and fine powdered in liquid nitrogen. Sonicated chromatin was incubated with anti-GFP magnetic beads GFP-Trap-MA (Chromotek) overnight. Immunoprecipitated DNA was purified with the QIAquick purification kit (Qiagen) and sent for sequencing. The Illumina Hiseq X Ten system was used for sequencing.
For ChIP-seq analysis, the pair-end reads were processed by Trimimomatic to trim the adapter sequences (Bolger et al., 2014). After the removal of low quality bases and the filter of short reads, clean reads were retained and mapped to the A. thaliana genome (TAIR10) by Bowtie 2 (version 2.2.8) (Langmead & Salzberg, 2012). Enriched peaks were identified by MACS (version 1.4) (Zhang et al., 2008), and the target genes were defined as the range from 1 kb upstream of transcription start site (TSS) to transcription end site (TES). The target genes of each peak were annotated by annotatePeak function in ChIPseeker package (Yu et al., 2015). The visualization of the average read coverage over gene body and additional 1.5 kb up- and down-stream of the TSS and TES was conducted by deepTools (version 2.4.1) (Ramirez et al., 2016). The statistical test of the overlap between two gene lists was performed by GeneOverlap package (Shen & Sinai, 2013).
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. The RNA-seq and ChIP-seq data have been deposited in the NCBI GEO under accession number: GSE176434.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- MS:
-
Murashige and Skoog
- NLS:
-
Nuclear localization signal
- NES:
-
Nuclear export signal
- DEGs:
-
Differentially expressed genes
- GO:
-
Gene ontology
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- AIR:
-
Alcohol insoluble residue
- ABSL:
-
Acetyl bromide soluble lignin
- ROS:
-
Reactive oxygen species
- DAB:
-
3, 3′-Diaminobenzidine
- H2DCF-DA:
-
2′,7′-dichlorofluorescein diacetate
- DPI:
-
Diphenyleneiodonium chloride
- DIC:
-
Differential interference contrast
- ChIP:
-
Chromatin immunoprecipitation
- TSS:
-
Transcription start site
- TES:
-
Transcription end site
- RT-PCR:
-
Reverse transcription-PCR
- IP-MS:
-
Immunoprecipitation and mass spectrometry
- split-LUC:
-
Split luciferase
- NPC:
-
Nuclear pore complex
- NE:
-
Nuclear envelope
- CHS:
-
Chalcone synthase
- IGV:
-
Integrative genomics viewer
References
Anderson CT, Kieber JJ (2020) Dynamic construction, perception, and remodeling of plant cell walls. Annu Rev Plant Biol 71(1):39–69. https://doi.org/10.1146/annurev-arplant-081519-035846
Bhattacharjee S, Lee LY, Oltmanns H, Cao HB, Veena Cuperus J, Gelvin SB (2008) IMPa-4, an Arabidopsis importin alpha isoform, is preferentially involved in Agrobacterium-mediated plant transformation. Plant Cell 20(10):2661–2680. https://doi.org/10.1105/tpc.108.060467
Blumenkr N, Asboehan G (1973) New method for quantitative-determination of uronic acids. Anal Biochem 54(2):484–489. https://doi.org/10.1016/0003-2697(73)90377-1
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Brasil JN, Cabral LM, Eloy NB, Primo LMF, Barroso-Neto IL, Grangeiro LPP, Gonzalez N, Inze D, Ferreira PCG, Hemerly AS (2015) AIP1 is a novel Agenet/Tudor domain protein from Arabidopsis that interacts with regulators of DNA replication, transcription and chromatin remodeling. BMC Plant Biol 15:207
Cao R, Tsukada Y-I, Zhang Y (2005) Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell 20(6):845–854. https://doi.org/10.1016/j.molcel.2005.12.002
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x
Coursey T, Milutinovic M, Regedanz E, Brkljacic J, Bisaro DM (2018) Arabidopsis histone reader EMSY-LIKE 1 binds H3K36 and suppresses geminivirus infection. J Virol 92(16):e00219–e00218
Derkacheva M, Liu S, Figueiredo DD, Gentry M, Mozgova I, Nanni P, Tang M, Mannervik M, Kohler C, Hennig L (2016) H2A deubiquitinases UBP12/13 are part of the Arabidopsis polycomb group protein system. Nature Plants 2(9):16126. https://doi.org/10.1038/nplants.2016.126
Ems-McClung SC, Zheng YX, Walczak CE (2004) Importin alpha/beta and Ran-GTP regulate XCTK2 microtubule binding through a bipartite nuclear localization signal. Mol Biol Cell 15(1):46–57. https://doi.org/10.1091/mbc.e03-07-0454
Endler A, Kesten C, Schneider R, Zhang Y, Ivakov A, Froehlich A, Funke N, Persson S (2015) A mechanism for sustained cellulose synthesis during salt stress. Cell 162(6):1353–1364. https://doi.org/10.1016/j.cell.2015.08.028
FAO (2011) The state of the world’s land and water resources for food and agriculture (SOLAW)—managing systems at risk. Food and agriculture Organization of the United Nations. Rome and Earthscan, London http://www.fao.org/ag/agl/agll/spush
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422(6930):442–446. https://doi.org/10.1038/nature01485
Foster CE, Martin TM, Pauly M (2010a) Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part II: carbohydrates. J Vis Exp 37:e1837
Foster CE, Martin TM, Pauly M (2010b) Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part I: lignin. J Vis Exp 37:e1745
Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA (2004) Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol 14(9):505–514. https://doi.org/10.1016/j.tcb.2004.07.016
Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW (2001) Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104(1):83–93. https://doi.org/10.1016/S0092-8674(01)00193-3
Hachet V, Kocher T, Wilm M, Mattaj IW (2004) Importin α associates with membranes and participates in nuclear envelope assembly in vitro. EMBO J 23(7):1526–1535. https://doi.org/10.1038/sj.emboj.7600154
Hamann T (2012) Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front Plant Sci 3:77
Jing Y, Shi L, Li X, Zheng H, Gao J, Wang M, He L, Zhang W (2019) OXS2 is required for salt tolerance mainly through associating with salt inducible genes, CA1 and Araport11, in Arabidopsis. Sci Rep 9(1):20341. https://doi.org/10.1038/s41598-019-56456-1
Klocko AD, Rountree MR, Grisafi PL, Hays SM, Adhvaryu KK, Selker EU (2015) Neurospora importin α is required for normal heterochromatic formation and DNA methylation. PLoS Genet 11(3):e1005083. https://doi.org/10.1371/journal.pgen.1005083
Kralemann LEM, Liu S, Trejo-Arellano MS, Muñoz-Viana R, Köhler C, Hennig L (2020) Removal of H2Aub1 by ubiquitin-specific proteases 12 and 13 is required for stable Polycomb-mediated gene repression in Arabidopsis. Genome Biol 21(1):144. https://doi.org/10.1186/s13059-020-02062-8
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with bowtie 2. Nat Methods 9(4):357–359. https://doi.org/10.1038/nmeth.1923
Law JA, Ausin I, Johnson LM, Vashisht AA, Zhu J-K, Wohlschlegel JA, Jacobsen SE (2010) A protein complex required for polymerase V transcripts and RNA-directed DNA methylation in Arabidopsis. Curr Biol 20(10):951–956. https://doi.org/10.1016/j.cub.2010.03.062
Law JA, Du JM, Hale CJ, Feng SH, Krajewski K, Palanca AMS, Strahl BD, Patel DJ, Jacobsen SE (2013) Polymerase IV occupancy at RNA-directed DNA methylation sites requires SHH1. Nature 498(7454):385–389. https://doi.org/10.1038/nature12178
Law JA, Vashisht AA, Wohlschlegel JA, Jacobsen SE (2011) SHH1, a homeodomain protein required for DNA methylation, as well as RDR2, RDM4, and chromatin remodeling factors, associate with RNA polymerase IV. PLoS Genet 7(7):e1002195. https://doi.org/10.1371/journal.pgen.1002195
Lee C, Teng Q, Huang W, Zhong R, Ye ZH (2009) The F8H glycosyltransferase is a functional paralog of FRA8 involved in glucuronoxylan biosynthesis in Arabidopsis. Plant Cell Physiology 50(4):812–827. https://doi.org/10.1093/pcp/pcp025
Lee Y, Choi D, Kende H (2001) Expansins: ever-expanding numbers and functions. Curr Opin Plant Biol 4(6):527–532. https://doi.org/10.1016/S1369-5266(00)00211-9
Li Y, Liu W, Zhong H, Zhang HL, Xia Y (2019) Redox-sensitive bZIP68 plays a role in balancing stress tolerance with growth in Arabidopsis. Plant J 100(4):768–783. https://doi.org/10.1111/tpj.14476
Lisso J, Altmann T, Müssig C (2006) The AtNFXL1 gene encodes a NF-X1 type zinc finger protein required for growth under salt stress. FEBS Lett 580(20):4851–4856. https://doi.org/10.1016/j.febslet.2006.07.079
Liu YL, Min JR (2016) Structure and function of histone methylation-binding proteins in plants. Biochemical J 473(12):1663–1680. https://doi.org/10.1042/BCJ20160123
Luo YJ, Wang ZJ, Ji HT, Fang H, Wang SF, Tian LN, Li X (2013) An Arabidopsis homolog of importin β is required for ABA response and drought tolerance. Plant J 75(3):377–389. https://doi.org/10.1111/tpj.12207
Maurer-Stroh S, Dickens NJ, Hughes-Davies L, Kouzarides T, Eisenhaber F, Ponting CP (2003) The Tudor domain 'Royal Family': Tudor, plant Agenet, chromo, PWWP and MBT domains. Trends Biochem Sci 28(2):69–74. https://doi.org/10.1016/S0968-0004(03)00004-5
Min JR, Zhang Y, Xu RM (2003) Structural basis for specific binding of polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev 17(15):1823–1828. https://doi.org/10.1101/gad.269603
Miyamoto Y, Yamada K, Yoneda Y (2016) Importin alpha: a key molecule in nuclear transport and non transport functions. J Biochem 160(2):69–75. https://doi.org/10.1093/jb/mvw036
Nachury MV, Maresca TJ, Salmon WG, Waterman-Storer CM, Heald R, Weis K (2001) Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 104(1):95–106. https://doi.org/10.1016/S0092-8674(01)00194-5
Nielsen PR, Nietlispach D, Mott HR, Callaghan J, Bannister A, Kouzarides T, Murzin AG, Murzina NV, Laue ED (2002) Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature 416(6876):103–107. https://doi.org/10.1038/nature722
Oka M, Yoneda Y (2018) Importin alpha: functions as a nuclear transport factor and beyond. Proc Japan Academy, Series B, Physical Biological Sciences 94(7):259–274. https://doi.org/10.2183/pjab.94.018
Palma K, Zhang Y, Li X (2005) An importin alpha homolog, MOS6, plays an important role in plant innate immunity. Curr Biol 15(12):1129–1135. https://doi.org/10.1016/j.cub.2005.05.022
Quimby BB, Dasso M (2003) The small GTPase Ran: interpreting the signs. Curr Opin Cell Biol 15(3):338–344. https://doi.org/10.1016/S0955-0674(03)00046-2
Ramirez F, Ryan DP, Gruning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dundar F, Manke T (2016) deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44(W1):W160–W165. https://doi.org/10.1093/nar/gkw257
Ran X, Zhao F, Wang Y, Liu J, Zhuang Y, Ye L, Qi M, Cheng J, Zhang Y (2020) Plant Regulomics: a data-driven interface for retrieving upstream regulators from plant multi-omics data. Plant J 101(1):237–248. https://doi.org/10.1111/tpj.14526
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140. https://doi.org/10.1093/bioinformatics/btp616
Roth C, Ludke D, Klenke M, Quathamer A, Valerius O, Braus GH, Wiermer M (2017) The truncated NLR protein TIR-NBS13 is a MOS6/IMPORTIN-alpha 3 interaction partner required for plant immunity. Plant J 92(5):808–821. https://doi.org/10.1111/tpj.13717
Saleh A, Alvarez-Venegas R, Avramova Z (2008) An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protoc 3(6):1018–1025. https://doi.org/10.1038/nprot.2008.66
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61(1):263–289. https://doi.org/10.1146/annurev-arplant-042809-112315
Scott TA, Melvin EH (1953) Determination of dextran with anthrone. Anal Chem 25(11):1656–1661. https://doi.org/10.1021/ac60083a023
Shani Z, Dekel M, Roiz L, Horowitz M, Kolosovski N, Lapidot S, Alkan S, Koltai H, Tsabary G, Goren R, Shoseyov O (2006) Expression of endo-1,4-beta-glucanase (cel1) in Arabidopsis thaliana is associated with plant growth, xylem development and cell wall thickening. Plant Cell Rep 25(10):1067–1074. https://doi.org/10.1007/s00299-006-0167-9
Shen L, Sinai M (2013) GeneOverlap: Test and visualize gene overlaps. R package version 1.6.0. http://shenlab-sinai.github.io/shenlab-sinai/
Shi HZ, Kim Y, Guo Y, Stevenson B, Zhu J-K (2003) The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell 15(1):19–32. https://doi.org/10.1105/tpc.007872
Somerville C (2006) Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol 22(1):53–78. https://doi.org/10.1146/annurev.cellbio.22.022206.160206
Tsugama D, Yoon HS, Fujino K, Liu S, Takano T (2019) Protein phosphatase 2A regulates the nuclear accumulation of the Arabidopsis bZIP protein VIP1 under hypo-osmotic stress. J Exp Bot 70(21):6101–6112. https://doi.org/10.1093/jxb/erz384
Tsukagoshi H (2016) Control of root growth and development by reactive oxygen species. Curr Opin Plant Biol 29:57–63. https://doi.org/10.1016/j.pbi.2015.10.012
Van Oosten MJ, Sharkhuu A, Batelli G, Bressan RA, Maggio A (2013) The Arabidopsis thaliana mutant air1 implicates SOS3 in the regulation of anthocyanins under salt stress. Plant Mol Biol 83(4–5):405–415. https://doi.org/10.1007/s11103-013-0099-z
Verslues PE, Guo Y, Dong CH, Ma W, Zhu J-K (2006) Mutation of SAD2, an importin beta-domain protein in Arabidopsis, alters abscisic acid sensitivity. Plant J 47(5):776–787. https://doi.org/10.1111/j.1365-313X.2006.02833.x
Wang P, Du Y, Hou YJ, Zhao Y, Hsu CC, Yuan F, Zhu X, Tao WA, Song CP, Zhu J-K (2015) Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc Nat Acad Sci USA 112(2):613–618. https://doi.org/10.1073/pnas.1423481112
Weber M, Deinlein U, Fischer S, Rogowski M, Geimer S, Tenhaken R, Clemens S (2013) A mutation in the Arabidopsis thaliana cell wall biosynthesis gene pectin methylesterase 3 as well as its aberrant expression cause hypersensitivity specifically to Zn. Plant J 76(1):151–164. https://doi.org/10.1111/tpj.12279
Wilbur JD, Heald R (2013) Mitotic spindle scaling during Xenopus development by kif2a and importin alpha. Elife 2:e00290. https://doi.org/10.7554/eLife.00290
Xu SL, Rahman A, Baskin TI, Kieber JJ (2008) Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20(11):3065–3079. https://doi.org/10.1105/tpc.108.063354
Yin X, Romero-Campero FJ, de Los Reyes P, Yan P, Yang J, Tian G, Yang X, Mo X, Zhao S, Calonje M, Zhou Y (2021) H2AK121ub in Arabidopsis associates with a less accessible chromatin state at transcriptional regulation hotspots. Nat Commun 12(1):315. https://doi.org/10.1038/s41467-020-20614-1
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2(7):1565–1572. https://doi.org/10.1038/nprot.2007.199
Yu G, Wang LG, He QY (2015) ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31(14):2382–2383. https://doi.org/10.1093/bioinformatics/btv145
Zhang C, Du X, Tang K, Yang Z, Pan L, Zhu P, Luo J, Jiang Y, Zhang H, Wan H, Wang X, Wu F, Tao WA, He XJ, Zhang H, Bressan RA, Du J, Zhu JK (2018) Arabidopsis AGDP1 links H3K9me2 to DNA methylation in heterochromatin. Nat Commun 9(1):4547. https://doi.org/10.1038/s41467-018-06965-w
Zhang SS, Sun L, Dong XR, Lu SJ, Tian WD, Liu JX (2016) Cellulose synthesis genes CESA6 and CSI1 are important for salt stress tolerance in Arabidopsis. J Integr Plant Biol 58(7):623–626. https://doi.org/10.1111/jipb.12442
Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9(9):R137. https://doi.org/10.1186/gb-2008-9-9-r137
Zhao CZ, Zayed O, Yu ZP, Jiang W, Zhu PP, Hsu CC, Zhang LR, Tao WA, Lozano-Duran R, Zhu J-K (2018a) Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc Nat Acad Sci USA 115(51):13123–13128. https://doi.org/10.1073/pnas.1816991115
Zhao CZ, Zayed O, Zeng FS, Liu CX, Zhang L, Zhu PP, Hsu CC, Tuncil YE, Tao WA, Carpita NC, Zhu JK (2019b) Arabinose biosynthesis is critical for salt stress tolerance in Arabidopsis. New Phytol 224(1):274–290. https://doi.org/10.1111/nph.15867
Zhao JF, Zhang WH, Zhao Y, Gong XM, Guo L, Zhu GL, Wang XC, Gong ZZ, Schumaker KS, Guo Y (2007) SAD2, an importin beta-like protein, is required for UV-B response in Arabidopsis by mediating MYB4 nuclear trafficking. Plant Cell 19(11):3805–3818. https://doi.org/10.1105/tpc.106.048900
Zhao S, Cheng LL, Gao YF, Zhang BC, Zheng XD, Wang L, Li PL, Sun QW, Li HT (2019a) Plant HP1 protein ADCP1 links multivalent H3K9 methylation readout to heterochromatin formation. Cell Res 29(1):54–66. https://doi.org/10.1038/s41422-018-0104-9
Zhao S, Zhang BC, Yang M, Zhu JS, Li HT (2018b) Systematic profiling of histone readers in Arabidopsis thaliana. Cell Rep 22(4):1090–1102. https://doi.org/10.1016/j.celrep.2017.12.099
Zheng M, Liu X, Lin J, Liu X, Wang Z, Xin M, Yao Y, Peng H, Zhou DX, Ni Z, Sun Q, Hu Z (2019) Histone acetyltransferase GCN5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J 97(3):587–602. https://doi.org/10.1111/tpj.14144
Zhu JH, Lee BH, Dellinger M, Cui XP, Zhang CQ, Wu S, Nothnagel EA, Zhu J-K (2010) A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. Plant J 63(1):128–140. https://doi.org/10.1111/j.1365-313X.2010.04227.x
Acknowledgements
We thank Prof. Xia Li from Huazhong Agricultural University for kindly providing GFP-NLS-CHS-NES(-)Rev plasmid.
Funding
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, Grant XDB27040101 (to J-K.Z.), Shanghai Pujiang Program, Grant 20PJ1414800 (to C.Z.), and National Natural Science Foundation of China, Grant No. 32070295 (to C.Z.).
Author information
Authors and Affiliations
Contributions
J.-K.Z. and C.Z. conceived and supervised this study. Z.W., M.W., H.S., and C.Z. designed the experiments; Z.W. and M.W. performed most of the experiments; M.W. constructed plasmids; C.Y. assisted in the analysis of cell wall content, and conducted ionomics assays; L.Z. performed ChIP assay; G.Q. conducted proteomics assays; L.P. analyzed ChIP-seq data; Q.Z. analyzed RNA-seq data; W.N. provided the plant material swo1–1; Z.W. wrote the manuscript with constructive input from all authors; and C.-P.S., J.-K.Z., and C.Z. revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Author C.-P.S., H.Z. and J.-K.Z. are members of the Editorial Board, but were not involved in the journal's review of, or decisions related to, this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1 Figure S1
Mutation in SWO1 gene results in salt-hypersensitivity. (a) Phenotypes of the wild type and swo1–1 seedlings grown on MS and MS + 100 mM NaCl media. Bar = 1 cm. (b) Quantification of the root lengths of the wild type and swo1–1 grown on MS and MS + 100 mM NaCl media. Data are means ± SD (n = 6); **represents significant differences between the wild type and swo1–1, P < 0.01 (Student’s t test). (c) Phenotypes of the wild type and swo1–1 grown on MS and MS + 325 mM mannitol media. (d) Quantification of the root lengths of the wild type and swo1–1 grown on MS and MS + 325 mM mannitol media. Data are means ± SD (n = 6). (e) Phenotypes of the wild type, swo1–1, and SWO1 complementation lines grown on MS and MS + 100 mM NaCl media. (f) Comparison of the root length of the wild type, swo1–1, and complementation plants after being transferred to MS and MS + 100 mM NaCl media. Values are means ± SD (n = 8). Different letters represent significant differences between different genotypes under the same treatment, P < 0.01 (one-way ANOVA).
Additional file 2 Figure S2
Characterization of swo1 mutants. (a) Schematic diagram shows the positions of the T-DNA insertions in different swo1 mutant alleles. Black rectangles and lines represent exons and introns, respectively. Arrows and red lines indicate the primers used for genotyping. (b) Genotyping of the swo1 mutant alleles using specific primers. (c) Semi-quantitative RT-PCR analysis of the transcript level of SWO1 in the wild type and swo1 mutant alleles. ACTIN2 was used as the internal control. (d) qRT-PCR analysis of the transcript level of SWO1 in the wild type and different swo1 mutant alleles. ACTIN2 was used as the internal control. Values are means ± SD (n = 3).
Additional file 3 Figure S3
SWO1 binds to genes involved in cell wall metabolism. IGV screenshots show the cell wall-associated genes that were bound by SWO1 in ChIP-seq assay and were differentially expressed in the swo1–2 after salt treatment for 14 h based on RNA-seq data.
Additional file 4 Figure S4
Both N- and C-termini are required for the localization and function of SWO1. (a) The left panel shows the localization of full-length and C-terminal of SWO1 protein in the transition zone of Arabidopsis roots. The right panel is the evaluation of fluorescence intensities across corresponding nuclei. The small white rounds in the left panel represent the zero position in the right panel. Scale bar = 20 μm. (b) RT-PCR analysis of the transcript levels of SWO1 N-terminal and C-terminal products in the wild type, swo1–2, and transgenic plants expressing full-length (SWO1-F), C-terminus (SWO1-C), and N-terminus (SWO1-N) of SWO1. ACTIN2 was used as the internal control. (c) qRT-PCR analysis of the transcript levels of SWO1 N-terminal and C-terminal products in the wild type, swo1–2, and transgenic plants expressing full-length and truncated SWO1. ACTIN2 was used as the internal control. Values are means ± SD (n = 3).
Additional file 5 Figure S5
Salt treatment does not affect the nuclear localization of SWO1. Time-course analysis of the subcellular localization of SWO1 after salt treatment from 0 min to 24 h in Arabidopsis roots. Scale bar = 20 μm.
Additional file 6 Figure S6
Characterization of higher-order mutants. qRT-PCR analysis of the transcript levels of IMPA1, IMPA2, and SWO1 in the wild type, swo1–2, impa1–1, impa2–1, swo1–2 impa1–1, swo1–2 impa2–1, impa1–1 impa2–1, and swo1–2 impa1–1 impa2–1 seedlings. ACTIN2 was used as the internal control. Values are means ± SD (n = 3).
Additional file 7 Figure S7
SWO1 is not required for the import of nuclear-localized proteins. (a) Arabidopsis protoplasts generated from the wild type, impa1–1 impa2–1, and kpnb1 were transiently transformed with the GFP-NLS-CHS-NES(−)Rev construct. Percentages of protoplasts emitting fluorescence from the nucleus were analyzed 16 h after transformation. Numbers of protoplasts used for analysis were shown on the columns (number of protoplasts emitting fluorescence from the nucleus/total number of transformed protoplasts). (b) Arabidopsis protoplasts generated from the wild type and the swo1–2 mutant were transiently transformed with the GFP-NLS-CHS-NES(−)Rev construct, and fluorescence was observed 16 h after transformation. Scale bar = 50 μm. (c) Subcellular localization of SWO1 in swo1–2 and impa1–1 impa2–1 mutants. Scale bar = 20 μm. (d) Subcellular localization of IMPA1 and IMPA2 in their own mutants or swo1–2 mutant. Scale bar = 20 μm.
Additional file 8 Figure S8
A proposed working model. SWO1 may function as a linker between NPC and specific chromatin regions. Under stress conditions, the imported importin-cargo complex can be captured by SWO1 in the nucleus, which facilitates the delivery of nuclear regulatory effectors to their target sites to regulate downstream gene expression.
Additional file 9 Table S1
Primers in this study.
Additional file 10 Table S2
RPKM values of genes in RNA-seq assay.
Additional file 11 Table S3
Shown are the class III peroxidases, cell wall loosening, and cell wall biogenesis and modification-related genes that are differentially expressed in swo1–2 after salt treatment for 14 h.
Additional file 12 Table S4
Peak information in SWO1 ChIP-seq assay.
Additional file 13 Table S5
NPC, NE components and the factors involved in epigenetic regulation were identified in SWO1 IP-MS.
Additional file 14 Table S6
Transcription factors that were identified in SWO1 IP-MS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Wang, M., Yang, C. et al. SWO1 modulates cell wall integrity under salt stress by interacting with importin ɑ in Arabidopsis. Stress Biology 1, 9 (2021). https://doi.org/10.1007/s44154-021-00010-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-021-00010-5