Abstract
In the Western-west area of El Minya Governorate, Egypt, the present study investigates the water quality and salinity of 33 groundwater samples. The primary aim is to conduct a comprehensive evaluation of water purity and gain insights into salinity dynamics. The investigation involves a combination of geochemical analysis and multivariate statistics to reveal significant findings that contribute to our understanding of groundwater characteristics in the region. The collected groundwater samples are analyzed to assess various properties, including pH levels, mineral content, hardness, and salinity. The prevalence of sodium (Na) and chloride (Cl) ions emerges as a noteworthy aspect, implying their derivation from deeper aquifers through mechanisms like upward seepage or reverse ion exchange. The observation is validated through Durov's plot analysis. Concerning the suitability of the water for consumption, the study raises concerns. Apart from magnesium (Mg) ions, all other parameters exceed the acceptable drinking water limits defined by the World Health Organization (WHO), indicating potential health implications related to water quality in the region. Furthermore, outcomes from the saturation index reveal an oversaturation of groundwater with evaporites and carbonates, with halite and sylvite minerals standing as exceptions to this trend. Negative chloroalkaline ratios offer insights into the origins of excessive Na and Cl ions in 72% of the samples, pointing towards processes such as rainy recharging and reverse ion exchange as contributors. Gibbs' chart analysis highlights evaporite dissolution as the predominant process influencing water composition. The study also utilizes principal component analysis, identifying the dissolution of halite as the primary source of Na, K, Cl, and SO4 ions, while the presence of calcium (Ca) and bicarbonate (HCO3) ions originates from the dissolution of carbonate minerals, thereby influencing water hardness and salinity levels. Considering the findings, we recommend the dilution of groundwater with fresh water, assuming the absence of additional contaminants. This step is essential for enhancing water safety for drinking purposes. This research not only advances our understanding of water quality and salinity dynamics in the region but also underscores the imperative for implementing sustainable water resource management strategies. Furthermore, we propose that future investigations delve into the broader environmental ramifications of these discoveries, thus contributing to a more holistic comprehension of the complexities associated with groundwater quality and salinity.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Groundwater plays a pivotal role in meeting essential water demands for domestic, industrial, and agricultural purposes, particularly in regions characterized by arid and semi-arid conditions [1]. However, the quality of groundwater in such areas faces degradation due to a complex interplay of factors. These include the dissolution of evaporites, variations in climate, surface runoff, unregulated fertilizer application, and the influx of saline waters from deep strata [2]. These activities, when combined, result in the generation of groundwater with a diverse composition of dissolved elements [3]. Thus, warranting a comprehensive understanding of the underlying processes.
Recent efforts have been directed toward the development of hydrogeochemical methodologies to comprehensively assess groundwater quality [4]. This involves the utilization of statistical and geostatistical models that aid in unraveling the intricate nature of groundwater characteristics. Mechanisms like adsorption, desorption, surface reduction, and cation exchange contribute to chemical interactions [5], particularly those involving ions such as calcium (Ca), magnesium (Mg), and sulfate (SO4) that influence the surface chemistry of rock substrates. Alterations in the predominant chemical makeup of groundwater are attributed to water–rock interactions, encompassing processes like mineral precipitation and dissolution [6], which in turn can significantly impact the salinity of the water [7]. The variability of replaceable solutes within aquatic systems can be attributed to diverse mineral content, dynamic kinematic conditions, and hydrological factors [8, 9], underscoring the complexity of groundwater composition dynamics.
Geochemical models have proven invaluable in both understanding and predicting the potential deterioration of groundwater resources [10]. To achieve an equilibrium portrayal of geochemical systems, an array of unique tools has been employed [11, 12]. These encompass models such as Durov, Gibbs, and end-member diagrams, as well as geo-statistical indices including the saturation index (SI) and chloro-alkaline indices (CAI), which facilitate the evaluation of interactions between rock and water. Further insights are derived by employing multivariate statistical techniques such as principal component analysis (PCA), allowing for a deeper interpretation of variable relationships. Prior scientific endeavors have successfully employed these models to elucidate the intricate geochemical processes governing interactions [6,7,8,9, 12,13,14,15,16,17,18,19,20,21,22,23,24,25], revealing the underlying mechanisms shaping groundwater quality.
Given the recent establishment of groundwater wells, the preservation of their purity holds the utmost significance. The principal objective of this study is to unravel and comprehend the geochemical processes that govern water chemistry and salinity within the research area. Through this endeavor, the research aims to contribute to a holistic understanding of groundwater quality dynamics, laying the foundation for informed water resource management strategies in the southern region of the Mediterranean basin.
1.1 Location
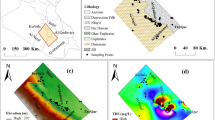
The study space is considered within the 1.5 million Feddan project, located in the western west area of El Minya district and spanning between the longitudes 29° 50–30° 15′ E and the latitudes 28° 10–28° 30′ N (Fig. 1). It acts as a section of the western limestone plateau. The portion included Pleistocene-Oligocene gravels, Quaternary sediments, Minia, Samalut, Makattam, and Moghra Formations, which constituted the stratigraphic series from base to top (Fig. 2) [26, 27]. It sources its water from the fractured Middle Eocene limestone wells, where the water-bearing layer is around 400 m thick and the water table is 70–110 m below the surface (Fig. 2) [28].
2 Material and methodology
Thirty-three samples taken of the groundwater in 2022 from the study site were filtered, acidified with nitric acid (pH < 2) [29], and stored in tight, pre-rinsed polypropylene containers (Fig. 1). Field measurements were recorded with the pH meter were pH, total dissolved solids (TDS), and electric conductivity (EC) values. The primary constituents that formed the water samples under evaluation were examined in the national water research center (NWRC) labs, and the ionic balance error (e%) was less than 5%.
Durov's diagram refers to the ion exchange condition and depicts the water type and dominating ions [30, 31]. Phreeqc Interactive version No. 3.4 was implemented to obtain the saturation index (SI) values of the dissolved minerals in water [13]. Positive scores determine the super-saturation, and negative signs denote the under-saturation [32].
The concentrations are supplied in epm to compute the chloro-alkaline indices (CAI) from the following equations (Eqs. 1, 2):
Positive results highlight the host rock's alkaline ions (Ca + Mg) being replaced with the alkali element of the groundwater (Na + K), indicating that forward ion exchange has taken place [33]. Negative readings signify the reverse exchange ion that provides surplus alkalis in the water [33]. Negative signs also explain the recharge from rainfall that seeps into wells [13, 34].
The Gibbs diagram (GD), which controls the chemistry of the water, identifies the geochemical processes in groundwater [13, 34]. These processes, like chemical weathering, dissolution, and precipitation, highlight how rocks and water interact [13, 35, 36]. Quantities of GA and GC are obtained using the following equations (Eqs. 3, 4), where the units of measurement are ppm.
Box-Whisker graphs and principal component analysis (PCA) apply using JASP Statistics version 0.16.4.0 and provide scaled results that aid in obtaining scientific interpretation.
3 Result and discussion
3.1 Hydrogeochemical characteristics
The descriptive data portrayed that the pH of the water differed from low acidic (6.2) to slightly basic (7.96; Table 1 and Fig. 3). EC values revealed that the water samples were overly mineralized (more than 1000 ppm; Table 1) [37]. TDS amounts fluctuated between slightly saline (1764 ppm) and moderately saline (3199 ppm; Table 1 and Fig. 3) [38]. Water samples were very hard (more than 300 ppm) according to TH levels (Table 1 and Fig. 3) [39]. According to ion averages, Cl and Na are dominant (563 and 897 ppm, respectively), which is proved by Durov's plot, where all water samples were projected in Field No. 9 except one, which was in Field No. 8 (Table 1 and Fig. 4). Durov's diagram's fields 8 and 9 proved that both Cl and Na predominated in the analyzed water [30, 31, 40]. It was indicated that groundwater had been mingled with Na-Cl water from deep aquifers by upward seepage and reverse ion exchange [13, 34, 41, 42].
All variables had asymmetrical patterns that were anomalous compared to WHO [43] standard allowed limits (SAL) for drinking water except alkalinity (Alk), magnesium, and HCO3 (Table 1 and Fig. 4), alluding to the fact that waters are unsafe to drink.
3.2 Geochemical modelling
3.2.1 Saturation index (SI) and chloro-alkaline Indices (CAI)
SI values depict amounts of soluble minerals in water [13, 32]. The waters were oversaturated with carbonate minerals (aragonite, calcite, and dolomite) and evaporites (anhydrite and gypsum), except for halite and sylvite minerals (Fig. 5). Hyper-saturated minerals in water tend to precipitate (Fig. 3), [13, 32]. Halite had a more meaningful impact than the other minerals on the salinity of waters, as supported by the positive correlation matrix results between TDS and soluble minerals, particularly halite (Table 2 and Fig. 5). It clarified Cl and Na prevailing because evaporite dissolves, notably halite minerals, which is reinforced by the significant link between halite and Na and Cl (r = 0.8; Table 2).
CAI shows the form of ion exchange that is most common in groundwater [13, 33]. Less than one-third of the samples revealed forward ion exchange between the earth's alkaline (Ca and Mg) in host rocks and alkalis (Na and K) in water, according to positive CAI ratios (Fig. 5), [13, 33]. Negative CAI values indicate that reverse ion exchange occurred in more than two-thirds of the samples, which results in a surplus of Na in the waters (Fig. 5) [13, 33]. The abundance of Na signified that the rain had recharged [13, 34].
3.2.2 Gibbs diagram (GD)
Dissolution, precipitation, and rock weathering are examples of geochemical processes where the predominant ones can control water chemistry, according to GD [35, 36]. GD determined that the evaporite dissolution is more controlling, causing an abundance of Na and Cl in the waters (Fig. 7) [35, 36]. Halite dissolution is the base source of such excesses, as evidenced by the close connection between it and Na and Cl (Table 2 and Fig. 6).
3.3 Multivariate statistics
3.3.1 Principal component analysis (PCA)
PC1 had a variance of 29.5%, referring to the dissolution of anhydrite and gypsum minerals, which are the prime sources of Ca and SO4 ions in the investigated water and have a moderate contribution to salinity (Table 3 and Fig. 7). PC2 indicates the dissolution of halite minerals, is the essential source of Cl and Na ions in the studied water and is the main contributor to the water's salinity with a variance of 22.7% (Table 3 and Fig. 7). PC3 indicates that dissolved carbonate minerals are the principal suppliers of HCO3 in the studied water and had a variation of 15.6%, not affecting salinity (Table 3 and Fig. 7). PC4 was estimated with a variance of 14.6%, revealing the decomposition of calcite and sylvite minerals are sources of Ca, K, and Cl ions in the analyzed water and contribute to water hardness and salinity (Table 3 and Fig. 7).
4 Conclusion
Water samples had low to medium salinity, extreme mineralization, a low acidic to weak basic pH, and much hard water. According to the classification provided by Durov's diagram, the predominance of Na and Cl signified water mixing with Na–Cl water due to upward water movement from deeper wells or via ion exchange. All variables were above the WHO guideline limit, except magnesium ions, which refer to tainted water. Si results revealed that all minerals, apart from halite and sylvite, were dissolving in excessively saturated waters. Negative CAI ratios proved reverse ion exchange had occurred, leading to an excess of Na and Cl in over two-thirds of the waters and the rainy recharge. According to GD, evaporated dissolution is the dominant process that led to the abundance of Na and Cl in water. PCA components demonstrated that the primary source of Na, K, Cl, and SO4 ions in waters was the dissolution of the evaporite minerals, notably halite. Also illustrated is that the principal source of Ca and HCO3 ions in water was the solution of carbonate minerals that contribute to salinity and hardness. Therefore, if no additional pollutants are present, the tested groundwater should be mixed with fresh water to limit its high salinity content and make it fit for drinking.
References
Li P, Wu J, Qian H (2013) Assessement of groundwater quality for irrigation purpose and identification of hydro-geochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69(7):2211–2225
Najib S, Fadili A, Mehdi K, Riss J, Makan A, Guessir H (2016) Salinization process and coastal groundwater in Chaouia, Morroco. J Afr Earth Sci 115:17–31
Moran-Ramirez J, Ruiz-Ledesma R, Mahlknecht J, Ramos-Leal JA (2016) Rockwater interactions and pollution processes in the volcanic aquifer system of Guadalajara, Mexico, using inverse geochemical modeling. Appl Geochem 68:79–94
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterisation of groundwater—a case study: Ain Azel plain (Algeria). Geoderma 156(3–4):390–398
Honarvar B, Rahimi A, Safari M, Khajehahmadi S, Karimi M (2020) Smart water effects on a crude oil–brine–carbonate rock (CBR) system: further suggestions on mechanisms and conditions. J Mol Liq 299:112173. https://doi.org/10.1016/j.molliq.2019.112173
Rendel PM, Mountain B, Feilberg KL (2022) Fluid–rock interaction during low-salinity water flooding of North Sea chalks. J Pet Sci Eng 214:110484. https://doi.org/10.1016/j.petrol.2022.110484
Mokhtari R, Anabaraonye BU, Afrough A, Mohammadkhani S, Feilberg KL (2022) Experimental investigation of low salinity water-flooding in tight chalk oil reservoirs. J Pet Sci Eng 208:109282. https://doi.org/10.1016/j.petrol.2021.109282
Huang T, Li Z, Long Y, Zhang F, Pang Z (2022) Role of desorption-adsorption and ion exchange in isotopic and chemical (Li, B, and Sr) evolution of water following water–rock interaction. J Hydrol 610:127800. https://doi.org/10.1016/j.jhydrol.2022.127800
Huang T, Li Z, Mayer B, Nightingale M, Li X, Li G, Long Y, Pang Z (2020) Identification of geochemical processes during hydraulic fracturing of a shale gas reservoir: a controlled field and laboratory water-rock interaction experiment. Geophys Res Lett 47:e2020GL090420
Li P, Qian H, Wu J, Ding J (2010) Geochemical modeling of groundwater in southern plain area of Pengyang County, Ningxia, China. Water Sci Eng 3(3):282–291
Helgeson CH (1968) Evaluation of irreversible reactions in geochemical processes involving minerals ans aqueous solutions. I. Thermodynamic relations. Geochem Cosmochim Acta 32:853–877
M’Nassria S, Lucas Y, Schafer G, Dridia L, Majdoub R (2019) Coupled hydrogeochemical modelling using KIRMAT to assess water-rock interaction in a saline aquifer in central-eastern Tunisia. Appl Geochem 102(2019):229–242. https://doi.org/10.1016/j.apgeochem.2019.01.018
Mohamed A, Asmoay A, Alshehri F, Abdelrady A, Othman A (2022) Hydro-geochemical applications and multivariate analysis to assess the water–rock interaction in arid environments. Appl Sci J 12(6343):1–13. https://doi.org/10.3390/app12136340
Mohamed A, Asmoay A, Alarifi Mohammed SM (2023) Simulation of surface and subsurface water quality in hyper-arid environments. Hydrology 10(4):86. https://doi.org/10.3390/hydrology10040086
Asmoay A, Mohamed A, Alshehri F, Linh NT, Al-Ansari N, Othman A (2023) Water quality assessment in dry regions using statistical methods. J King Saud Univ-Sci 35(5):102665. https://doi.org/10.1016/j.jksus.2023.102665
Bouteraa O, Mebark A, Bouaicha F, Nouaceur Z, Laigne B (2019) Groundwater quality assessment using multivariate analysis, geostatistical modeling, and water quality index (WQI): a case of study in the Boumerzoug-El Khroub valley of Northeast Algeria. Acta Geochim 38(6):796–814. https://doi.org/10.1007/s11631-019-00329-x
Barkat A, Bouaicha F, Bouteraa O, Mester T, Ata B, Balla D, Rahal Z, Szabó G (2021) Assessment of complex terminal groundwater aquifer for different use of Oued Souf valley (Algeria) using multivariate statistical methods, geostatistical modeling, and water quality index. Water 13(11):1609. https://doi.org/10.3390/w13111609
Asmoay A, Mabrouk W (2023) Appraisal of rock–water interaction and frailty of groundwater to corrosion and salinization, northwestern Gulf of Suez, Egypt. J Umm Al-Qura Univ Appl Sci. https://doi.org/10.1007/s43994-023-00075-0
Benmarce K, Hadji R, Hamed Y, Zahri F, Zighmi K, Hamad A, Gentilucci M, Ncibi K, Besser H (2023) Hydrogeological and water quality analysis of thermal springs in the Guelma region of North-Eastern Algeria: a study using hydrochemical, statistical, and isotopic approaches. J Afr Earth Sci 205:105011
Brahmi S, Baali F, Hadji R, Brahmi S, Hamad A, Rahal O, Zerrouki H, Saadali B, Hamed Y (2021) Assessment of groundwater and soil pollution by leachate using electrical resistivity and induced polarization imaging survey, case of Tebessa municipal landfill, NE Algeria. Arab J Geosci 14(4):1–13
Hamad A, Abdeslam I, Fehdi C, Badreddine S, Mokadem N, Legrioui R et al (2021) Vulnerability characterization for multi-carbonate aquifer systems in semiarid climate, case of Algerian–Tunisian transboundary basin. Int J Energy Water Resour 6:67–80
Hamad A, Baali F, Hadji R, Zerrouki H, Besser H, Mokadem N, Legrioui R, Hamed Y (2018) Hydrogeochemical characterization of water mineralization in Tebessa-Kasserine karst system (Tuniso-Algerian Transboundary basin). Euro-Mediterr J Environ Integr 3(1):7
Hamed Y, Hadji R, Ahmadi R, Ayadi Y, Shuhab K, Pulido-Bosch A (2023) Hydrogeological investigation of karst aquifers using an integrated geomorphological, geochemical, GIS, and remote sensing techniques (Southern Mediterranean Basin—Tunisia). Environ Dev Sustain 2023:1–33
Ncibi K, Mastrocicco M, Colombani N, Busico G, Hadji R, Hamed Y, Shuhab K (2022) Differentiating nitrate origins and fate in a semi-arid basin (Tunisia) via geostatistical analyses and groundwater modelling. Water 14(24):4124
Mohamadi A, Dumdum A, Bouaicha F, Menani M (2021) Evaluation of the quality of groundwater for its appropriateness for irrigation purposes using Water Quality Index (WQI), Mchira-Teleghma aquifer case study, northeastern Algeria. Sustain Water Resour Manag 7:97. https://doi.org/10.1007/s40899-021-00571-3
Said R (1990) The geology of Egypt. Balkema Publ. Comp, Rotterdam
Shabana AR (2010) Hydrogiological studies on the area West Deir Mouas-Mallawi, El Minia governorate-Egypt. Egypt J Geol 54:61–78
Ibrahem SMM, Elalfy M, Hagras MA (2020) Groundwater potentials of Eocene Limestone Aquifer in West-West El-Minya Area, Egypt. Egypt Desert Res 70(1):59–82
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington
Sajjala SR, Al Dawery SK, Ahmed A, Al Sakiti AHH (2019) A comparative study for quality of local and imported commercially available bottled water brands. Int J Hum Cap Urban Manag 4(2):77–86
Kechiched R, Nezli IE, Foufou A, Belksier MS, Benhamida SA, Djeghoubbi R, Slamene N, Zaimeche OI (2020) Fluoride-bearing groundwater in the complex terminal aquifer (a case study in Hassi Messaoud area, southern Algeria): hydrochemical characterization and spatial distribution assessed by indicator kriging. Sustain Water Resour Manag 6:54
Deutsch WJ (1997) Groundwater Geochemistry: fundamentals and applications to contamination. Lewis Publishers, New York
Schoeller H (1977) Geochemistry of groundwater, chap. 15. In: Brown RH (ed) Groundwater studies an international guide for research and practice. UNESCO, Paris, pp 1–18
Collins AG (1975) Geochemistry of oilfield waters. Elsevier, Amsterdam
Gibbs RJ (1970) Mechanisms controlling World’s water chemistry. Science 170(3962):1088–1090
Marandia A, Shand P (2018) Groundwater chemistry and the Gibbs diagram. Appl Geochem 97:209–212
Detay M (1997) Water wells: implementation, maintenance, and restoration. Wiley, London
Hem JD (1992) Study and interpretation of chemical characteristics of natural water (3d edn). U.S. Geological Survey Water-Supply Paper 2254, 263 pp
Boyd CE (2000) Water quality: an introduction. Kluwer, Boston
Lloyd JW, Heathcote JA (1985) Natural inorganic hydrochemistry in relation to groundwater, an introduction. Clarence Press, Oxford
Asmoay ASA (2017) Hydrogeochemical studies on the water resources and soil characteristics in the Western Bank of the River Nile between Abu Qurqas and Dayr Mawas, El Minya Governorate, Egypt. Ph.D. Thesis, Fac Sci, Al-Azhar Univ, Egypt
Sabet H, El-Gohary A, Salman S, Asmoay A (2017) Evaluation of surface water for different uses in the area between Abu Qurqas - Dyer Mawas districts, El Minya Governorate, Egypt. Int J Innov Sci Eng Technol 4(1):120–128
WHO (2022) Guidelines for drinking-water quality: fourth edition incorporating the first and second addenda. ISBN 978-92-4-004506-4
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
AA: Collecting the samples, and chemical analysis of samples. Conceptualization, collecting the data, interpretation, visualization and writing the main draft, revising, and editing the manuscript, preparing location map and land cover map, and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declare no competing financial interests.
Data availability
All the data are provided within the manuscript.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asmoay, A. Evaluating groundwater quality and salinity dynamics in the Western-west area of El Minya Governorate, Egypt, based on geochemical modelling and multivariate analysis. J.Umm Al-Qura Univ. Appll. Sci. 10, 91–101 (2024). https://doi.org/10.1007/s43994-023-00081-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00081-2